Abstract
The proportions of Haemophilus influenzae resistant to ampicillin and other β-lactam antibiotics have been low in Sweden compared to other countries in the Western world. However, a near-doubled proportion of nasopharyngeal Swedish H. influenzae isolates with resistance to β-lactams has been observed in the last decade. In the present study, the epidemiology and mechanisms of antimicrobial resistance of H. influenzae isolates from blood and cerebrospinal fluid in southern Sweden from 1997 to 2010 (n = 465) were studied. Antimicrobial susceptibility testing was performed using disk diffusion, and isolates with resistance to any tested β-lactam were further analyzed in detail. We identified a significantly increased (P = 0.03) proportion of β-lactam-resistant invasive H. influenzae during the study period, which was mainly attributed to a significant recent increase of β-lactamase-negative β-lactam-resistant isolates (P = 0.04). Furthermore, invasive β-lactamase-negative β-lactam-resistant H. influenzae isolates from 2007 and onwards were found in higher proportions than the corresponding proportions of nasopharyngeal isolates in a national survey. Multiple-locus sequence typing (MLST) of this group of isolates did not completely separate isolates with different resistance phenotypes. However, one cluster of β-lactamase-negative ampicillin-resistant (BLNAR) isolates was identified, and it included isolates from all geographical areas. A truncated variant of a β-lactamase gene with a promoter deletion, blaTEM-1-PΔ dominated among the β-lactamase-positive H. influenzae isolates. Our results show that the proportions of β-lactam-resistant invasive H. influenzae have increased in Sweden in the last decade.
INTRODUCTION
Invasive disease caused by the respiratory pathogen Haemophilus influenzae has in the past been synonymous with disease by encapsulated H. influenzae type b (Hib), a cause of meningitis and epiglottitis, mainly in children (6). Following the introduction of the conjugated Hib vaccine in the early 1990s (introduced in the National Swedish Childhood Immunization Schedule in 1992), a rapid decline in invasive Hib disease occurred (23). Invasive disease by non-type b isolates of H. influenzae, including nontypeable Haemophilus influenzae (NTHi) and encapsulated serotypes other than Hib, has mainly been considered an opportunistic infection. In the last decade, however, a number of reports indicated increasing incidence rates of invasive non-type b Haemophilus disease that were not merely related to infections in immunocompromised individuals (1, 3, 35). A similar increase of invasive disease by non-type b H. influenzae in Sweden during the years 1997 to 2009 was recently confirmed by us (26). Importantly, we found that both NTHi and Haemophilus influenzae type f (Hif) often cause severe sepsis in individuals with no evidence of immune suppression. More than 70% of bacteremic cases also had concurrent pneumonia (26). From our study and others, it is evident that the epidemiology of invasive H. influenzae disease in general has changed. Invasive H. influenzae disease mainly affected children in the pre-Hib vaccine era, but now it affects both the very young and the very old, and cases are most commonly seen in older adults.
Resistance to ampicillin in H. influenzae was first described in 1974 (17). In Sweden, as in many other countries, ampicillin is the main drug of choice in proven H. influenzae infections and the primary empirical treatment choice for respiratory tract infections, where H. influenzae can be suspected. Ampicillin resistance in H. influenzae is now globally widespread, with incidence rates varying from 8 to 30% in different European countries and North America to more than 50% in some East Asian countries (12, 13).
The nomenclature of resistant H. influenzae is complex, and since definitions vary between different studies and regions, the definitions used by us are outlined in Table 1. Isolates with resistance to ampicillin can be sorted into two main categories: those that carry a β-lactamase, and those that do not. The most common mechanism of β-lactam resistance in H. influenzae is by TEM-1 or ROB-1 β-lactamases (7), and such isolates are denoted β-lactamase positive, ampicillin resistant (BLPAR). The commonly used term β-lactamase negative, ampicillin resistant (BLNAR) is used for isolates with ampicillin resistance but no evidence of β-lactamase production. After this definition was established, it was concluded that ampicillin resistance in such isolates was due to key mutations in the ftsI gene (encoding penicillin-binding protein 3 [PBP-3]) that lowered the affinity for β-lactams (36). Subsequently, it became clear that some isolates had such mutations but were not ampicillin resistant according to phenotype testing. Isolates with key mutations in PBP-3 regardless of resistance phenotype are designated genomic BLNAR (gBLNAR), a group of isolates that overlaps with, but does not match, the BLNAR group (34, 36).
Table 1.
Study definitions of the different types of β-lactam-resistant invasive H. influenzae isolatesa
| Abbreviation | Name | Study definition | n |
|---|---|---|---|
| BLPAR | β-Lactamase positive, ampicillin resistantb | Resistance to penicillin according to disk diffusion testing, using SRGAc breakpoints for the study period; nitrocefin positive | 45 |
| BLNAR | β-Lactamase negative, ampicillin resistant | MIC of ampicillin, ≥2 mg/liter; nitrocefin negative | 11d |
| gBLNAR | Genomic β-lactamase negative, ampicillin resistant | Substitutions in PBP-3: genotype I, Arg517His; genotype II, Asn526Lys; genotype III, Met377Ile, Ser385Thr, Leu389Phe, and Asn 526Lys; nitrocefin negative | 16d |
| BLNBR | β-Lactamase negative, β-lactam resistant | Resistance to one or more tested β-lactam antibiotics (penicillin V/G, ampicillin, a cephalosporin, or a carbapenem) according to SRGA breakpoints; nitrocefin negative | 43 |
| BLPACR | β-Lactamase positive, amoxicillin-clavulanate resistant | Resistance to ampicillin or penicillin and a tested cephalosporin according to SRGA breakpoints; nitrocefin positive | 3 |
The BLNAR and gBLNAR groups have substantial overlap and are both subsets of the BLNBR group.
All studied isolates that were nitrocefin positive had an ampicillin MIC of ≥2 mg/liter.
SRGA, Swedish Reference Group for Antibiotics.
Group sizes (n) are from a total of 465 tested isolates, but since only a portion of isolates were available for Etest and sequencing, the numbers for BLNAR and gBLNAR were obtained from fewer isolates and are therefore not comparable to the other group sizes.
Clinical isolates that are susceptible to ampicillin but resistant to other β-lactams are consequently not included in the BLNAR definition. However, β-lactam antibiotics other than ampicillin are often used empirically in infections where H. influenzae can be the pathogen. Due to this, resistance of H. influenzae to β-lactam antibiotics other than ampicillin needs to be considered. For many years, the screening method for identification of β-lactam-resistant H. influenzae in Sweden has been disk diffusion testing for penicillin and cefaclor/loracarbef followed by a nitrocefin β-lactamase test. Even though penicillin rarely is an alternative for treatment of H. influenzae infections, experience suggests that this method is suitable for resistance surveillance, allowing for sensitive monitoring of β-lactam resistance. In this study, we refer to the β-lactamase-negative isolates with resistance (according to disk diffusion test screening) to any tested β-lactam antibiotic as β-lactamase negative, β-lactam resistant (BLNBR). This term includes the BLNAR isolates as a subset. Finally, isolates with both a β-lactamase and chromosomally derived resistance are defined as β-lactamase positive, amoxicillin-clavulanate resistant (BLPACR).
The epidemiological trends of antimicrobial resistance in H. influenzae vary in different areas of the world. The proportions of β-lactam-resistant isolates in general, and specifically BLNARs, are high in Japan and its neighboring countries, as described in several reports (10, 11, 28). In Europe, reports are less consistent, with some reports suggesting increasing proportions of isolates with ampicillin resistance (14, 32), albeit at a lower level than in Japan. In contrast, a recent Spanish report showed a decrease in proportions of ampicillin-resistant strains (24), demonstrating the local differences in resistance epidemiology. The proportion of β-lactam-resistant H. influenzae has been consistent, and comparatively low, in Sweden. However, in the last decade a 2-fold increase of β-lactam-resistant strains has been observed in the yearly national surveillance of Swedish nasopharyngeal H. influenzae isolates (http://www.smi.se/upload/stat/haemophilus-influenzae-99-09.gif). The aim of the current study was to investigate the epidemiology, mechanisms, and clonality of antimicrobial resistance in invasive H. influenzae in Sweden from 1997 to 2010.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The collection comprised clinical H. influenzae isolates from three densely populated regions in Sweden, i.e., Skåne County, Stockholm, and Gothenburg. All isolates from blood and cerebrospinal fluid collected between 1997 and 2010 (n = 465) were registered, and available isolates (n = 301) were stored at −70°C. Bacteria were cultured on chocolate blood agar plates and incubated for 18 h at 35°C in a humid atmosphere containing 5% CO2.
DNA preparation and capsule typing by PCR.
In order to release bacterial DNA, 5 bacterial colonies were heated in sterile distilled water at 96°C for 10 min. To amplify the capsule transport gene, a bexA PCR was performed on all available strains (n = 301) (5). To further increase the sensitivity, all available strains were screened for bexB by PCR using the primers 5′-TTGTGCCTGTGCTGGAAGGTTATG-3′ and 5′-GGTGATTAACGCGTTGCTTATGCG-3′ (annealing temperature, 54°C), resulting in a product size of 567 bp. Strains positive for bexA and/or bexB were further tested for capsule type by using specific primers against types b, a, d and f, c, and e cap loci in sequential order (5). Whenever a strain had previously been capsule typed by bex/cap PCR, the result was included in the analysis in case the strain was not available (n = 21). Results from serotyping by agglutination with antisera were not used, since this method is considered inferior in specificity compared with PCR (29). For all saved isolates from 1997 to 2009, a PCR to exclude the presence of Haemophilus haemolyticus isolates was performed (21). However, instead of a nested PCR, an initial PCR with primers 16S3′ and 16SNor was performed (26). If a product of correct size was not obtained, isolates were subjected to 16S rRNA sequencing. Since not a single isolate of H. haemolyticus was identified, the procedure was discontinued in 2010.
Antimicrobial susceptibility testing.
The disk diffusion method was used for antimicrobial susceptibility testing (4). Although not all strains were available for further analysis, all the clinical isolates were or had been tested for resistance to penicillin V, ampicillin, and trimethoprim-sulfamethoxazole. The majority of strains had been tested for resistance to tetracycline (95%), a cephalosporin (cefaclor/loracarbef and cefuroxime-axetil or cefotaxime; 98%), and a fluoroquinolone (nalidixic acid/ciprofloxacin/moxifloxacin or levofloxacin; 86%). Only a few isolates had been tested for resistance to a carbapenem (imipenem/meropenem; 39%), chloramphenicol (6%), or an aminoglycoside (4%). Antimicrobial susceptibility was interpreted according to Swedish Reference Group for Antibiotics (SRGA) breakpoints of the study period (www.srga.org/ZONTAB/Zontab2a.htm and www.srga.org/ZONTAB/Zontab2b.htm). Isolates were defined as β-lactam resistant according to SRGA breakpoints for penicillin V (10 μg) or for another tested β-lactam. All isolates with β-lactam resistance according to these breakpoints were or had been tested for β-lactamase production by using a commercial disk test (Céfinase disks; bioMérieux, Marcy l'Etoile, France). The cefinase disks contain nitrocefin, which is a chromogenic cephalosporin. Since susceptibility testing for amoxicillin-clavulanate was not routinely performed, the identification of true BLPACR isolates (β-lactamase positive, amoxicillin-clavulanate resistant) was not possible. The definition refers to isolates with both β-lactamase production and chromosomal resistance, and since the TEM-1 or ROB-1 β-lactamases of H. influenzae do not confer resistance to cephalosporins, BLPACR isolates were defined as those β-lactamase-positive isolates with resistance to a tested cephalosporin. β-Lactam-resistant isolates were thereby defined as BLPAR, BLNBR, or BLPACR based on results from nitrocefin testing and cefaclor (30 μg)/loracarbef (10 μg) tests, respectively. E-tests for ampicillin (Biodisk, Solna, Sweden) were performed on all available β-lactam-resistant isolates.
PCR and sequencing for detection of blaTEM and blaROB.
All available β-lactam-resistant isolates that tested positive for β-lactamase production were subjected to PCR to detect the specific β-lactamase gene. First, a blaTEM-1 PCR was performed, and on TEM-1 PCR-negative isolates, a blaROB-1 PCR was then performed (30). Since the blaTEM-1 PCR resulted in products of two distinct sizes, blaTEM PCR products from representative isolates were sent for sequencing and compared to known blaTEM-1 variants (20, 34). The sequenced isolates were included as controls in the blaTEM-1 PCR.
PBP-3 sequencing.
All available isolates that were either defined as BLNBR or BLPACR based on the method described above were subjected to an ftsI PCR, amplifying the transmembranous part of PBP-3 by using primers 5′-CCTTTCGTTGTTTTAACCGCA-3′ and 5′-AGCTGCTTCAGCATCTTG-3′ (annealing temperature, 52°C), resulting in a product size of 770 bp. All products were sent for sequencing, analyzed for amino acid substitutions, and compared to the wild-type H. influenzae RdKW20 PBP-3 by using the CLC-DNA workbench (CLC bio, Aarhus, Denmark).
Multilocus sequence Typing (MLST).
All available BLNBR and BLPACR isolates were sequence typed using PCR primers and conditions according to the H. influenzae protocol described on the MLST.net web page (http://haemophilus.mlst.net/). Sequences were trimmed manually, concatenated, and aligned using ClustalX (19). A best-fitting nucleotide substitution model was estimated using the Akaike information criterion corrected for small sample sizes (AICc) as implemented in jModeltest 0.1.0 (25). A neighbor-joining (NJ) tree was constructed in PAUP* version 4.0b10 (33) using the AICc model (HKY+I+G). Support for internal branches was obtained with 1,000 bootstrap replicates in PAUP*. The resulting phylogenetic tree was visualized using FigTree version 1.3.1 (http://tree.bio.ed.ac.uk/software/figtree).
Data sorting and estimates of population at risk.
The laboratories in Stockholm, Gothenburg, and Malmö/Lund (Skåne County) kept complete records of all H. influenzae isolates from blood and cerebrospinal fluid (n = 465). Due to variations in storage routines, not all strains had survived during the years. Of the 465 isolates, 340 were or had been serotyped by PCR. If less than 50% of isolates from one laboratory were or had been serotyped by PCR in a year, all results from that laboratory were excluded from the serotype epidemiology analysis for that particular year, and the population data were adjusted accordingly. From the isolates defined as BLPAR or BLPACR, 69% (33/48) were available for detailed study. From the isolates defined as BLNBR or BLPACR, 80% (36/46) were available for further study. All population data by region and year were collected from the Swedish Central Statistics Agency (www.scb.se).
Statistical analyses.
To test the significance of the increase in proportions of H. influenzae β-lactam resistance, trend tests using yearly proportions of each type of resistance as a dependent variable in linear regression analyses were initially performed. These analyses gave significance levels of the increase and confidence intervals (CIs). We had a priori knowledge that the data set was skewed toward the end of the study period, and considering the fact that the dependent variable was binomial, logistic regressions were also performed on the three data sets. After plotting the three data sets, the assumption of a linear relation of data used in both the linear and logistic regressions could not be assumed for the BLNBR data set nor the data set with all ampicillin-resistant isolates. The curve fit of these two data sets suggested that a quadratic polynomial regression should be used. For the BLNBR data set, a cubic equation (a third-degree polynomial equation) fit the data almost equally well. For these two data sets, quadratic logistic regressions were performed with centered squared years and centered years used as covariates. Years, and not exact dates, were used as time points, since we know that there is a seasonal variation in H. influenzae disease. The most conservative estimate of significance was used. The data were analyzed using PASW statistics version 20.0.
RESULTS
Increasing numbers and proportions of invasive β-lactam-resistant H. influenzae in Sweden from 1997 to 2010.
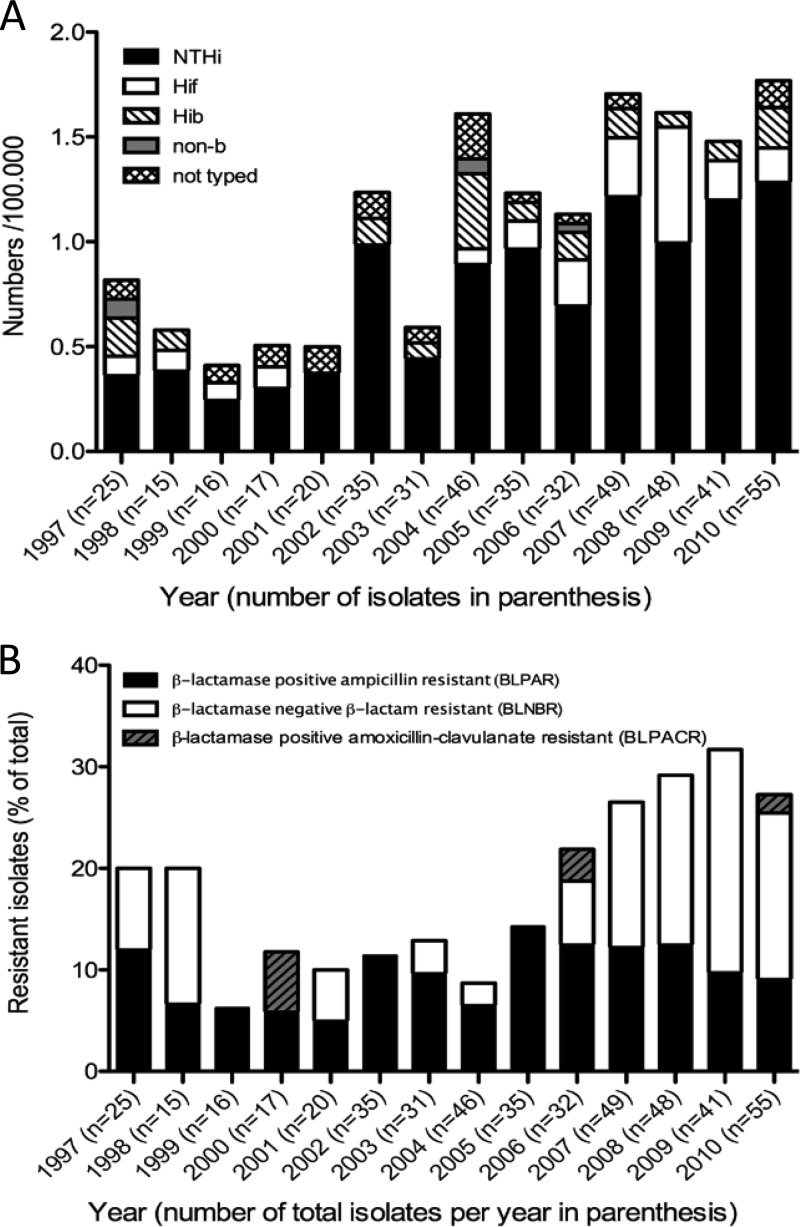
We recently observed an increase of invasive H. influenzae disease in Sweden from 1997 to 2009 (26), which was in parallel with similar epidemiological findings in North America as well as in Europe (18, 35). In the present study, results from 2010 were also included. Since 2010 holds the highest incidence per 100,000 individuals during the study period, a continued increasing incidence trend is suggested (Fig. 1A). The increase was dominated by NTHi.
Fig 1.
Incidence of invasive Haemophilus influenzae as well as β-lactam-resistant invasive H. influenzae increased from 1997 to 2010. (A) The incidence of all H. influenzae strains, as well of NTHi and Hif strains, increased during the observation period. For all years, laboratories in which less than 50% of strains had been or could be capsule typed by PCR were excluded. The denotion “non-b” reflects a small group of isolates that had been serotyped by PCR against only bexA and capB and were sorted as encapsulated but not Hib. The denotation “not typed” describes isolates that were included in the analysis but not available for capsule typing by PCR. (B) The proportion of β-lactam-resistant isolates as a percentage of all invasive isolates is shown per study year. BLPAR isolates, BLNBR isolates, and BLPACR isolates are shown separately. The total proportion increased significantly throughout the study period, as did the proportion of β-lactamase-negative β-lactam-resistant isolates.
As revealed by disk diffusion, 91 out of 465 H. influenzae isolates were defined as β-lactam resistant, of which 43 isolates were β-lactamase negative. The total numbers of isolates for each group are shown in Table 1. The absolute numbers (ranging from 1 to 5 for 1997 to 2000 to 12 to 15 for 2007 to 2010) as well as the proportion of β-lactam-resistant invasive H. influenzae isolates increased (Fig. 1B). The increase in proportion of β-lactam-resistant isolates was significant in a linear regression (P = 0.01; 95% CI, 0.36 to 2.26), as was the increase of BLNBR isolates (P = 0.04; 95% CI, 0.08 to 1.94), whereas the increase in BLPAR isolates was not statistically significant (P = 0.13; 95% CI, 0.11 to 0.72). Since the plots of the data sets, except for BLPAR data, suggested a quadratic equation, a logistic regression of the data using a quadratic regression was performed. The observations were confirmed, and the increase of the BLNBR isolates (P = 0.02) as well as the increase of all β-lactam-resistant isolates (P = 0.03) remained significant. A logistic regression of the BLPAR data set further stressed that these isolates did not increase in incidence (P = 0.67). β-Lactam resistance in Swedish H. influenzae isolates appeared almost exclusively in NTHi isolates, since only eight encapsulated strains displayed this characteristic during the study period.
We also studied the susceptibility to other antimicrobial agents. The proportion of isolates resistant to trimethoprim-sulfamethoxazole varied from 6 to 20% per year, and no trend suggesting increasing incidence rates was seen throughout the study period. This contrasts to the national nasopharyngeal surveillance data, where an increasing trend of resistance to the folic acid antagonists has been observed (http://www.smi.se/upload/stat/haemophilus-influenzae-99-09.gif). Finally, resistance to fluoroquinolones and tetracycline remained low during the study period, at 2.1% and 1.9%, respectively.
The gene variant with a promoter deletion, blaTEM-1-PΔ, dominates among BLPAR isolates.
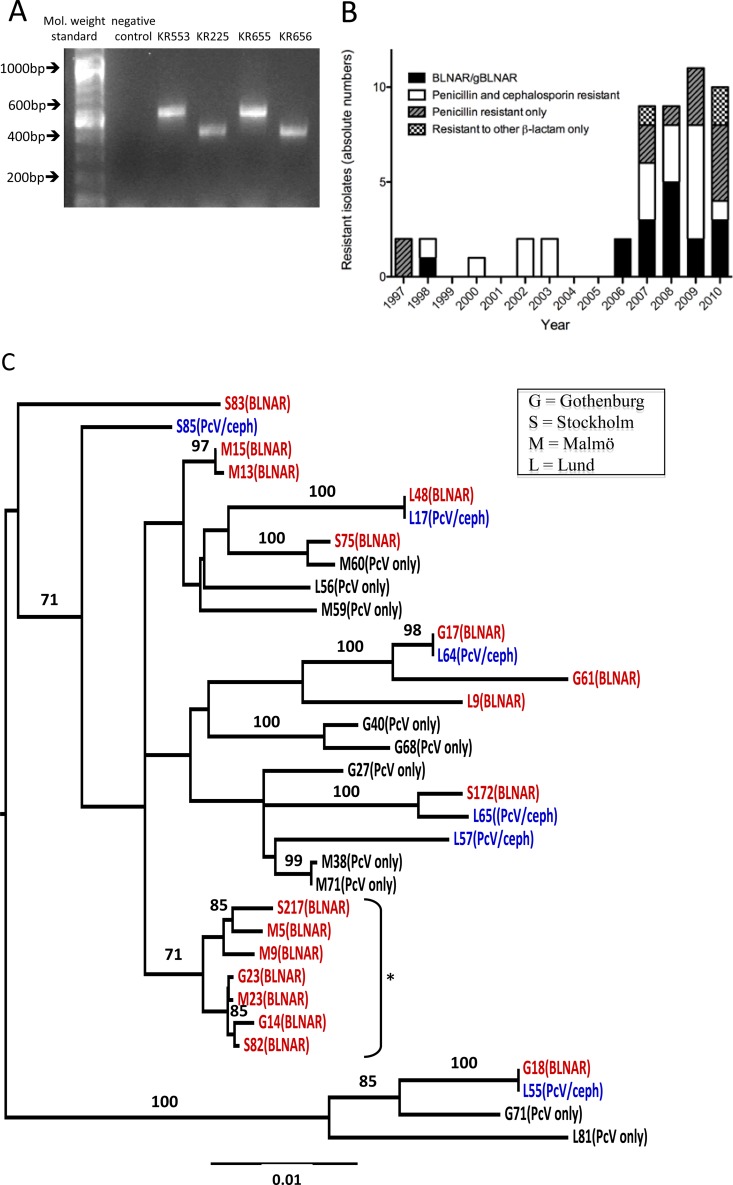
All identified β-lactamase-positive isolates (BLPAR or BLPACR) (Table 1) that were available for further analysis (n = 33) were resistant to ampicillin (the MIC for ampicillin ranged from 4 to 256 mg/liter). The corresponding β-lactamase gene was defined by PCR in 30 out of 33 isolates, and we found that blaTEM-1 dominated (n = 29). Only one isolate carrying the blaROB-1 gene was found. The gene product encoding TEM-1 was detected in two variants, resulting in different DNA products when using the same primer pair (Fig. 2A). After sequencing, it was clear that the larger product (600 bp) represented the wild-type blaTEM-1 gene, whereas the smaller product represented a blaTEM-1 gene with a 135-bp deletion in the promoter region. This corresponded to the blaTEM-1-PΔ gene previously described in Spain by Molina and colleagues (20). In our clinical collection, the variant blaTEM-1-PΔ dominated during the study period (18 had the blaTEM-1-PΔ gene, whereas 11 isolates carried the wild-type blaTEM-1 gene). The median MIC for ampicillin, however, was the same for the two identified blaTEM gene variants. Finally, we found three β-lactamase-positive (as revealed by nitrocefin testing), ampicillin-resistant H. influenzae isolates (BLPAR) from 2009 and 2010 that were negative for both blaTEM and blaROB genes when we used the described primers.
Fig 2.
Two variants of blaTEM-1 and a steep increase of β-lactamase-negative invasive isolates with a cluster of BLNAR isolates were identified. (A) The agarose gel shows an example of a blaTEM-1 PCR result from four different invasive NTHi strains with β-lactamase production. The lanes are, from left to right, molecular weight standard, negative control, and the clinical NTHi isolates KR553, KR225, KR655, and KR656. Sequencing revealed that the products of KR553 and KR655 are blaTEM-1 wild type, whereas the products of KR225 and KR656 are representative of the blaTEM-1-PΔ. (B) The recent increase of H. influenzae isolates with a β-lactamase-negative, β-lactam-resistant phenotype. The absolute numbers of invasive BLNBR isolates during 1997 to 2010, sorted by resistance phenotype, are shown. The black bars show BLNAR isolates, and the white bars show isolates resistant to penicillin and a cephalosporin. The striped gray bars show isolates resistant to penicillin only, while the checked bars show isolates resistant to only a cephalosporin or a carbapenem. (C) A neighbor-joining phylogenetic tree was constructed based on concatenated MLST data from all available invasive BLNBR isolates. The BLNAR isolates are indicated in red. Isolates with penicillin and cephalosporin (PcV/ceph) resistance are indicated in blue, while isolates with only penicillin (PcV only) resistance are shown in black. The prefix letter of the isolate name indicates the laboratory where the isolate was isolated: G, Gothenburg; S, Stockholm; M, Malmö; L, Lund. Clusters with >70% bootstrap support are indicated with their bootstrap values, and one cluster of seven gBLNAR/BLNAR isolates, including isolates from all three geographical areas of the study, is indicated by an asterisk.
Amino acid substitutions in PBP-3 are found mainly in BLNAR isolates and are less common in other BLNBR strains.
A total of 46 isolates were defined as β-lactamase negative, β-lactam resistant (BLNBR), or BLPACR (Table 1). Of these isolates, 12 were penicillin resistant only, and 34 isolates were resistant to penicillin and another tested β-lactam. Of the total 46 isolates, 36 were available for further testing, and these were subjected to an ampicillin Etest followed by PBP-3 sequencing. Several of the isolates were true BLNAR (11/36; ampicillin MIC, ≥2 mg/liter) or gBLNAR (16/36) (amino acid substitutions Arg517His or Asn526Lys). In Table 2, we show all variants of PBP-3 that were identified among the BLNBR isolates and the correlating MIC ranges for ampicillin. Genotype II dominated among BLNAR isolates, and a correlation between the BLNAR genotypes and ampicillin resistance phenotype was confirmed. However, several isolates that were resistant to other β-lactams but susceptible to ampicillin did not have BLNAR-defining substitutions in PBP-3. Seven BLNBR isolates did not have any mutations at all in PBP-3. These findings imply that mechanisms other than β-lactamase production and substitutions in PBP-3 contribute to β-lactam resistance in H. influenzae.
Table 2.
Amino acid substitutions in PBP-3 of 36 invasive β-lactamase-negative β-lactam-resistant H. influenzae isolatesa
| BLNAR genotype | n | Amino acid substitution |
Ampicillin MICb (range; mg/liter) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asp 350 | Ala 368 | Asp 373 | Met 377 | Ala 395 | Ala 437 | Ile 449 | Ile 475 | Gly 490 | Ala 502 | Arg 517 | Asn 526 | Ala 530 | Val 547 | Asn 569 | |||
| I | 1 | His | 1 | ||||||||||||||
| IIb | 8 | Asn | Ile | Val | Lys | Ile | Ser | 0.5–4 | |||||||||
| IIb | 2 | Asn | Ile | Glu | Val | Lys | Ile | Ser | 2 | ||||||||
| IIb | 1 | Asn | Gly | Val | Lys | Ile | Ser | 2 | |||||||||
| IIb | 1 | Ile | Val | Lys | Ile | 8 | |||||||||||
| IId | 2 | Val | Lys | Ile | Ser | 2 | |||||||||||
| II | 1 | Asn | Glu | Lys | Ser | 0.5 | |||||||||||
| —c | 3 | Asn | Ile | Ser | 0.25–0.5 | ||||||||||||
| — | 2 | Asn | Ser | Ile | Ser | 0.5 | |||||||||||
| — | 2 | Asn | 0.5 | ||||||||||||||
| — | 1 | Leu | 1 | ||||||||||||||
| — | 2 | Thr | 0.25–0.5 | ||||||||||||||
| — | 3 | Ile | 0.25 | ||||||||||||||
| — | 7 | (No substitutiond) | 0.25–256 | ||||||||||||||
All gBLNAR variants are highlighted in boldface.
MICs were determined by Etest.
—, the isolate was not gBLNAR.
Two strains produced β-lactamases (BLPACR) and therefore there was a broad MIC range.
A marked increase of β-lactamase-negative β-lactam-resistant isolates was found from 2007 and onwards (Fig. 2B), with consistent yearly proportions above 10%. In the years 1997 and 1998, the proportion of BLNBR isolates was relatively high, but this was based on a very limited number of isolates. This makes the data from these years less reliable and more difficult to interpret. For comparison, the definitions were also adjusted to the definition used in the national surveillance program described earlier, which only includes isolates resistant to both penicillin and cefaclor/loracarbef in the BLNBR group. From 2007 and onwards, we observed consistently higher proportions of β-lactamase-negative β-lactam-resistant invasive isolates than the proportions seen in the national surveillance data for nasopharyngeal isolates, for which numbers never reached 5%.
Identification of a cluster of BLNAR genotype IIb isolates with limited genetic variation.
To identify putative clusters, MLST based upon 7 different genes was performed on the invasive BLNBR isolates. Even though alleles were shared, all analyzed isolates had different ST profiles, as revealed by the MLST. The clonal relation of the BLNBR isolates was analyzed using concatenated MLST sequences. In the resulting neighbor-joining analysis, clusters supported by bootstrap values of >70% were considered well supported (Fig. 2C). The phylogenetic analysis identified several clusters with bootstrap support of 70% or more, of which one cluster contained 7 BLNAR isolates (Fig. 2C). Interestingly, this BLNAR cluster comprised isolates from all three distinct geographical areas in the study, all from the period 2008 to 2010. Furthermore, all of the isolates in the cluster had identical PBP-3 sequences, belonging to genotype IIb according to the classification of Dabernat and colleagues (2). Even though the numbers are small, together these findings suggest a clonal spread of this particular cluster.
DISCUSSION
This study identifies an increase in proportions of β-lactam resistance among invasive H. influenzae isolates in Sweden during the years 1997 to 2010. The proportions of β-lactam-resistant isolates reached 30% in the final years of the study period. The observed increase was not mainly due to an increase of β-lactamase-producing isolates, but among these a blaTEM-1 variant with a promoter deletion dominated (i.e., blaTEM-1-PΔ). The increase was mainly due to a recent rise in BLNBR isolates. Since such isolates have a potential for resistance to multiple antibiotics (34), the observation is of concern. Not all of the BLNBR isolates displayed true BLNAR phenotypes, but most isolates were resistant to multiple β-lactam antibiotics. Our study also confirms a strong, but not perfect, correlation between BLNAR-defining amino acid substitutions and the ampicillin resistance phenotype established in earlier studies (2, 9, 31, 36). However, it is evident that other mechanisms than PBP-3 mutations or β-lactamase production contribute to β-lactam resistance in H. influenzae. A few such mechanisms, including disrupted repression of the acrR efflux pump, have been suggested (15).
Since the study design was retrospective, our study has limitations. Not all isolates were available for detailed study, and since the absolute numbers of H. influenzae isolates were limited, the statistical calculations as well as the indications from the MLST analysis should be interpreted with caution. Furthermore, the reliability of the disk diffusion method for defining precise levels of β-lactam resistance in H. influenzae has been questioned. However, as a primary screening method for resistance in clinical isolates, when followed by a detailed examination, the disk diffusion method was considered suitable. Previous reports that have studied clonal relations of resistant H. influenzae have used pulsed-field gel electrophoresis (PFGE) (9, 32), and PFGE is a common method for studying clonal relations in local outbreaks with a limited geographical distribution. Even though all methods have limitations, we believe that MLST is advantageous, with its benefits of a high resolution power and the possibility of international comparisons.
Acquisition of antimicrobial resistance is often thought to imply a fitness cost and thereby theoretically reduced bacterial fitness and virulence. However, evidence points to antimicrobial resistance in Gram-negative bacteria that can be linked to a higher degree of virulence (27), possibly due to cocarriage of resistance and virulence genes. The explanation for the increase of the proportion of resistant invasive H. influenzae isolates is likely to be multifactorial. Selection pressure from liberal use of antibiotics for upper airway infections may be a contributing factor, and there is support for this mechanism from earlier reports (8). Moreover, a contribution of the spread of dominant clones of H. influenzae with antimicrobial resistance should be considered. Such patterns were suggested in earlier studies (12, 16). The MLST results from the present study of invasive isolates suggest a spread of one BLNAR clone with close genetic relation, but the absolute number of isolates was too small to fully conclude this as a fact. Two observations strengthening this indication is that the cluster was comprised of isolates from all three geographical areas of the study, and all of the isolates of this cluster had identical PBP-3 sequences. Among the BLPAR isolates, the reason for the spread and domination of the blaTEM-1-PΔ variant needs further investigation.
The finding of higher proportions of β-lactamase-negative β-lactam-resistant H. influenzae invasive isolates, including BLNAR, than that found in surveillance of nasopharyngeal disease carriage strains is intriguing. Since not all isolates were tested for cephalosporins or carbapenems, and since not all isolates were available for PBP-3 sequencing, the numbers in this group may be an actual underestimate. The possibility of a higher invasive capacity of resistant strains cannot be excluded, and such suggestions have been made for BLNAR isolates in earlier work (22). Since the study is skewed toward metropolitan areas of Sweden, however, the risk of the results reflecting local Swedish differences in resistance epidemiology also has to be considered. Interestingly, when the BLNBR data set was statistically examined, the curve could be fitted almost equally well with a cubic equation as the quadratic one used in this analysis. One may argue that a cubic equation, with a reduction in the rate of increase at the end of the study period, may be a more plausible estimate. The coming years will show which model best predicts future incidence rates.
To assess the relevance of studying H. influenzae resistance to all β-lactams, and not only to ampicillin, in a clinical setting, we registered the initial antibiotic given to the patients in 106 cases of H. influenzae sepsis in the county of Skåne (data not shown). The majority (53%) were primarily given an expanded- or broad-spectrum cephalosporin. Interestingly, 28% were given benzylpenicillin, 15% were given a carbapenem, and only one single patient was administered ampicillin as a starting antibiotic. This observed empirical treatment strategy reflects the clinical need to consider resistance of H. influenzae to also β-lactams other than ampicillin, most notably cephalosporins and benzylpenicillin.
To harmonize resistance testing, a novel disk diffusion method to detect β-lactam resistance in H. influenzae was issued by the European Committee on Antimcrobial Susceptibility Testing (EUCAST; www.eucast.org) in 2011. The new method sorts β-lactam-resistant isolates by using benzylpenicillin disks (1 U) in Mueller-Hinton agar. Preliminary results from our laboratory suggest a higher incidence of β-lactamase-negative β-lactam-resistant nasopharyngeal H. influenzae isolates in 2011. Whether this reflects a true increase of β-lactam resistance in H. influenzae or merely improved diagnostics is unclear for the time being. Since the two methods are not entirely interchangeable, only results from the one used during the study period (1997 to 2010) were included in the present study. Regardless of the specific method utilized, it is clear that the proportion of β-lactam-resistant H. influenzae in Sweden is no longer low, as roughly 30% of invasive isolates displayed β-lactam resistance in the final years of this study. These results call for continued surveillance and active measures to restrain the use of unnecessary antibiotics in upper airway infections.
ACKNOWLEDGMENTS
This work was supported by grants from the Alfred Österlund Foundation, the Anna and Edwin Berger Foundation, the Anna-Lisa and Sven-Erik Lundgren Foundation, the Capio Research Foundation, the Greta and Johan Kock Foundation, the Gyllenstiernska Krapperup Foundations, the Physiographical Society, the Swedish Medical Research Council (grant number 521-2010-4221), the Cancer Foundation at the University Hospital in Malmö, and the Skåne County Council's research and development foundation.
We are grateful to Elisabeth Ek, Sahlgrenska University, Gothenburg, for help with Gothenburg isolates, to Marta Brant, Medical Microbiology, Malmö, for technical support, and to Fredrik Nilsson at FoU Region Skåne, Lund, for statistical assistance.
Footnotes
Published ahead of print 11 June 2012
REFERENCES
- 1. Brown VM, et al. 2009. Invasive Haemophilus influenzae disease caused by non-type b strains in northwestern Ontario, Canada, 2002–2008. Clin. Infect. Dis. 49:1240–1243 [DOI] [PubMed] [Google Scholar]
- 2. Dabernat H, et al. 2002. Diversity of beta-lactam resistance-conferring amino acid substitutions in penicillin-binding protein 3 of Haemophilus influenzae. Antimicrob. Agents Chemother. 46:2208–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dworkin MS, Park L, Borchardt SM. 2007. The changing epidemiology of invasive Haemophilus influenzae disease, especially in persons ≥ 65 years old. Clin. Infect. Dis. 44:810–816 [DOI] [PubMed] [Google Scholar]
- 4. Ericsson H. 1960. The paper disc method for determination of bacterial sensitivity to antibiotics. Studies on the accuracy of the technique. Scand. J. Clin. Lab. Invest. 12:408–413 [DOI] [PubMed] [Google Scholar]
- 5. Falla TJ, et al. 1994. PCR for capsular typing of Haemophilus influenzae. J. Clin. Microbiol. 32:2382–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Falla TJ, et al. 1993. Population-based study of non-typable Haemophilus influenzae invasive disease in children and neonates. Lancet 341:851–854 [DOI] [PubMed] [Google Scholar]
- 7. Farrell DJ, Morrissey I, Bakker S, Buckridge S, Felmingham D. 2005. Global distribution of TEM-1 and ROB-1 beta-lactamases in Haemophilus influenzae. J. Antimicrob. Chemother. 54:773–776 [DOI] [PubMed] [Google Scholar]
- 8. Garcia-Cobos S, et al. 2008. Antibiotic resistance in Haemophilus influenzae decreased, except for beta-lactamase-negative amoxicillin-resistant isolates, in parallel with community antibiotic consumption in Spain from 1997 to 2007. Antimicrob. Agents Chemother. 52:2760–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garcia-Cobos S, et al. 2007. Ampicillin-resistant non-beta-lactamase-producing Haemophilus influenzae in Spain: recent emergence of clonal isolates with increased resistance to cefotaxime and cefixime. Antimicrob. Agents Chemother. 51:2564–2573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goto H, Shimada K, Ikemoto H, Oguri T. 2009. Antimicrobial susceptibility of pathogens isolated from more than 10,000 patients with infectious respiratory diseases: a 25-year longitudinal study. J. Infect. Chemother. 15:347–360 [DOI] [PubMed] [Google Scholar]
- 11. Hasegawa K, et al. 2004. Rapidly increasing prevalence of beta-lactamase-nonproducing, ampicillin-resistant Haemophilus influenzae type b in patients with meningitis. Antimicrob. Agents Chemother. 48:1509–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hotomi M, et al. 2007. Genetic characteristics and clonal dissemination of beta-lactamase-negative ampicillin-resistant Haemophilus influenzae strains isolated from the upper respiratory tract of patients in Japan. Antimicrob. Agents Chemother. 51:3969–3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jacobs MR. 2003. Worldwide trends in antimicrobial resistance among common respiratory tract pathogens in children. Pediatr. Infect. Dis. J. 22:S109–S119 [DOI] [PubMed] [Google Scholar]
- 14. Jansen WT, Verel A, Beitsma M, Verhoef J, Milatovic D. 2008. Surveillance study of the susceptibility of Haemophilus influenzae to various antibacterial agents in Europe and Canada. Curr. Med. Res. Opin. 24:2853–2861 [DOI] [PubMed] [Google Scholar]
- 15. Kaczmarek FS, et al. 2004. Genetic and molecular characterization of beta-lactamase-negative ampicillin-resistant Haemophilus influenzae with unusually high resistance to ampicillin. Antimicrob. Agents Chemother. 48:1630–1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karlowsky JA, et al. 2002. Antimicrobial surveillance of Haemophilus influenzae in the United States during 2000–2001 leads to detection of clonal dissemination of a beta-lactamase-negative and ampicillin-resistant strain. J. Clin. Microbiol. 40:1063–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khan W, Ross S, Rodriguez W, Controni G, Saz AK. 1974. Haemophilus influenzae type B resistant to ampicillin. A report of two cases. JAMA 229:298–301 [PubMed] [Google Scholar]
- 18. Ladhani S, et al. 2010. Invasive Haemophilus influenzae disease, Europe, 1996–2006. Emerg. Infect. Dis. 16:455–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Larkin, et al. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948 [DOI] [PubMed] [Google Scholar]
- 20. Molina JM, Cordoba J, Monsoliu A, Diosdado N, Gobernado M. 2003. Haemophilus influenzae and betalactam resistance: description of bla TEM gene deletion. Rev. Esp. Quimioter. 16:195–203 (In Spanish.) [PubMed] [Google Scholar]
- 21. Murphy, et al. 2007. Haemophilus haemolyticus: a human respiratory tract commensal to be distinguished from Haemophilus influenzae. J. Infect. Dis. 195:81–89 [DOI] [PubMed] [Google Scholar]
- 22. Okabe T, et al. 2010. An amino acid substitution in PBP-3 in Haemophilus influenzae associate with the invasion to bronchial epithelial cells. Microbiol. Res. 165:11–20 [DOI] [PubMed] [Google Scholar]
- 23. Peltola H. 2000. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin. Microbiol. Rev. 13:302–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Perez-Trallero E, et al. 2010. Antimicrobial resistance among respiratory pathogens in Spain: latest data and changes over 11 years (1996–1997 to 2006–2007). Antimicrob. Agents Chemother. 54:2953–2959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Posada D. 2008. jModeltest: phylogenetic model averaging. Mol. Biol. Evol. 25:1253–1256 [DOI] [PubMed] [Google Scholar]
- 26. Resman F, et al. 2011. Invasive disease by Haemophilus influenzae in Sweden 1997–2009; evidence of increasing incidence and clinical burden of non-type b strains. Clin. Microbiol. Infect. 17:1638–1645 [DOI] [PubMed] [Google Scholar]
- 27. Sahly H, et al. 2008. Extended-spectrum beta-lactamase production is associated with an increase in cell invasion and expression of fimbrial adhesins in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 52:3029–3034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sakata H, et al. 2009. Nationwide survey of the development of drug-resistance in the pediatric field: drug sensitivity of Haemophilus influenzae in Japan. J. Infect. Chemother. 15:402–409 [DOI] [PubMed] [Google Scholar]
- 29. Satola SW, Collins JT, Napier R, Farley MM. 2007. Capsule gene analysis of invasive Haemophilus influenzae: accuracy of serotyping and prevalence of IS1016 among nontypeable isolates. J. Clin. Microbiol. 45:3230–3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Scriver SR, et al. 1994. Determination of antimicrobial susceptibilities of Canadian isolates of Haemophilus influenzae and characterization of their beta-lactamases. Canadian Haemophilus Study Group. Antimicrob. Agents Chemother. 38:1678–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Skaare D, et al. 2010. Mutant ftsI genes in the emergence of penicillin-binding protein-mediated beta-lactam resistance in Haemophilus influenzae in Norway. Clin. Microbiol. Infect. 16:1117–1124 [DOI] [PubMed] [Google Scholar]
- 32. Skoczynska A, Kadlubowski M, Wasko I, Fiett J, Hryniewicz W. 2007. Resistance patterns of selected respiratory tract pathogens in Poland. Clin. Microbiol. Infect. 13:377–383 [DOI] [PubMed] [Google Scholar]
- 33. Swofford DL. 2000. PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4. Sinauer Associates, Sunderland, MA [Google Scholar]
- 34. Tristram S, Jacobs MR, Appelbaum PC. 2007. Antimicrobial resistance in Haemophilus influenzae. Clin. Microbiol. Rev. 20:368–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tsang RS, et al. 2007. Characterization of invasive Haemophilus influenzae disease in Manitoba, Canada, 2000–2006: invasive disease due to non-type b strains. Clin. Infect. Dis. 44:1611–1614 [DOI] [PubMed] [Google Scholar]
- 36. Ubukata K, et al. 2001. Association of amino acid substitutions in penicillin-binding protein 3 with beta-lactam resistance in beta-lactamase-negative ampicillin-resistant Haemophilus influenzae. Antimicrob. Agents Chemother. 45:1693–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]