Abstract
A wound biofilm model was created by adapting a superficial infection model. Partial-thickness murine wounds were inoculated with methicillin-resistant Staphylococcus aureus (MRSA). Dense biofilm communities developed at the wound surface after 24 h as demonstrated by microscopy and quantitative microbiology. Common topical antimicrobial agents had reduced efficacy when treatment was initiated 24 h after inoculation compared to 4 h after inoculation. This model provides a rapid in vivo test for new agents to treat wound biofilm infections.
TEXT
A growing body of evidence suggests bacterial biofilms are a significant cause of nonhealing wounds (4, 5, 7). Studies of infected full-thickness wounds in mice and rabbits have demonstrated that biofilms can contribute to delayed healing, but limited data regarding antimicrobial treatment of wound biofilms are available (1, 2, 8–10). A murine superficial skin infection model in which partial-thickness wounds were inoculated with Staphylococcus aureus and treated beginning 4 h after inoculation has been described previously (3, 6). Marketed topical antimicrobial ointments were shown to be effective in reducing the wound staphylococcal burden after 4 days of treatment. While performing a similar model to test topical antimicrobial therapies, we found that allowing the infection to become established for 24 h prior to initiating treatment enabled biofilm formation, resulting in a useful model for evaluating the efficacy of in vivo wound biofilm treatment.
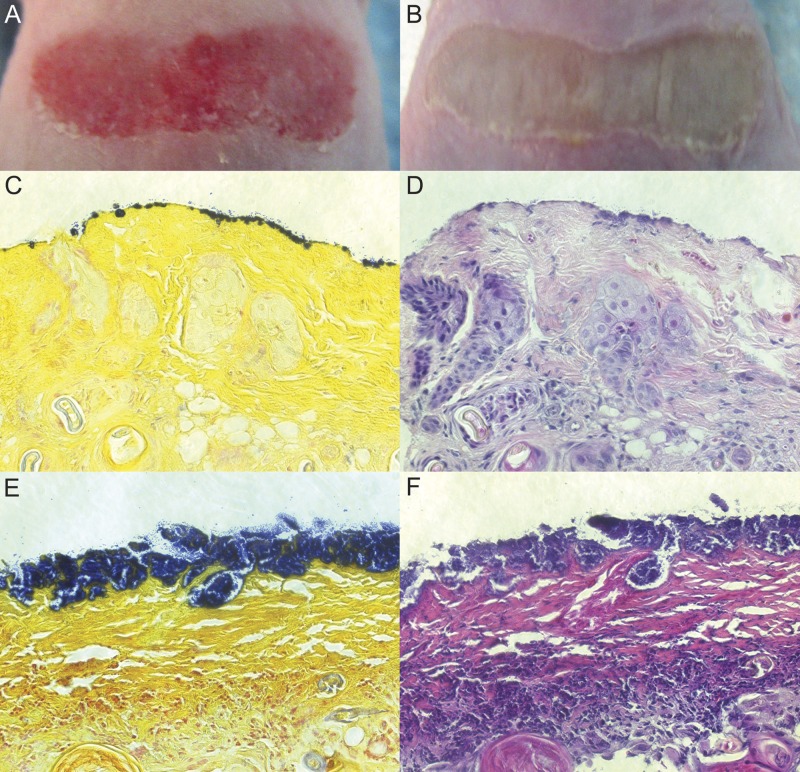
Several adaptations were made to the model, and all procedures were approved by the UNT HSC Institutional Animal Care and Use Committee. Hairless SKH1 mice were used to expedite wounding without dehairing and to better maintain secondary dressings to ensure a moist wound environment. To facilitate a robust, consistent infection, transient neutropenia was induced by an intraperitoneal dose of 150 mg of cyclophosphamide per kg of body weight 4 days prior to the study (11). After anesthetization and skin preparation, a partial-thickness wound was introduced by repeated touching of a rotary tool with a sterile sanding attachment to the back of each mouse until skin tissue was red and glistening. A representative wound is shown in Fig. 1A. This technique for generating partial-thickness wounds was found in pilot studies to be more efficient than tape stripping. The wounds were gently cleaned and inoculated with 2 × 107 CFU of multidrug-resistant methicillin-resistant Staphylococcus aureus (MRSA) strain ATCC 33592. The wounds were dressed with a bandage moistened with saline (to maintain a moist wound environment) and covered with a tubular mesh dressing secured with a strip of elastic tape. A visible change in the wound surface was apparent after 24 h, and it persisted with a similar appearance for two additional days (Fig. 1B).
Fig 1.
Wound photographs and visible light microscopy of wound tissue. Photographs of the dorsum after wounding (A) and 2 days after MRSA inoculation (B) showed the alteration in the wound surface resulting from MRSA. Gram-stained (C and E) and H&E-stained (D and F) tissue sections were imaged at ×400 magnification. MRSA-inoculated wounds were sampled either 4 h (C and D) or 24 h (E and F) after inoculation.
The nature of the infection present at different time points was examined by quantitative microbiology and microscopy. Bacterial counts performed on processed wound biopsy samples indicated that the geometric mean MRSA bioburden was 5 × 109 CFU/g tissue 4 h after inoculation and increased to 2 × 1010 CFU/g after 24 h, remaining stable thereafter over an additional 2 days. Samples taken for histological analysis were fixed with formalin and embedded in paraffin, and sections were either stained with hematoxylin and eosin (H&E) or Gram stained. Images of samples taken 4 h after inoculation (Fig. 1C and D) showed small clusters of MRSA (Fig. 1C, blue) at the wound surface. After 24 h, a dense MRSA community covered much of the wound surface (Fig. 1E and F). The tissue encompassing the MRSA biofilm and immediately beneath it was predominately fibrous matrix material, and immune cell accumulation was evident beneath this fibrous layer (Fig. 1F).
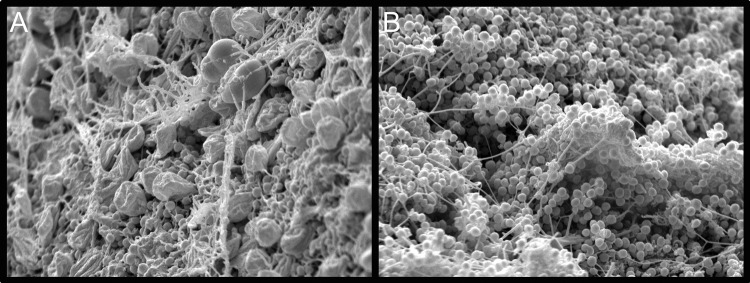
To further explore the characteristics of the MRSA infection, scanning electron microscopy (SEM) was performed on samples taken 4 and 24 h after inoculation. Specimens for SEM were transferred to buffered 2.5% glutaraldehyde, postfixed in 1% osmium tetroxide, dehydrated, dried, and coated with gold in an automatic sputter coater. Samples were viewed in a Philips XL 30 ESEM scanning electron microscope with a LaB6 source operating in high-vacuum mode at 15 kV. Micrographs taken 4 h after MRSA inoculation (Fig. 2A) showed cocci invading the host matrix amid host cells. After 24 h, large portions of the wound surface were covered by a dense MRSA biofilm community (Fig. 2B), including distinct matrix fibers and amorphous encasing material.
Fig 2.
Scanning electron micrographs of the wound tissue surface either 4 h (A) or 24 h (B) after inoculation with MRSA. Images were taken at ×4,000 magnification.
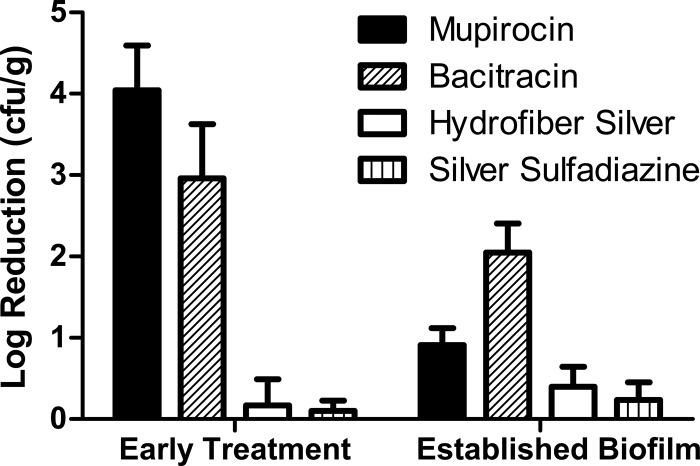
The difference in biofilm character of the MRSA infection at 24 h compared to 4 h was also found to reflect a distinct response to topical antimicrobial therapy (Fig. 3). Test articles for these studies included mupirocin calcium (Bactroban; GlaxoSmithKline), bacitracin zinc USP, silver sulfadiazine (Silvadene; Monarch Pharmaceuticals), and a silver dressing (Aquacel Ag; ConvaTec). Application to the wound consisted of 0.1 ml for creams and ointments, and the silver dressing was cut to size and moistened with saline. The wounds were further dressed as described above and gently cleaned with saline prior to reapplying treatment. After 2 days of treatment, the mice were humanely euthanized and sampled using biopsy punches, and the samples were processed to determine the quantitative tissue bioburden. Results for each treatment and time are based on 12 to 18 mice from at least two independent experiments. When treatment was initiated 4 h after MRSA inoculation, two common topical antibiotics (mupirocin and bacitracin) were effective in reducing the MRSA bioburden (>3-log reduction). The efficacy of the two antibiotics was significantly reduced (P < 0.05 by analysis of variance [ANOVA]) when treatment was initiated 24 h after inoculation, consistent with the observed biofilm character of the infection at this time point. It is interesting to note that while mupirocin was significantly more efficacious than bacitracin when treatment was initiated at 4 h, bacitracin was superior when treatment started at 24 h. The silver dressing and silver sulfadiazine cream were not effective in reducing the MRSA bioburden whether treatment was early or late.
Fig 3.
Log reduction in the wound tissue bioburden. Treatment for 2 days with different topical agents was initiated 4 h (early treatment) or 24 h (established biofilm) after MRSA inoculation.
The combined microscopy, quantitative microbiology, and efficacy data demonstrate that we have developed a MRSA wound biofilm model. The changes in efficacy observed when biofilm was allowed to form prior to treatment suggest an important difference between this model and published studies in which treatment was initiated shortly after wound inoculation (3, 6). The model is straightforward and rapid to perform and provides robust, reproducible endpoints. The model may also be expanded to additional species, as preliminary experiments with Pseudomonas aeruginosa resulted in a bioburden of >108 CFU/g with bacterial communities present in the wound tissue (data not shown). Although wound healing was not measured as an endpoint in these studies, visual observations indicated that animals inoculated with MRSA exhibited delayed healing compared to uninoculated controls. The contribution of the biofilm nature of the infection to this delayed healing is uncertain but may merit future study, given evidence that biofilms are an important factor in many chronic wounds (4, 5, 7). Additional endpoints for the effects of antimicrobials could be explored such as histology to follow wound healing or molecular analyses to understand the host response. This model should be beneficial in guiding development of novel antibiofilm agents that better address infection in chronic wounds.
ACKNOWLEDGMENTS
We thank the staff of the UNT HSC Department of Laboratory Animal Medicine for support of the animal model, Laurie Miller and Chris Gilpin of the Molecular and Cellular Imaging Facility at the University of Texas Southwestern Medical Center for assistance with SEM, Sarah Ramsay for the processing of histology samples, and David Valtierra for his preliminary work with P. aeruginosa.
This work was funded by Healthpoint Biotherapeutics.
Footnotes
Published ahead of print 29 May 2012
REFERENCES
- 1. Agostinho AM, et al. 2011. An in vitro model for the growth and analysis of chronic wound MRSA biofilms. J. Appl. Microbiol. 111:1275–1282 [DOI] [PubMed] [Google Scholar]
- 2. Gurjala AN, et al. 2011. Development of a novel, highly quantitative in vivo model for the study of biofilm-impaired cutaneous wound healing. Wound Repair Regen. 19:400–410 [DOI] [PubMed] [Google Scholar]
- 3. Hurdle JG, Yendapally R, Sun D, Lee RE. 2009. Evaluation of analogs of reutericyclin as prospective candidates for treatment of staphylococcal skin infections. Antimicrob. Agents Chemother. 53:4028–4031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. James GA, et al. 2008. Biofilms in chronic wounds. Wound Repair Regen. 16:37–44 [DOI] [PubMed] [Google Scholar]
- 5. Kirketerp-Møller K, et al. 2008. The distribution, organization, and ecology of bacteria in chronic wounds. J. Clin. Microbiol. 46:2717–2722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kugelberg E, et al. 2005. Establishment of a superficial skin infection model in mice by using Staphylococcus aureus and Streptococcus pyogenes. Antimicrob. Agents Chemother. 49:3435–3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Percival SL, Hill KE, Malic S, Thomas DW, Williams DW. 2011. Antimicrobial tolerance and the significance of persister cells in recalcitrant chronic wound biofilms. Wound Repair Regen. 19:1–9 [DOI] [PubMed] [Google Scholar]
- 8. Schierle CF, De la Garza M, Mustoe TA, Galiano RD. 2009. Staphylococcal biofilms impair wound healing by delaying reepithelialization in a murine cutaneous wound model. Wound Repair Regen. 17:354–359 [DOI] [PubMed] [Google Scholar]
- 9. Seth AK, et al. 2012. Treatment of Pseudomonas aeruginosa biofilm-infected wounds with clinical wound care strategies: a quantitative study using an in vivo rabbit ear model. Plast. Resconstr. Surg. 129:262e–274e [DOI] [PubMed] [Google Scholar]
- 10. Zhao G, et al. 2010. Delayed wound healing in diabetic (db/db) mice with Pseudomonas aeruginosa biofilm challenge: a model for the study of chronic wounds. Wound Repair Regen. 18:467–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zuluaga AF, et al. 2006. Neutropenia induced in outbred mice by a simplified low-dose cyclophosphamide regimen: characterization and applicability to diverse experimental models of infectious diseases. BMC Infect. Dis. 6:55. [DOI] [PMC free article] [PubMed] [Google Scholar]