Abstract
Posaconazole oral suspension, a marketed extended-spectrum triazole with proven efficacy as antifungal treatment and prophylaxis, should be taken with food to maximize absorption. New tablet and capsule formulations have been developed in an attempt to optimize absorption and bioavailability. The aims of this exploratory open-label, partially randomized, 2-part, 4-way, single-dose crossover study in 16 healthy adults were to characterize pharmacokinetics for posaconazole tablet and capsule formulations relative to those for posaconazole oral suspension under fasted and fed conditions and to assess safety and tolerability. Under fasted conditions, posaconazole exposures (area under the curve [AUC]) for the tablet and capsule formulations were similar (mean AUC from time zero to infinity [AUC0–∞], tablet A, 11,700 ng · h/ml [coefficient of variation {CV}, 26%]; tablet B, 11,300 ng · h/ml [CV, 22%]; capsule, 11,000 ng · h/ml [CV, 25%]) and were substantially higher than the exposure for the oral suspension (mean AUC0–∞, 3,420 ng · h/ml [CV, 44%]). Tablets and capsule showed less variability in exposure than the oral suspension. In fed subjects, tablets and capsule resulted in similar AUC values (mean AUC0–∞, tablet A, 11,900 ng · h/ml [23%]; tablet B, 12,400 ng · h/ml [CV, 25%]; capsule, 12,300 ng · h/ml [CV, 28%]) and slightly higher exposure than the oral suspension (mean AUC0–∞, 8,750 [CV, 24%]). Median times to the maximum concentration of drug in plasma were 4 to 5 h (fasted conditions) and 6 to 8 h (fed conditions). Mean half-lives values were similar for all formulations under fed and fasted conditions (23.1 to 29.2 h). Consistent with previous data, exposure for the oral suspension increased 2.5- to 3-fold when it was given with a high-fat meal. Conversely, exposures for tablets and capsule were not markedly affected by food. All formulations of posaconazole at 100 mg were safe and well tolerated.
INTRODUCTION
Posaconazole oral suspension is a marketed extended-spectrum triazole with demonstrated efficacy as antifungal prophylaxis for invasive fungal disease (IFD) in high-risk patients (3, 19) and as treatment for refractory IFD (15, 21).
The pharmacokinetics of posaconazole have been extensively studied in healthy volunteers and patients at risk for IFD (1, 4, 8, 10, 12, 18). Several studies have demonstrated that the bioavailability of posaconazole oral suspension is significantly enhanced when it is given with food, particularly a high-fat meal (5, 11). Patients at risk for IFD may be unable to eat because of mucositis, nausea, or neutropenic enterocolitis (7, 13, 16). In these patients who are unable to eat, absorption of posaconazole oral suspension may be enhanced by dividing posaconazole doses or by administering the drug with a liquid nutritional supplement or acidic beverage (9, 11).
In an attempt to further optimize absorption and bioavailability without dividing the dose or taking posaconazole with food or a nutritional supplement, new solid oral (tablet and capsule) formulations have been developed. The main objective behind developing these new formulations was to further enhance absorption under fasted conditions with no significant food effect, so that posaconazole can be given with or without food. These new formulations may also be future alternatives for the marketed oral suspension in patients at risk for low absorption of posaconazole.
The objectives of this exploratory study were to characterize posaconazole pharmacokinetics for 1 new capsule formulation and 2 new tablet formulations relative to the marketed oral suspension in healthy subjects under fasted and fed conditions and to assess the safety and tolerability of the posaconazole capsule and tablet formulations. To the best of our knowledge, this is the first report of a new solid oral formulation of posaconazole showing a significant increase in exposure in fasted healthy subjects compared with that of the currently marketed oral suspension formulation.
(This study was previously presented at the 51st Interscience Conference on Antimicrobial Agents and Chemotherapy, 17 to 20 September 2011, Chicago, IL.)
MATERIALS AND METHODS
Study design.
This was a phase I, single-center, open-label, partially randomized, 2-part, exploratory study (fed and fasted), with each part containing a 4-way, single-dose (100-mg) crossover of posaconazole oral suspension, 2 tablet formulations, and 1 capsule formulation (Fig. 1). The tablet and capsule formulations each contained 100 mg posaconazole in a solid dispersion formed by dissolving posaconazole in a pH-sensitive polymer matrix using hot-melt extrusion technology. The drug substance was mixed with hypromellose acetate succinate and ascorbic acid (1:3:0.08, wt/wt/wt) for the hot-melt extrusion process. Capsules and tablets were made from the resulting solid dispersion powder. For the capsule formulation, capsules were filled with milled solid dispersion powder. For the tablet formulations, the milled solid dispersion powder was mixed with small amounts of excipients such as microcrystalline cellulose and low-substituted hydroxypropyl cellulose (tablet A) and polyvinylpyrrolidone and croscarmellose sodium (tablet B). These formulations were designed to inhibit the release of posaconazole until the drug reached the elevated-pH environment of the small intestine, maximizing systemic absorption.
Fig 1.
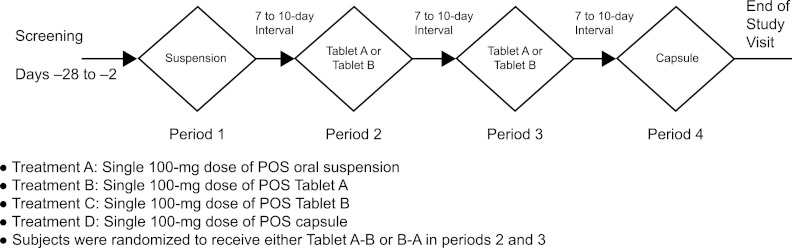
Study design of part 1 (fasted) and part 2 (fed). POS, posaconazole.
In both parts, subjects received single oral doses of posaconazole at 100 mg at each of the 4 periods. In period 1, subjects received oral suspension; in periods 2 and 3, subjects were randomly allocated to receive one of the tablet formulations (either tablet A-B or tablet B-A); in period 4, subjects received the capsule (Fig. 1). There was a 7- to 10-day interval between each period, and all subjects were confined to the study center on the day before each dosing day. All dosing was in the morning. In part 1 of the study, subjects received posaconazole after an overnight 10-hour fast. Subjects then received the treatments in the same sequence under fed conditions in part 2; subjects received posaconazole during a standardized high-fat breakfast containing approximately 54 g fat.
Subjects.
The study was conducted in accordance with the principles of good clinical practice and was approved by the appropriate institutional review board and regulatory agencies. Written informed consent was obtained from each subject before enrollment. In this exploratory study, sample size was based on empirical considerations and no statistical hypothesis was tested.
Sixteen healthy male and female participants aged 18 to 55 years inclusive with a body mass index of 19 to 32 kg/m2 inclusive were enrolled. Clinical laboratory test results (hematology, blood chemistry, and urinalysis) were within normal limits or clinically acceptable to the investigator/sponsor. Female subjects had a negative pregnancy test at screening and on admission and were postmenopausal, surgically sterilized, using a medically accepted method of contraception for 2 months, or abstaining from sexual intercourse before the screening period. Male subjects agreed to use a medically accepted method of contraception or to abstain from sexual intercourse during the study and for 1 month after stopping the study drug. Subjects could not have had any surgical or medical condition that might significantly alter the absorption, distribution, metabolism, or excretion of any drug, including a history or the presence of inflammatory bowel disease, ulcers, gastrointestinal or rectal bleeding; major gastrointestinal tract surgery; pancreatic injury or pancreatitis; liver disease or liver injury; or impaired renal function. Subjects could not have had a history of any infectious disease within 4 weeks before drug administration or a positive result for hepatitis B surface antigen, hepatitis C antibodies, HIV, or drugs with a high potential for abuse. Subjects were excluded if they had a history of alcohol or drug abuse in the past 2 years, had donated blood in the past 60 days, had previously received posaconazole, or had participated in a clinical study within 30 days. Subjects could not smoke more than 10 cigarettes or use an equivalent amount of tobacco per day. Use of any concomitant drug was prohibited from 14 days before screening to throughout the study period.
Pharmacokinetic evaluations.
During parts 1 and 2, blood samples (3 ml) were collected on each dosing day at predetermined time points: 0 h (predose) and 0.5, 1, 2, 3, 4, 5, 6, 8, 12, 24, 48, 72, 96, 120, 144, and 168 h postdose. Plasma samples were assayed for posaconazole concentration using validated liquid chromatography (17) with tandem mass spectrometric detection with a calibration range of 5 to 5,000 ng/ml, precision of 5.4 to 13.1, and accuracy of 0.8 to 5.0.
Primary pharmacokinetic parameters were area under the curve from time zero to the time of the final quantifiable sample (AUC0–t), maximum plasma concentration (Cmax), and time to Cmax (Tmax). Additional pharmacokinetic parameters were terminal-phase half-life (t1/2), area under the curve from time zero to infinity (AUC0–∞), apparent total body clearance (CL/F), and apparent volume of distribution (V/F). For this exploratory study, posaconazole plasma concentrations and pharmacokinetic parameters were listed and summarized by treatment group using descriptive statistics and graphics.
Safety analyses.
Safety assessments included reporting of adverse events (AEs); determination of vital signs; physical examination; and electrocardiographic (ECG), hematology, and blood chemistry analyses.
RESULTS
Subject demographics and disposition.
Sixteen subjects (8 males and 8 females) with a mean age of 31.4 years were enrolled; subject demographics are shown in Table 1. Fifteen subjects completed the study; 1 subject withdrew after part 1 of the study because of a mild AE (toothache), which was considered unlikely related to study medication.
Table 1.
Subject demographics
| Characteristic | Valuea |
|---|---|
| No. (%) of subjects by: | |
| Gender | |
| Male | 8 (50) |
| Female | 8 (50) |
| Race | |
| White | 10 (63) |
| Black | 4 (25) |
| Asian | 1 (6) |
| Age (yr) | |
| Mean (SD) | 31.4 (7.13) |
| Median (range) | 31.0 (19–45) |
| Body mass index (kg/m2) | |
| Mean (SD) | 26.1 (3.00) |
| Median (range) | 25.4 (21.3–30.5) |
Data are for all subjects (n = 16).
Pharmacokinetic evaluations. (i) Fasted conditions.
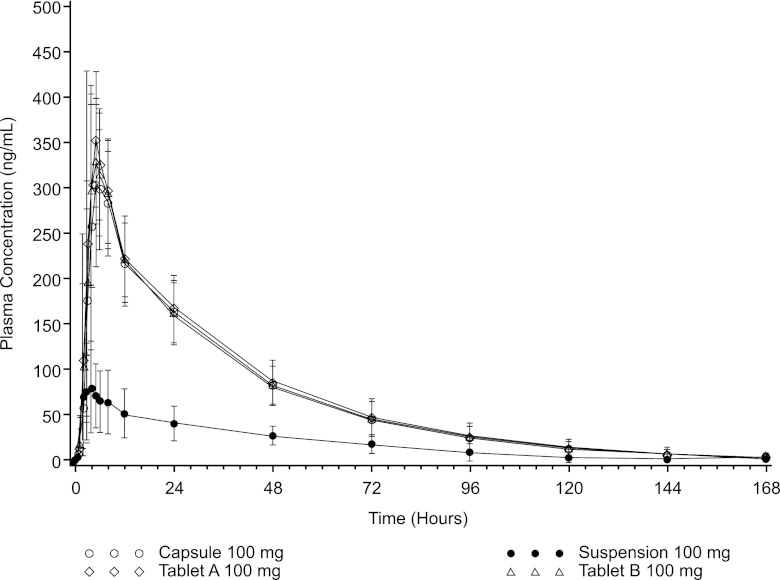
In fasted subjects, posaconazole tablets and capsule showed substantially higher exposure than the oral suspension, with enhanced absorption being the apparent primary contributor to the effect, as half-life remained unchanged (Table 2). Whereas posaconazole exposures (AUC) for the tablet and capsule formulations were similar (mean AUC0–∞, tablet A, 11,700 ng · h/ml [coefficient of variation }CV{, 26%]; tablet B, 11,300 [CV, 22%]; capsule, 11,000 ng · h/ml [CV, 25]), they were substantially higher than those for the oral suspension (mean AUC0–∞, 3,420 ng · h/ml [CV, 44%]) (Table 2 and Fig. 2). Similarly, Cmaxs were comparable for the tablet and capsule formulations but substantially higher than the Cmax for the oral suspension (mean Cmax, tablet A, 385 ng/ml [CV, 28%]; tablet B, 358 ng/ml [CV, 23%]; capsule, 335 ng/ml [CV, 27%]; oral suspension, 84 ng/ml [CV, 62%]). Tablets and capsule also showed less variability in exposure than the oral suspension; peak and total exposure CV values were approximately 25% for the tablet and capsule formulations but approximately 45% to 60% for the posaconazole oral suspension (Table 2). Median Tmax values were 5 h for the tablets and capsule and 4 h for the oral suspension. CL/F and V/F were higher for the oral suspension under fasting conditions, due to demonstrated poor absorption of the oral suspension with a low relative bioavailability under fasting conditions, than under fed conditions (5).
Table 2.
Plasma pharmacokinetic parameters of 100 mg posaconazole under fasted conditionsa
| Formulation | AUC0–t (ng · h/ml) | AUC0–∞ (ng · h/ml) | Cmax (ng/ml) | Tmaxb (h) | t1/2 (h) | CL/F (liters/h) | V/F (liters) |
|---|---|---|---|---|---|---|---|
| Oral suspension | 2,970 (50) | 3,420 (44)c | 84.0 (62) | 4.00 (2.00, 8.00) | 29.2 (31)c | 34.0 (38)c | 1,450 (54)c |
| Tablet A | 11,400 (26) | 11,700 (26) | 385 (28) | 5.00 (3.00, 6.00) | 26.1 (28) | 9.16 (29) | 345 (45) |
| Tablet B | 11,000 (22) | 11,300 (22) | 358 (23) | 5.00 (2.00, 8.00) | 25.0 (25) | 9.25 (22) | 331 (34) |
| Capsule | 10,700 (26) | 11,000 (25) | 335 (27) | 5.00 (4.00, 8.00) | 25.1 (27) | 9.67 (26) | 349 (36) |
Data are arithmetic means (percent CV) for 16 subjects. AUC0–t, area under the curve from time zero to the time of the final quantifiable sample; AUC0–∞, area under the curve from time zero to infinity; CL/F, apparent total body clearance; Cmax, maximum plasma concentration; CV, coefficient of variation; t1/2, terminal-phase half-life; Tmax, time to Cmax; V/F, apparent volume of distribution.
Data represent median (minimum, maximum).
n = 15.
Fig 2.
Mean (±SD) posaconazole (100 mg) plasma concentrations versus time (fasted conditions).
(ii) Fed conditions.
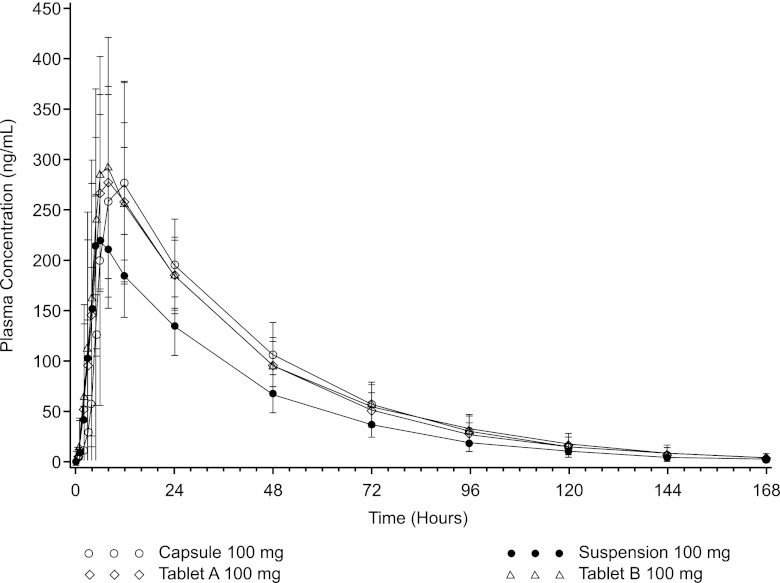
In fed subjects, tablets and capsule again resulted in similar AUC values (mean AUC0–∞, tablet A, 11,900 ng · h/ml [CV, 23%]; tablet B, 12,400 ng · h/ml [CV, 25%]; capsule, 12,300 ng · h/ml [CV, 28%]) and had higher exposures than the oral suspension (mean AUC0–∞, 8,750 ng · h/ml [CV, 24%]) (Table 3 and Fig. 3). The Cmaxs of the tablet and capsule formulations were comparable and were higher than the Cmax of the oral suspension (mean Cmax, tablet A, 327 ng/ml [CV, 23%]; tablet B, 348 ng/ml [CV, 32%]; capsule, 330 ng/ml [CV, 34]; oral suspension, 243 [CV, 18%]). The median Tmax was 6 to 8 h.
Table 3.
Plasma pharmacokinetic parameters of 100 mg posaconazole under fed conditionsa
| Formulation | AUC0–t (ng · h/ml) | AUC0–∞ (ng · h/ml) | Cmax (ng/ml) | Tmaxb (h) | t1/2 (h) | CL/F (liters/h) | V/F (liters) |
|---|---|---|---|---|---|---|---|
| Oral suspension | 8,470 (25) | 8,750 (24) | 243 (18) | 6.00 (5.00, 12.0) | 25.1 (35) | 12.1 (26) | 427 (39) |
| Tablet A | 11,700 (24) | 11,900 (23) | 327 (23) | 8.00 (3.00, 24.0) | 23.7 (21) | 8.97 (32) | 305 (34) |
| Tablet B | 12,100 (25) | 12,400 (25) | 348 (32) | 6.00 (3.00, 24.0) | 25.3 (24) | 8.64 (32) | 305 (27) |
| Capsule | 12,000 (28) | 12,300 (28) | 330 (34) | 8.00 (5.00, 24.0) | 23.1 (21) | 8.85 (34) | 290 (33) |
Data are arithmetic means (percent CV) for 15 subjects. AUC0–t, area under the curve from time zero to the time of the final quantifiable sample; AUC0–∞, area under the curve from time zero to infinity; CL/F, apparent total body clearance; Cmax, maximum plasma concentration; CV, coefficient of variation; t1/2, terminal-phase half-life; Tmax, time to Cmax; V/F, apparent volume of distribution.
Data represent median (minimum, maximum).
Fig 3.
Mean (±SD) posaconazole (100 mg) plasma concentrations versus time (fed conditions).
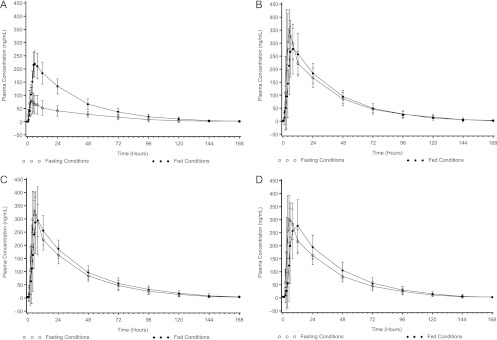
Consistent with previous data (5, 11), posaconazole exposure for the oral suspension was significantly increased (2.5- to 3-fold) when given with a high-fat meal than when administered in the fasted state (Fig. 4A); in contrast, exposures for tablets and capsule were not markedly affected by food (Fig. 4B to D).
Fig 4.
Mean (±SD) posaconazole (100 mg) plasma concentrations versus time under fed and fasted conditions for different formulations. (A) Oral suspension; (B) tablet A; (C) tablet B; (D) capsule.
Safety assessments.
A single 100-mg oral dose of posaconazole was safe and well tolerated when administered as an oral suspension, a tablet, or a capsule. There were no serious AEs. The incidence of treatment-emergent AEs was comparable between the different posaconazole formulations under both fasted and fed conditions. Only 1 subject reported a treatment-emergent AE of headache that was considered by the investigator to be possibly related to treatment (posaconazole oral suspension under fed conditions). All other treatment-emergent AEs were considered likely unrelated to treatment. All AEs were mild to moderate in severity. One subject discontinued prematurely because of a mild AE (toothache) that was likely unrelated to study drug. There were no clinically significant abnormalities in laboratory safety test, vital sign, or ECG results during the study.
DISCUSSION
Results of the current study show that under fasted conditions, posaconazole solid formulations (tablets or capsule) yielded substantially higher mean drug exposures than the posaconazole oral suspension. Under fed conditions, posaconazole solid formulations (tablets or capsule) still yielded higher drug exposures than the posaconazole oral suspension. Furthermore, under fasted conditions, the tablet and capsule formulations had less intersubject variability in peak and total exposures than the posaconazole oral suspension.
The effect of food on the bioavailability of azole antifungals varies among agents. Food does not significantly affect the pharmacokinetics of fluconazole (6) but significantly decreases voriconazole absorption (14). When administered with a meal, absorption of the capsule formulation of itraconazole is increased (2, 22), whereas absorption of the oral solution of itraconazole is decreased (20) relative to that in the fasted state. Because itraconazole capsules and posaconazole oral suspension are lipophilic and have poor solubility in water, the presence of food may enhance their solubility (2, 8). A previous study on the effect of food on posaconazole bioavailability in healthy adults reported that mean Cmax and AUC values were approximately 4-fold greater when posaconazole was administered with a high-fat meal and 3- and 2.6-fold greater, respectively, when administered with a nonfat meal than when administered under fasted conditions (5). Consistent with these historic data, this study showed that a high-fat meal increased both the mean peak and total exposures of posaconazole oral suspension. However, exposures for the posaconazole tablet and capsule formulations were not markedly affected by food, suggesting that new tablet and capsule formulations could be given without regard to food.
Although the plasma cutoff level for breakthrough IFD for posaconazole has not yet been defined (10), posaconazole plasma levels may be important to maintain efficacy for both treatment and prophylaxis. For example, a positive association between exposure and response was reported in a nonrandomized trial on posaconazole salvage treatment for invasive aspergillosis (21). The increased exposures of the tablet and capsule formulations compared with the oral suspension, especially under fasted conditions, may benefit patients at risk for low absorption of posaconazole, such as those who cannot tolerate food and thus are unable to take the currently marketed oral suspension with food. It is also possible that the significantly improved bioavailability of new solid oral formulations of posaconazole under fasted conditions could reduce the dosing frequency from the currently recommended two, three, and four times a day for the oral suspension formulation to once daily for the new solid oral formulations.
In summary, posaconazole tablets and capsule yielded substantially improved exposure over the posaconazole oral suspension formulation under fasted conditions, with relatively less variability and minimal food effect. Thus, new solid oral tablet and capsule formulations can be given with or without food. These data have important implications and warrant further clinical studies to characterize the pharmacokinetics and support development of these new solid oral formulations. Posaconazole was safe and well tolerated by healthy subjects when administered orally under fasted and fed conditions as a single 100-mg dose of oral suspension, tablet A, tablet B, or capsule.
ACKNOWLEDGMENTS
This study was funded by Merck Sharp & Dohme Corp., Whitehouse Station, NJ.
Medical writing and editorial assistance were provided by Sheena Hunt and Susan Quiñones of ApotheCom, Yardley, PA, with our original work. This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ.
L.M. and M.M. are currently employees of Merck Sharp & Dohme Corp. G.K. and E.O. were employees of Merck Sharp & Dohme Corp. when the study was conducted.
Footnotes
Published ahead of print 21 May 2012
REFERENCES
- 1. AbuTarif MA, Krishna G, Statkevich P. 2010. Population pharmacokinetics of posaconazole in neutropenic patients receiving chemotherapy for acute myelogenous leukemia or myelodysplastic syndrome. Curr. Med. Res. Opin. 26:397–405 [DOI] [PubMed] [Google Scholar]
- 2. Barone JA, et al. 1993. Food interaction and steady-state pharmacokinetics of itraconazole capsules in healthy male volunteers. Antimicrob. Agents Chemother. 37:778–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cornely OA, et al. 2007. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N. Engl. J. Med. 356:348–359 [DOI] [PubMed] [Google Scholar]
- 4. Courtney R, Pai S, Laughlin M, Lim J, Batra V. 2003. Pharmacokinetics, safety, and tolerability of oral posaconazole administered in single and multiple doses in healthy adults. Antimicrob. Agents Chemother. 47:2788–2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Courtney R, Wexler D, Radwanski E, Lim J, Laughlin M. 2004. Effect of food on the relative bioavailability of two oral formulations of posaconazole in healthy adults. Br. J. Clin. Pharmacol. 57:218–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Debruyne D. 1997. Clinical pharmacokinetics of fluconazole in superficial and systemic mycoses. Clin. Pharmacokinet. 33:52–77 [DOI] [PubMed] [Google Scholar]
- 7. Gorschluter M, et al. 2005. Neutropenic enterocolitis in adults: systematic analysis of evidence quality. Eur. J. Haematol. 75:1–13 [DOI] [PubMed] [Google Scholar]
- 8. Gubbins PO, et al. 2006. Pharmacokinetics and safety of oral posaconazole in neutropenic stem cell transplant recipients. Antimicrob. Agents Chemother. 50:1993–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Krishna G, et al. 2009. Effect of varying amounts of a liquid nutritional supplement on the pharmacokinetics of posaconazole in healthy volunteers. Antimicrob. Agents Chemother. 53:4749–4752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Krishna G, Martinho M, Chandrasekar P, Ullmann AJ, Patino H. 2007. Pharmacokinetics of oral posaconazole in allogeneic hematopoietic stem cell transplant recipients with graft-versus-host disease. Pharmacotherapy 27:1627–1636 [DOI] [PubMed] [Google Scholar]
- 11. Krishna G, Moton A, Ma L, Medlock MM, McLeod J. 2009. The pharmacokinetics and absorption of posaconazole oral suspension under various gastric conditions in healthy volunteers. Antimicrob. Agents Chemother. 53:958–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Krishna G, et al. 2008. Pharmacokinetics of oral posaconazole in neutropenic patients receiving chemotherapy for acute myelogenous leukemia or myelodysplastic syndrome. Pharmacotherapy 28:1223–1232 [DOI] [PubMed] [Google Scholar]
- 13. Pille S, Bohmer D. 1998. Options for artificial nutrition of cancer patients. Strahlenther. Onkol. 174(Suppl 3):S52–S55 [PubMed] [Google Scholar]
- 14. Purkins L, Wood N, Kleinermans D, Greenhalgh K, Nichols D. 2003. Effect of food on the pharmacokinetics of multiple-dose oral voriconazole. Br. J. Clin. Pharmacol. 56:17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Raad, et al. 2006. Posaconazole as salvage treatment of invasive fusariosis in patients with underlying hematologic malignancy and other conditions. Clin. Infect. Dis. 42:1398–1403 [DOI] [PubMed] [Google Scholar]
- 16. Sansone-Parsons A, et al. 2006. Effect of a nutritional supplement on posaconazole pharmacokinetics following oral administration to healthy volunteers. Antimicrob. Agents Chemother. 50:1881–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shen JX, Krishna G, Hayes RN. 2007. A sensitive liquid chromatography and mass spectrometry method for the determination of posaconazole in human plasma. J. Pharm. Biomed. Anal. 43:228–236 [DOI] [PubMed] [Google Scholar]
- 18. Ullmann AJ, et al. 2006. Pharmacokinetics, safety, and efficacy of posaconazole in patients with persistent febrile neutropenia or refractory invasive fungal infection. Antimicrob. Agents Chemother. 50:658–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ullmann AJ, et al. 2007. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N. Engl. J. Med. 356:335–347 [DOI] [PubMed] [Google Scholar]
- 20. Van de Velde V, et al. 1996. Effect of food on the pharmacokinetics of a new hydroxypropyl-beta-cyclodextrin formulation of itraconazole. Pharmacotherapy 16:424–428 [PubMed] [Google Scholar]
- 21. Walsh TJ, et al. 2007. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin. Infect. Dis. 44:2–12 [DOI] [PubMed] [Google Scholar]
- 22. Zimmermann T, Yeates RA, Laufen H, Pfaff G, Wildfeuer A. 1994. Influence of concomitant food intake on the oral absorption of two triazole antifungal agents, itraconazole and fluconazole. Eur. J. Clin. Pharmacol. 46:147–150 [DOI] [PubMed] [Google Scholar]