Abstract
The objective of this study was to analyze the impact of MIC values within the susceptible range of antibiotics on the outcomes of patients with Gram-negative infections. The PubMed and Scopus electronic databases were searched. We identified 13 articles (1,469 patients) that studied the impact of antibiotic MICs on the outcomes of infections; β-lactams were studied in 10 of them. Infections due to Salmonella enterica strains with high fluoroquinolone MICs were associated with more treatment failures than those due to strains with low MICs (relative risk [RR], 5.75; 95% confidence interval [CI], 1.77 to 18.71). Among non-Salmonella enterobacteriaceae, there was no difference in treatment failures depending on the MIC value (RR, 1.18; 95% CI, 0.71 to 1.97); however, a higher all-cause mortality was observed for patients infected with strains with high MICs (RR, 2.03; 95% CI, 1.05 to 3.92). More treatment failures were observed for patients infected with nonfermentative Gram-negative bacilli when strains had high MICs (RR, 5.54; 95% CI, 2.72 to 11.27). The mortality rate for patients with infections with Gram-negative nonfermentative bacilli with high MICs was also higher than for those with low MICs (RR, 2.39; 95% CI, 1.19 to 4.81). The limited available data suggest that there is an association between high MICs, within the susceptible range, and adverse outcomes for patients with Gram-negative infections.
INTRODUCTION
Antibiotic resistance has been an issue of debate since the introduction of antibiotics into clinical practice in the 1940s. At the beginning, it was demonstrated that antibiotics could inhibit bacterial growth in vitro in specific, minimal concentrations (MICs); since then, this value has been used to denote susceptibility in vivo and to guide clinical practice. However, it was not always possible to predict the clinical outcome of an infection based solely on the MIC. Moreover, the acquisition of resistant mechanisms either by mutations or through interbacterial communication has rendered bacteria more tolerant to antibiotics and more difficult to treat. As a result, susceptibility breakpoints kept changing over time (20). With time, several pharmacodynamic parameters have been associated more precisely with patient or infection outcomes for specific antibiotics.
Despite these facts, susceptibility according to in vitro MICs continues to be a key factor in decision making. However, a recent meta-analysis reported that patients infected with vancomycin-susceptible Staphylococcus aureus isolates with vancomycin MICs of >1 μg/ml had more treatment failures and higher mortality rates than patients infected with isolates with vancomycin MICs of ≤1 μg/ml (data not shown). Moreover, the Clinical and Laboratory Standards Institute (CLSI) acknowledges that more treatment failures are expected for patients with typhoid fever treated with fluoroquinolones if the “susceptible” pathogen is resistant to nalidixic acid (4).
Therefore, it is evident that the designations “sensitive,” “intermediately sensitive,” and even (to a lesser extent) “resistant” according to the MIC value do not fully reciprocate their meaning. In this context, we sought to review systematically the available evidence in order to examine whether high MIC values, within the susceptible range, are associated with worse outcomes than lower MIC values in infections caused by Gram-negative bacteria.
MATERIALS AND METHODS
Literature search.
A systematic search of the literature in the PubMed and Scopus databases was performed in January 2012. The following search pattern was applied to articles published from January 1990 onwards: MIC or MICs or “MIC” or “MICs,” acinetobacter or baumannii or pseudomonas or aeruginosa or klebsiella or enterobacteriaceae or haemophilus or moraxella or neisseria or gram negative, and outcome or response or impact or influence or effect or efficacy or effectiveness or failure or cure or mortality or outcomes or prolonged or improved or prognosis. Furthermore, the references of relevant articles were hand searched to identify additional potentially eligible studies. Articles published in a language other than English, Spanish, German, French, Italian, or Greek were not evaluated.
Study selection.
Any published article reporting clinical or microbiological outcomes of patients with infections due to antibiotic-susceptible Gram-negative isolates (defined as susceptible according to current CLSI and European Committee on Antimicrobial Susceptibility Testing [EUCAST] criteria [4; http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/EUCAST_breakpoints_v_2.0_120101.pdf]) stratified by antibiotic MIC (any testing method could be used) and receiving the corresponding antimicrobial treatment was considered eligible for our review. If the CLSI and EUCAST criteria did not match the lower value that was considered the breakpoint or if comparative data could not be extracted for this value (the EUCAST usually has lower breakpoints for Gram-negative bacteria), alternative breakpoints were used. Studies reporting patients with infection at any site were eligible for inclusion. Case reports were not eligible for inclusion in the review. Abstracts reported for conferences were excluded.
Data extraction.
Literature searches, study selection, and data extraction were performed independently by 2 investigators (G.S.T. and K.Z.V.). Any disagreement was resolved by consensus in meetings with all investigators. The extracted data included the characteristics of each study (study design, country, and time period when the study was conducted), its patient population (number of evaluated patients or episodes as well as age, gender, comorbidity, and empirical or initial treatment of the patients), the studied infection(s) and pathogens, the testing method performed for the determination of susceptibility, as well as clinical outcomes.
Definitions and outcomes.
The primary outcomes of this review were all-cause (30-day or in-hospital) mortality and treatment failure (clinical or microbiological, as assessed by each study's investigators). In general, treatment failure could be defined as a persistence of symptoms/signs, failure to eradicate the implicated pathogen (as indicated by repetitive specimen cultures), infection recurrence, or death.
All patients were allocated into 2 groups (high versus low MICs), depending on the MIC values of the isolated bacteria. Patients with typhoid fever were grouped into the high-MIC group when the ciprofloxacin or ofloxacin MIC was ≥0.125 μg/ml. For other Gram-negative bacteria, the group of patients with infections due to isolates with high MICs included those with isolates with the upper MIC value (breakpoint) within the susceptible range and those with isolates with an MIC value 1 dilution lower; the remaining isolates composed the low-MIC group. Patients infected with strains that were resistant to the administered antibiotics were not included. If data for the grouping of patients into the above-mentioned populations were not available, isolates were allocated to the closest relevant group.
Statistical analysis.
Pooled risk ratios (RRs) and 95% confidence intervals (CIs) were calculated for all outcomes. Statistical heterogeneity between studies was assessed by using a χ2 test (a P value of <0.10 was defined to indicate significant heterogeneity) and the I2 value. The Mantel-Haenszel random-effects model (REM) was used for all analyses. Publication bias was assessed by the funnel plot method. The meta-analysis was performed with Review Manager for Windows, version 5.1. Two analyses were performed for enterobacteriaceae and nonfermentative Gram-negative bacteria: one using the CLSI 2011 breakpoints and one using lower available breakpoints.
RESULTS
Figure 1 shows the selection process for the included articles. The electronic search provided 3,177 articles. Thirteen articles were included; data for 1,469 patients were ultimately eligible, from 2 articles on typhoid fever (5, 15), 7 on other enterobacteriaceae, (1, 8, 11, 16–19), 5 on nonfermentative Gram-negative bacilli (1, 3, 8, 22, 23), and 2 on other Gram-negative bacteria (6, 8). The characteristics of the included studies are presented in Table 1. One study provided data for enterobacteriaceae, Pseudomonas aeruginosa, and other Gram-negative bacteria (8), and another one provided data for Acinetobacter baumannii and enterobacteriaceae (1). β-Lactams were the antibiotics studied in all but three studies, in which fluoroquinolones and tigecycline were studied. Publication bias was detected in analyses of both treatment failure and mortality.
Fig 1.
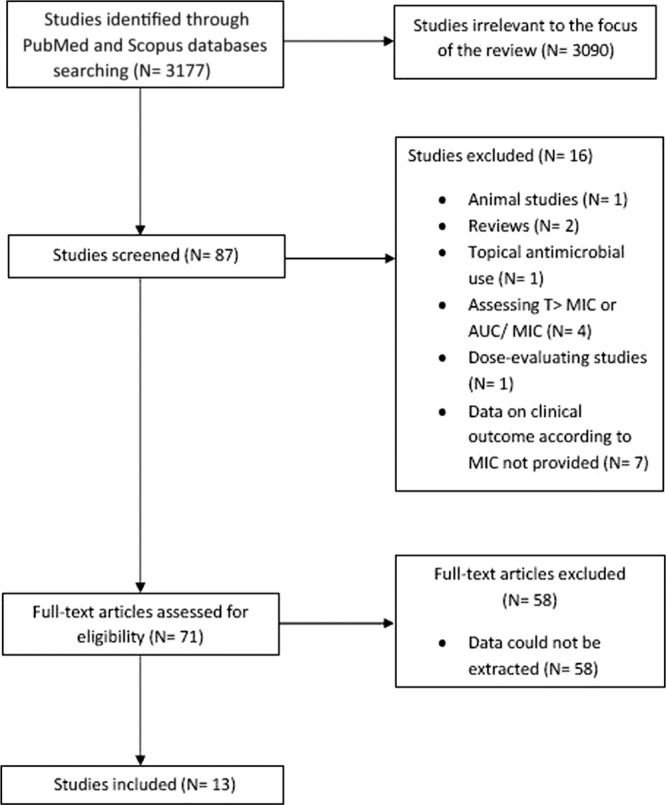
Flow diagram of the article selection process. AUC, area under the concentration-time curve; T>MIC, time above MIC.
Table 1.
Characteristics and outcomes of patients with Gram-negative infections included in the systematic review, according to isolated pathogensa
| Organism and reference | Study design, location, yr of study | No. of enrolled patientse | Characteristics of patients | MIC testing method(s) | Bacterial pathogen(s) studied | Infection type(s) and/or site(s) | CLSI 2011 breakpoint(s) | Outcome(s) according to CLSI breakpoints and no. of patients with isolates with high MICs/no. of patients with isolates with low MICs (%) | Outcomes according to the lower breakpoints available and no. of patients with isolates with high MICs/no. of patients with isolates with low MICs (%) | Description |
|---|---|---|---|---|---|---|---|---|---|---|
| Salmonella enterica | ||||||||||
| 15 | Post hoc analysis, Vietnam, 1991–2000 | 540 | Patients with uncomplicated typhoid fever | Disk diffusion, agar plate dilution | S. enterica | Typhoid fever | Ofloxacin, ≤2 μg/ml S, ≥8 μg/ml R | Failure for 37/117 (32) vs 17/423 (4) | Failure for 37/117 (32) vs 17/423 (4) | Patients with isolates with DFS were treated with higher doses and for longer periods; 1 patient died |
| 5 | Retrospective, United States, 1999–2002 | 87 | Hospitalized patients with typhoid fever | Broth microdilution | S. enterica | Typhoid fever | Ciprofloxacin, ≤1 μg/ml S, ≥4 μg/ml R | For any antibiotic, failure for 4/24 (17) vs 2/46 (4); for fluoroquinolones, failure for 2/11 (18) vs 1/10 (10) | For any antibiotic, failure for 4/24 (17) vs 2/46 (4); for fluoroquinolones, failure for 2/11 (18) vs 1/10 (10) | Travel to South Asia was predictive of DFS (P = 0.005); median time to defervescence was higher for isolates with DFS (92 vs 72 h; P = 0.01); no deaths were reported |
| Enterobacteriaceae other than Salmonella | ||||||||||
| 17 | Retrospective, United States, 2005–2008 | 21 | Health care-associated bacteremia due to ESBL-producing strains | Etest | Enterobacter cloacae | Bacteremia | PTZ, ≤16 μg/ml S, ≥64 μg/ml R; CFP, ≤8 μg/ml S, ≥32 μg/ml R; CPM, ≤1 μg/ml S, ≥4 μg/ml R | For PTZ 8 ≤ MIC ≤ 16 vs MIC ≤ 4 μg/ml, CFP 4 ≤ MIC ≤ 8 vs MIC ≤ 2 μg/ml, and CPM 0.5 ≤ MIC ≤ 1 vs MIC ≤ 0.25 μg/ml, failure for 1/1 (100) vs 7/15 (47) and death for 1/1 (100) vs 3/15 (20) | For PTZ 4 ≤ MIC ≤ 8 vs MIC ≤ 2 μg/ml, CFP 0.5 ≤ MIC ≤ 1 vs MIC ≤ 0.25 μg/ml, and CPM MIC ≤ 0.25 vs 0.5≤ MIC ≤ 1 μg/ml, failure for 5/5 (100) vs 2/9 (47) and death for 3/5 (60) vs 0/9 (0) | Treatment with carbapenems was associated with fewer treatment failures |
| 19 | Post hoc analysis of 6 prospective studies, Spain | 192 | Hospitalized adults with ESBL-positive E. coli bacteremia | NR | E. coli | Bacteremia | PTZ, ≤16 μg/ml S, ≥64 μg/ml R | For 8≤ MIC ≤ 16 vs MIC ≤ 4 μg/ml, death for 3/13 (23) vs 1/22 (5) | For 4 ≤ MIC ≤ 8 vs MIC ≤ 2 μg/ml, death for 3/10 (30) vs 0/18 (0) | Treatment with carbapenems was not associated with lower mortality |
| 19 | Post hoc analysis of 6 prospective studies, Spain | 192 | Hospitalized adults with ESBL-positive E. coli bacteremia | NR | E. coli | Bacteremia | AMC, ≤8 μg/ml S, ≥32 μg/ml R | For MIC = 8 vs MIC ≤ 4 μg/ml, death for 2/25 (8) vs 1/12 (8) | For MIC = 8 vs MIC ≤ 4 μg/ml, death for 2/25 (8) vs 1/12 (8) | Treatment with carbapenems was not associated with lower mortality |
| 1 | Retrospective, United States, 2004–2006 | 18 | Hospitalized adults with nosocomial infections | Etest | Klebsiella pneumoniae, E. coli, E. cloacae | VAP, UTI, bacteremia, abscess, DFI, SSTI | Tigecycline, ≤2 μg/ml S, ≥8 μg/ml R | For 1 ≤ MIC ≤ 2 vs MIC < 1 μg/ml, failure for 3/3 (100) vs 2/3 (67) and death for 0/3 (0) vs 2/2 (100) | For 1 ≤ MIC ≤ 2 vs MIC < 1 μg/ml, failure for 3/3 (100) vs 2/3 (67) and death for 2/2 (100) vs 0/3 (0) | None; no data for tigecycline breakpoints by EUCAST |
| 18 | Prospective, Spain, 2002–2003 | 37 | Outpatients with ESBL-producing E. coli infections | Broth microdilution | E. coli | Cystitis | AMC, ≤8 μg/ml S, ≥32 μg/ml R | For MIC ≤ 4 vs MIC = 8 μg/ml, failure for 2/14 (14) vs 1/18 (6) | For MIC = 8 vs MIC ≤ 4 μg/ml, failure for 2/14 (14) vs 1/18 (6) | None; EUCAST and CLSI breakpoints are the same |
| 11 | Retrospective, Belgium, 1994–2000 | 44 | ICU patients with ESBL-producing Enterobacter aerogenes infections | Agar dilution | E. aerogenes | UTI, VAP, bacteremia, IAIs | CFP, ≤8 μg/ml S, ≥32 μg/ml R | For MIC ≤ 2 vs 4 ≤ MIC ≤ 8 μg/ml, failure for 1/3 (33) vs 6/13 (46) and death for 2/3 (67) vs 7/13 (54) | For 0.5 ≤ MIC ≤ 1 vs MIC ≤ 0.25 μg/ml, failure for 1/4 (25) vs 0/1 (0) and death for 0/4 (0) vs 0/1 (0) | For patients with isolates with intermediate susceptibility, both treatment failure and death were found for 1/3 patients (33%) |
| 11 | Retrospective, Belgium, 1994–2000 | 44 | ICU patients with ESBL-producing E. aerogenes infections | Agar dilution | E. aerogenes | UTI, VAP, bacteremia, IAIs | CPM, ≤1 μg/ml S, ≥4 μg/ml R | For 0.5 ≤ MIC ≤ 1 vs MIC ≤ 0.25 μg/ml, failure for 0/4 (0) vs 5/16 (31) and death for 0/4 (0) vs 6/16 (38) | For 0.5 ≤ MIC ≤ 1 vs MIC ≤ 0.25 μg/ml, failure for 0/4 (0) vs 5/16 (31) and death for 0/4 (0) vs 6/16 (38) | For isolates with intermediate susceptibility, both treatment failure and death were found for 1/1 patients (100%) |
| 16 | Prospective, international, 1996–1997, review of cases | 36 | Patients with ESBL-producing enterobacteriaceae | Etest | K. pneumoniae, E. coli, Klebsiella oxytoca | Bacteremia, VAP, HAP, SBP, SSTIs | Various cephalosporinsb | Failure for 2/6 (33) vs 5/11 (45)c and death for 3/7 (43) vs 1/11 (9) | Failure for 3/7 (43) vs 3/8 (38)c and death for 4/8 (50) vs 0/8 (0) | The remaining 18 cases could not be used in the analysis after the revision of breakpoints, since the isolates were classified as resistant |
| 8 | Post hoc analysis of 17 RCTs, international | >2,000; exact no. not specified | Hospitalized and outpatients | NR | K. pneumoniae, E. coli, E. cloacae, Citrobacter freundii | Pneumonia, UTIs, IAIs, meningitis, SSTIs | Meropenem, ≤1 μg/ml S, ≥4 μg/ml R | For 0.5 ≤ MIC ≤ 1 vs MIC ≤ 0.25 μg/ml, failure for 0/9 (0) vs 24/330 (7) | For 0.5 ≤ MIC ≤ 1 vs MIC ≤ 0.25 μg/ml, failure for 0/9 (0) vs 24/330 (7) | Since the majority of treatment failures occurred when low MICs were encountered, the adverse outcomes were due to severe or worsening underlying disease and not due to resistance |
| Nonfermenting Gram-negative bacteria | ||||||||||
| 23 | Retrospective, Japan, 2008–2009 | 73 | Hospitalized adults with nosocomial infections | Broth microdilution | P. aeruginosa | HAP, bacteremia | PTZ, ≤64 μg/ml S, ≥128 μg/ml R | For 64 ≥ MIC ≥ 32 vs MIC ≤ 16 μg/ml, failure for 16/25 (64) vs 4/48 (8) | For 8 ≥ MIC ≥ 16 vs MIC ≤ 4 μg/ml, failure for 2/21 (10) vs 0/13 (0) | Microbiological eradication was the endpoint of the study |
| 3d | Retrospective, United States, NA | 176 | NA | Broth microdilution | P. aeruginosa | Bacteremia | CFP, ≤8 μg/ml S, ≥32 μg/ml R | For 8 ≥ MIC ≥ 4 vs MIC ≤ 2 μg/ml, death for 9/19 (47) vs 4/17 (24) | For 8 ≥ MIC ≥ 4 vs MIC ≤ 2 μg/ml, death for 4/17 (24) vs 9/19 (47) | MIC ≥ 8 was independent predictor of mortality along with APACHE II score, creatinine clearance rate of <60 ml/min, and renal replacement therapy; data for lower MICs according to EUCAST breakpoints could not be extracted |
| 22 | Retrospective, United States, 2002–2006 | 83 | Hospitalized adults with nosocomial infections | NR | P. aeruginosa | Bacteremia | PTZ, ≤64 μg/ml S, ≥128 μg/ml R | For 64 ≥ MIC ≥ 32 vs MIC ≤ 16 μg/ml, death for 6/7 (86) vs 3/10 (30) | For 64 ≥ MIC ≥ 32 vs MIC ≤ 16 μg/ml, death for 6/7 (86) vs 3/10 (30) | Treatment with PTZ was associated with higher mortality than control antibiotics for MIC ≥ 32 μg/ml; data for lower MICs according to EUCAST breakpoints could not be extracted |
| 1 | Retrospective, United States, 2004–2006 | 18 | Hospitalized adults with nosocomial infections | Etest | A. baumannii | VAP, UTI, bacteremia, abscess, DFI, SSTI | Tigecycline, ≤2 μg/ml S, ≥8 μg/ml R | For 1 < MIC ≤ 2 vs MIC ≤ 1, failure for 1/2 (50) vs 0/2 (0) and death for 0/2 vs 0/2 | For 1 < MIC ≤ 2 vs MIC ≤ 1 μg/ml, failure for 1/2 (50) vs 0/2 (0) and death for 0/2 vs 0/2 | Among patients with A. baumannii infections, those with S isolates were less likely to die than those with I isolates (0/4 vs 4/5; P = 0.048); no data for tigecycline breakpoints from EUCAST |
| 8 | Post hoc analysis of 17 RCTs, international | >2,000; exact no. not specified | Hospitalized and outpatients | NR | P. aeruginosa | Pneumonia, UTIs, IAIs, meningitis, SSTIs | Meropenem, ≤4 μg/ml S, ≥16 μg/ml R | For 2 ≤ MIC ≤ 4 vs MIC ≤ 1 μg/ml, failure for 3/7 (43) vs 7/66 (11) | For 1 ≤ MIC ≤ 2 vs MIC < 1 μg/ml, failure for 4/16 (25) vs 6/55 (11) | None |
| Haemophilus influenzae | ||||||||||
| 8 | Post hoc analysis of 17 RCTs, international | >2,000; exact no. not specified | Hospitalized and outpatients | NR | H. influenzae | Pneumonia, meningitis, SSTIs | Meropenem, ≤0.5 μg/ml S | For MIC ≤ 0.125 vs 0.25 ≤ MIC ≤ 0.5, failure for 0/6 (0) vs 2/83 (2) | For 0.25 ≤ MIC ≤ 0.5 vs MIC ≤ 0.125 μg/ml, failure for 0/6 (0) vs 2/83 (2) | None |
| 6 | RCT, Israel, 1994–1995 | 266 | Children <3 yr old, outpatients | Etest, broth microdilution | H. influenzae | Acute otitis media | Cefaclor, ≤8 μg/ml S, ≥32 μg/ml R | For 4 ≤ MIC ≤ 8 vs MIC ≤ 2 μg/ml, failure for 5/10 (50) vs 16/44 (36) | For 4 ≤ MIC ≤ 8 vs MIC ≤ 2 μg/ml, failure for 5/10 (50) vs 16/44 (36) | Microbiological eradication was the endpoint of the study; data for lower MICs according to EUCAST breakpoints could not be extracted |
| 6 | RCT, Israel, 1994–1995 | 266 | Children <3 yr old, outpatients | Etest, broth microdilution | H. influenzae | Acute otitis media | Cefuroxime, ≤4 μg/ml S, ≥16 μg/ml R | For 2 ≤ MIC ≤ 4 vs MIC ≤ 1 μg/ml, failure for 2/6 (33) vs 4/38 (11) | For 2 ≤ MIC ≤ 4 vs MIC ≤ 1 μg/ml, failure for 2/6 (33) vs 4/38 (11) | Data for lower MICs according to EUCAST breakpoints could not be extracted |
Studies appearing twice in the table provided data for more than one group of Gram-negative bacteria. Abbreviations: AMC, amoxicillin-clavulanate; CFP, cefepime; CLSI, Clinical and Laboratory Standards Institute; CPM, carbapenems; DFI, diabetic foot infection; DFS, decreased fluoroquinolone susceptibility (MIC ≥ 0.125 μg/ml); ESBL, extended-spectrum β-lactamases; HAP, hospital-acquired pneumonia; IAIs, inta-abdominal infections; ICU, intensive care unit; I, intermediately sensitive; NA, not applicable; NR, not reported; OR, odds ratio; PTZ, piperacillin-tazobactam; RCT, randomized controlled trial; RR, relative risk/risk ratio; R, resistant; S, sensitive; SBP, spontaneous bacterial peritonitis; SSTI, skin and soft tissue infection; UTI, urinary tract infection; VAP, ventilator-associated pneumonia.
Including ceftriaxone, ceftazidime, cefotaxime, cefepime, cefmetazole, and cefoxitin.
The outcome of treatment for one patient could not be determined because he died 1 day after treatment onset due to bowel necrosis.
The study also included patients with other Gram-negative bacterial infections, but data could not be extracted.
The numbers of patients from whom data could be extracted were usually lower than the numbers of enrolled patients.
Enterobacteriaceae.
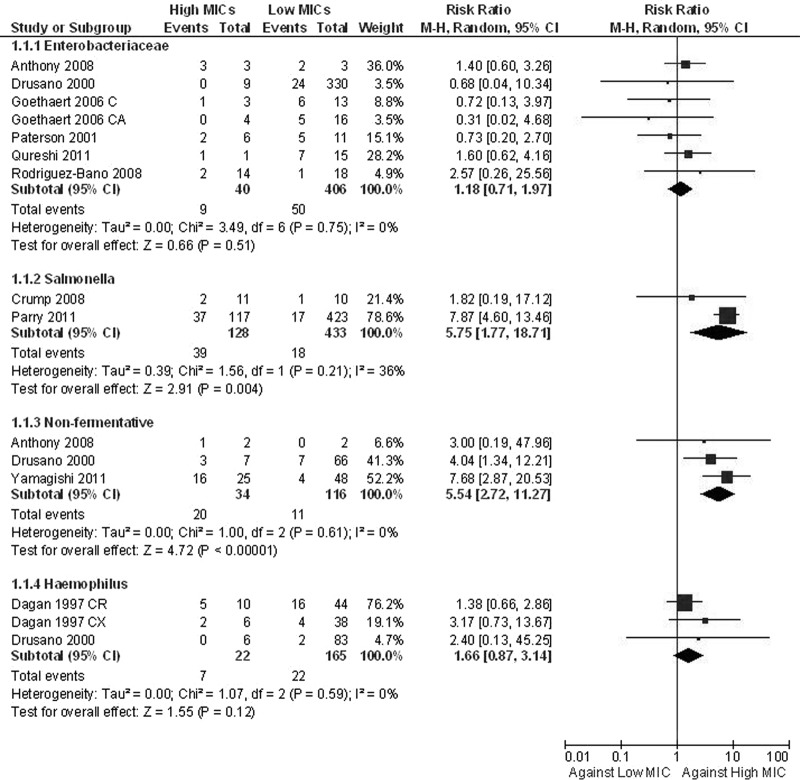
The articles on typhoid fever showed that when Salmonella enterica strains with MICs of ≥0.125 μg/ml were the causative microorganisms, more treatment failures were encountered than when with MICs were <0.125 μg/ml (RR, 5.75; 95% CI, 1.77 to 18.71) (Fig. 2) (5, 15). All patients were treated with fluoroquinolones. One death was reported in these two available articles. In addition, patients infected by isolates with decreased fluoroquinolone susceptibilities (MIC ≥ 0.125 μg/ml) were treated with higher doses (13 to 18 mg/kg of body weight versus 11 mg/kg), and the duration of antibiotic administration was longer (3 versus 7 days); the median time to defervescence was also higher for these patients.
Fig 2.
Forest plot depicting the risk ratios (RR) of treatment failure for patients with infection with high-MIC versus low-MIC Gram-negative isolates. Vertical line, “no-difference” point between the two regimens; squares, risk ratios; diamonds, pooled risk ratios for all studies; horizontal lines, 95% CIs; M-H, Mantel-Haenszel.
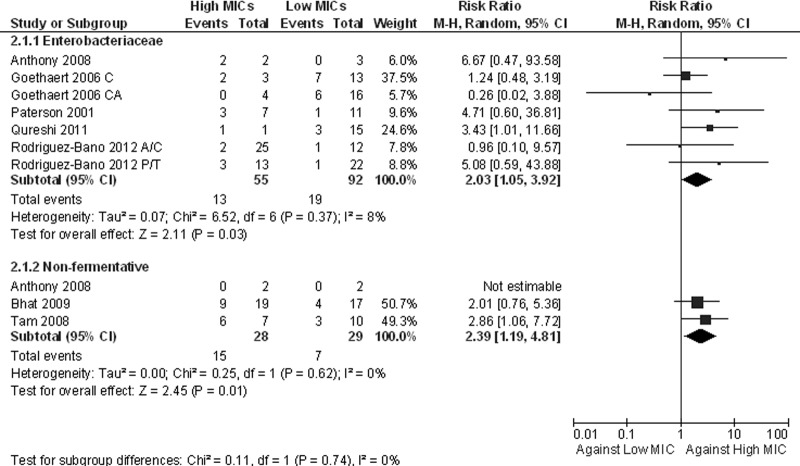
Seven studies reported outcomes for patients with infections due to enterobacteriaceae other than Salmonella spp. (1, 8, 11, 16–19). Several β-lactams were used, including cephalosporins, carbapenems, and β-lactams/β-lactamase inhibitors. None of the individual studies reported a difference in outcomes between infections by strains with high and infections by strains with low MICs. The pooling of the data from those studies according to CLSI breakpoints showed that there was no difference in treatment failures depending on the MIC value (RR, 1.18; 95% CI, 0.71 to 1.97) (Fig. 2); there was also no difference when the analysis was restricted to the five studies specifying that only extended-spectrum-β-lactamase (ESBL)-producing microorganisms were included (RR, 1.11; 95% CI, 0.58 to 2.13). However, a higher mortality rate was observed for patients infected with strains with high MICs (RR, 2.03; 95% CI, 1.05 to 3.92) (Fig. 3); when the analysis was restricted to the studies with ESBL-producing enterobacteriaceae, the difference in mortality was not statistically significant (RR, 1.89; 95% CI, 0.94 to 3.83). When the lower breakpoints were applied, fewer patients were included in the analyses, and no significant differences in both treatment failures (RR, 1.60; 95% CI, 0.93 to 2.73) and mortality rates (RR, 3.30; 95% CI, 0.92 to 11.79) were noted.
Fig 3.
Forest plot depicting the risk ratios (RR) of all-cause mortality of patients with infection with high-MIC versus low-MIC Gram-negative isolates. Vertical line, “no-difference” point between the two regimens; squares, risk ratios; diamonds, pooled risk ratios for all studies; horizontal lines, 95% CIs; M-H, Mantel-Haenszel.
Nonfermentative bacilli.
Data for P. aeruginosa infections were provided by 4 articles (3, 8, 22, 23). Yamagishi et al. reported previously that the rate of microbiological failure was higher when the piperacillin-tazobactam value was 64 ≥ MIC ≥ 32 μg/ml than when the MIC was ≤16 μg/ml (Fig. 2) (23). More treatment failures were also reported in a retrospective analysis of data from randomized trials on meropenem (Fig. 2) (8). Only four patients with A. baumannii infections were included in that review. In the primary study, which included 9 patients with A. baumannii infections, those with sensitive isolates were less likely to die than those with intermediately sensitive strains (0/4 versus 4/5; P = 0.048) (1). The pooling of the data on nonfermentative Gram-negative bacilli according to CLSI criteria showed that more treatment failures were observed for patients infected with strains with high MICs (RR, 5.54; 95% CI, 2.72 to 11.27) (Fig. 2). When lower breakpoints were used, fewer patients were included in the analysis, and no significant difference was noted (RR, 2.46; 95% CI, 0.91 to 6.63).
Tam et al. reported previously that there were higher mortality rates for patients infected with P. aeruginosa isolates with piperacillin-tazobactam values of 64 ≥ MIC ≥ 32 μg/ml than for patients infected with isolates with MICs of ≤16 (P = 0.04, Fig. 3); in addition, those authors noted that patients treated with piperacillin-tazobactam had higher mortality rates than those treated with control antibiotics (carbapenems, fluoroquinolones, aminoglycosides, and cephalosporins) when they were infected with isolates with piperacillin-tazobactam MICs of ≥32 μg/ml (P = 0.004) (22). The mortality rate for patients with infections with Gram-negative nonfermentative bacilli with high MICs was higher than that for patients with isolates with low MICs (RR, 2.39; 95% CI, 1.19 to 4.81). For this analysis, data regarding the lower breakpoints could not be extracted.
Other Gram-negative organisms.
The two studies that reported the outcomes of patients with Haemophilus influenzae infections reported that there was no difference in treatment failures between patients infected by strains with high MICs and those infected by strains with low MICs; the pooling of the data from those studies did not change the results (RR, 1.66; 95% CI, 0.87 to 3.14) (Fig. 2) (6, 8). Again, data according to the lower breakpoints could not be extracted. Data for mortality were not available.
DISCUSSION
The limited data regarding the outcomes of infections due to Gram-negative bacteria according to the MIC value suggested that high MIC values within the currently accepted “susceptible” range were associated with worse outcomes. This was more evident for S. enterica and P. aeruginosa infections, for which more treatment failures were reported for strains with high MICs of fluoroquinolones and piperacillin-tazobactam or meropenem, respectively. In addition, data from two studies showed that the mortality rate was also higher for patients infected with P. aeruginosa strains with high MICs. The data for enterobacteriaceae other than S. enterica showed that there was no difference in reported treatment failures, but the reported mortality rate was higher for patients infected with enterobacteriaceae with high MICs of various antibiotics.
The CLSI reports annually the breakpoints for susceptibility of the most important bacteria. Since 2010, the European Committee on Antimicrobial Susceptibility Testing has reported its own breakpoints. During the last few years, several changes have been made, usually toward the lowering of the MIC for susceptibility. These changes are in concordance with the message conveyed by this review, that lower MICs are generally associated with better outcomes. This is a particularly important practical point. For example, it is necessary for the clinician to recognize that when treating a patient with typhoid fever, a common differential for the returning traveler and also endemic in many countries, with a quinolone antibiotic, he or she should be alert for potential deterioration despite the fact that the bacterium is susceptible to the antibiotic or should even consider the use of an alternative antibiotic agent from the outset.
Another important point that has to be taken into account when interpreting MIC data to make clinical decisions, especially when using the EUCAST breakpoints (http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/EUCAST_breakpoints_v_2.0_120101.pdf), is that for a significant number of pathogens, the MIC value pertains to the maximum antibiotic dose (i.e., 18 g of piperacillin-tazobactam for P. aeruginosa). Nevertheless, different doses of an antibiotic have to be considered occasionally for different MICs for the same bacterium (i.e., Streptococcus pneumoniae) (10, 16). A potential therapeutic implication in the future regarding the association of MICs with infection outcomes is that in cases of infection by bacteria with high MICs in the “susceptible” range, physicians should pay attention to parameters such as the antibiotic dose provided (i.e., the maximum dose), the duration of antibiotic infusion (i.e., 3-h extended-duration infusion for β-lactams rather than 1-h infusions), prescribing according to weight, or even consideration of the provision of an alternative antibiotic agent or a combination regimen (9, 13).
Two studies that provided data for outcomes for patients according to the MIC value have been published (3, 7). Both of those studies analyzed various bacteria, including enterobacteriaceae and nonfermentative Gram-negative bacteria, and provided data for the whole cohort. One of those studies reported the outcomes for patients treated with levofloxacin; patients were divided into three groups, those with infections due to bacteria with MICs of ≤0.25 μg/ml, MICs of 0.5 μg/ml, and MICs of 1 or 2 μg/ml (7). No difference in mortality was observed between these groups in the whole cohort, which included patients treated with monotherapy and combination therapy. However, a borderline significantly lower mortality rate was observed for patients infected with strains with MICs of ≤0.5 μg/ml than when the MIC was between 1 and 2 μg/ml (6/167 [3.5%] versus 2/10 [20%]; P = 0.05) in the levofloxacin monotherapy group. In addition, high MIC values were associated with longer hospitalizations after culture results were obtained (approximately 5.7 days). Data for specific bacteria could not be extracted from that study, so the data were not included in this analysis.
The second study reported the outcomes for patients with Gram-negative bacteremia treated with cefepime (3): patients infected with strains with MICs of ≥8 μg/ml had a higher mortality rate than patients infected with strains with MICs of ≤4 μg/ml (17/31 [55%] versus 35/145 [24%]; P < 0.001). Mortality rates were similar between patients infected with strains with MICs of 8 μg/ml and those infected with strains with MICs of ≥16 μg/ml (56% and 53%, respectively); in addition, mortality rates were similar among patients infected with strains with MICs of ≤1, 2, and 4 μg/ml (23%, 28%, and 27%, respectively). Finally, independent predictors of mortality in that study were an MIC of ≥8 μg/ml, the APACHE II score, a creatinine clearance rate of <60 ml/min, and continuous renal replacement therapy. Data regarding patients with infections due to P. aeruginosa could be extracted and were included in this analysis; data regarding other pathogens could not be extracted.
Although increasing resistance or decreased susceptibility to broad-spectrum cephalosporins has been reported for Neisseria spp. and especially Neisseria gonorrhoeae, we could not find any article that provided data for increasing treatment failures with increasing MICs within the susceptible range. However, articles that reported only treatment success or treatment failure for patients with susceptible isolates have been published (2, 12).
This systematic review has some limitations. First, the definition of high- and low-MIC groups was arbitrary. Although more comparisons could be attempted by stratifying patients by more MIC values (e.g., by the MIC breakpoint and then a dilution lower, etc.), the available data did not allow for further meaningful comparisons. Second, most of the studies included in the review were retrospective and were not designed to study our hypothesis (the principle of the relationship between treatment failure and/or mortality and high MICs within the susceptible range). In addition, most of those studies included only a small number of patients, which decreased the power of this analysis.
Third, several studies were performed more than 10 years ago; it can be postulated that the frequency of infections due to pathogens with high MICs was lower and, therefore, that a greater difference between the studied populations was not evident. However, the limited data suggest that our hypothesis may be valid. Fourth, the populations included in the review were rather heterogeneous: community-, health care-, hospital-, and intensive care unit (ICU)-associated infections were studied together. However, the analyses performed included only patients who received β-lactams for a group of Gram-negative bacteria (e.g., enterobacteriaceae and nonfermentative bacilli, etc.) or fluoroquinolones for S. enterica.
Fifth, the association between treatment failures or mortality and the underlying disease or severity of the infection could not be studied. We could not retrieve data regarding comorbidity or disease severity for the majority of the included patients, nor could we perform a sensitivity analysis or metaregression to identify potential confounders. Therefore, a causal relationship cannot be proven. Sixth, the dose of the administered antibiotics or the mode of administration (intermittent, extended, or continuous) was not provided in the majority of the studies. There are some data to show that the dose and the mode of administration may affect patient outcomes, especially for the treatment of resistant bacteria (14, 21). Finally, this analysis included different bacteria and antibiotics in different settings and countries within a period of 15 years. Thus, the results of this meta-analysis may not be representative of all antibiotics for two reasons: first because most of the studied antibiotics were β-lactams (which might mean that this hypothesis is not true for other classes of antibiotics, e.g., aminoglycosides or fluoroquinolones) and second because the current breakpoints for a given antibiotic might be truly high while for other antibiotics, even within the same class, the breakpoints might have been set appropriately.
In conclusion, the limited available data suggest that there is an association between high MIC values within the currently accepted susceptible range and adverse outcomes of infections. Since most of the studies were retrospective, included a small number of patients, and did not provide data for confounding factors, the association of high MICs and adverse outcomes requires confirmation in larger, prospective studies.
ACKNOWLEDGMENT
We have no conflicts of interest and no funding.
Footnotes
Published ahead of print 21 May 2012
REFERENCES
- 1. Anthony KB, et al. 2008. Clinical and microbiological outcomes of serious infections with multidrug-resistant gram-negative organisms treated with tigecycline. Clin. Infect. Dis. 46:567–570 [DOI] [PubMed] [Google Scholar]
- 2. Bercion R, et al. 2008. Acute bacterial meningitis at the ‘Complexe Pediatrique’ of Bangui, Central African Republic. J. Trop. Pediatr. 54:125–128 [DOI] [PubMed] [Google Scholar]
- 3. Bhat SV, et al. 2007. Failure of current cefepime breakpoints to predict clinical outcomes of bacteremia caused by gram-negative organisms. Antimicrob. Agents Chemother. 51:4390–4395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clinical and Laboratory Standards Institute 2011. Performance standards for antimicrobial testing: twenty-first informational supplement. CLSI document M100-S21. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 5. Crump JA, et al. 2008. Clinical response and outcome of infection with Salmonella enterica serotype Typhi with decreased susceptibility to fluoroquinolones: a United States Foodnet multicenter retrospective cohort study. Antimicrob. Agents Chemother. 52:1278–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dagan R, et al. 1997. Bacteriologic response to oral cephalosporins: are established susceptibility breakpoints appropriate in the case of acute otitis media? J. Infect. Dis. 176:1253–1259 [DOI] [PubMed] [Google Scholar]
- 7. Defife R, Scheetz MH, Feinglass JM, Postelnick MJ, Scarsi KK. 2009. Effect of differences in MIC values on clinical outcomes in patients with bloodstream infections caused by gram-negative organisms treated with levofloxacin. Antimicrob. Agents Chemother. 53:1074–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Drusano GL, Lode H, Edwards JR. 2000. Meropenem: clinical response in relation to in vitro susceptibility. Clin. Microbiol. Infect. 6:185–194 [DOI] [PubMed] [Google Scholar]
- 9. Falagas ME, Karageorgopoulos DE. 2010. Adjustment of dosing of antimicrobial agents for bodyweight in adults. Lancet 375:248–251 [DOI] [PubMed] [Google Scholar]
- 10. Falagas ME, Siempos II, Bliziotis IA, Panos GZ. 2006. Impact of initial discordant treatment with beta-lactam antibiotics on clinical outcomes in adults with pneumococcal pneumonia: a systematic review. Mayo Clin. Proc. 81:1567–1574 [DOI] [PubMed] [Google Scholar]
- 11. Goethaert K, et al. 2006. High-dose cefepime as an alternative treatment for infections caused by TEM-24 ESBL-producing Enterobacter aerogenes in severely-ill patients. Clin. Microbiol. Infect. 12:56–62 [DOI] [PubMed] [Google Scholar]
- 12. Lo JY, et al. 2008. Ceftibuten resistance and treatment failure of Neisseria gonorrhoeae infection. Antimicrob. Agents Chemother. 52:3564–3567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lorente L, et al. 2009. Clinical cure of ventilator-associated pneumonia treated with piperacillin/tazobactam administered by continuous or intermittent infusion. Int. J. Antimicrob. Agents 33:464–468 [DOI] [PubMed] [Google Scholar]
- 14. Lorente L, Lorenzo L, Martin MM, Jimenez A, Mora ML. 2006. Meropenem by continuous versus intermittent infusion in ventilator-associated pneumonia due to gram-negative bacilli. Ann. Pharmacother. 40:219–223 [DOI] [PubMed] [Google Scholar]
- 15. Parry CM, et al. 2011. The influence of reduced susceptibility to fluoroquinolones in Salmonella enterica serovar Typhi on the clinical response to ofloxacin therapy. PLoS Negl. Trop. Dis. 5:e1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Paterson DL, et al. 2001. Outcome of cephalosporin treatment for serious infections due to apparently susceptible organisms producing extended-spectrum beta-lactamases: implications for the clinical microbiology laboratory. J. Clin. Microbiol. 39:2206–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qureshi ZA, et al. 2011. Risk factors and outcome of extended-spectrum beta-lactamase-producing Enterobacter cloacae bloodstream infections. Int. J. Antimicrob. Agents 37:26–32 [DOI] [PubMed] [Google Scholar]
- 18. Rodriguez-Bano J, et al. 2008. Community infections caused by extended-spectrum beta-lactamase-producing Escherichia coli. Arch. Intern. Med. 168:1897–1902 [DOI] [PubMed] [Google Scholar]
- 19. Rodriguez-Bano J, Navarro MD, Retamar P, Picon E, Pascual A. 2012. Beta-lactam/beta-lactam inhibitor combinations for the treatment of bacteremia due to extended-spectrum beta-lactamase-producing Escherichia coli: a post hoc analysis of prospective cohorts. Clin. Infect. Dis. 54:167–174 [DOI] [PubMed] [Google Scholar]
- 20. Rodriguez-Bano J, Picon E, Navarro MD, Lopez-Cerero L, Pascual A. 2011. Impact of changes in CLSI and EUCAST breakpoints for susceptibility in bloodstream infections due to extended-spectrum beta-lactamase-producing Escherichia coli. Clin. Microbiol. Infect. [Epub ahead of print.]. doi:10.1111/j.1469-0691.2011.03673.x [DOI] [PubMed] [Google Scholar]
- 21. Taccone FS, Cotton F, Roisin S, Vincent JL, Jacobs F. 2012. Optimal meropenem concentrations to treat multidrug-resistant Pseudomonas aeruginosa septic shock. Antimicrob. Agents Chemother. 56:2129–2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tam VH, et al. 2008. Outcomes of bacteremia due to Pseudomonas aeruginosa with reduced susceptibility to piperacillin-tazobactam: implications on the appropriateness of the resistance breakpoint. Clin. Infect. Dis. 46:862–867 [DOI] [PubMed] [Google Scholar]
- 23. Yamagishi Y, et al. 2012. Investigation of the clinical breakpoints of piperacillin-tazobactam against infections caused by Pseudomonas aeruginosa. J. Infect. Chemother. 18:127–129 [DOI] [PubMed] [Google Scholar]