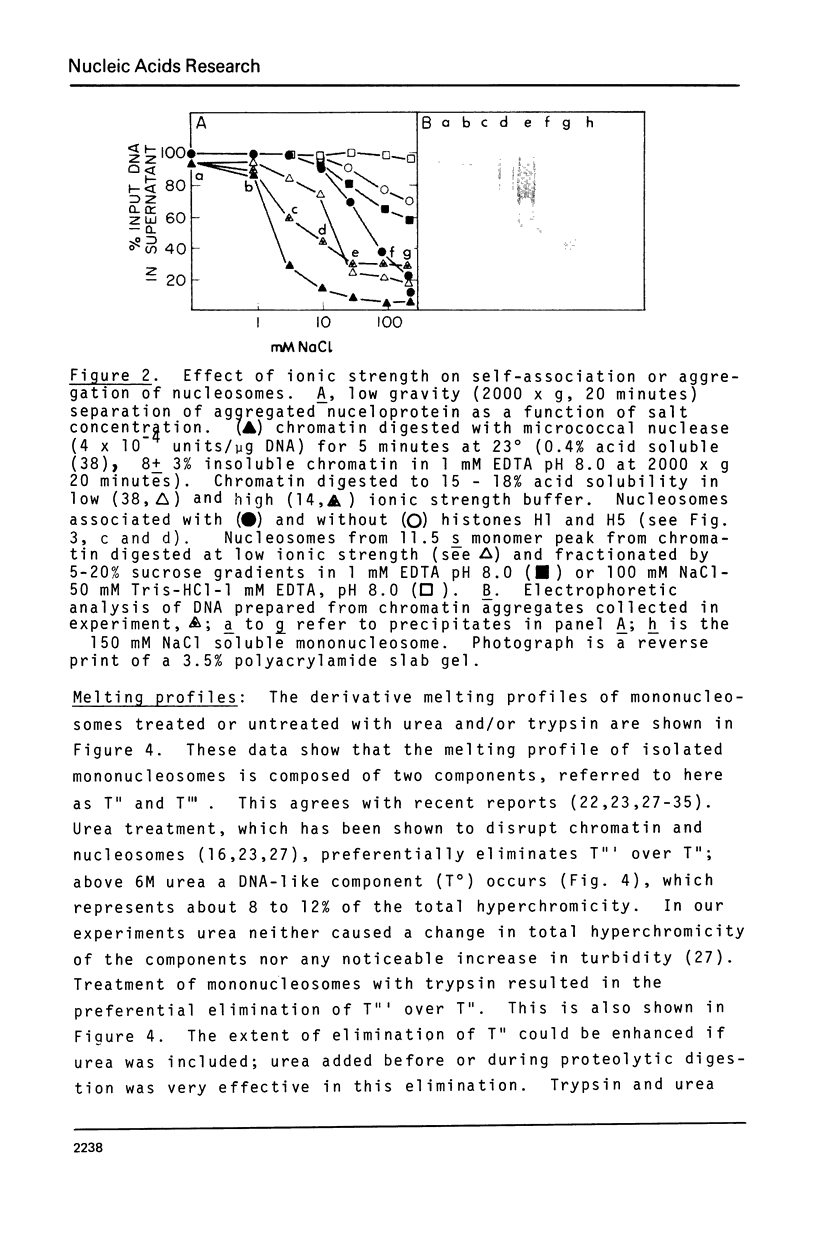
Abstract
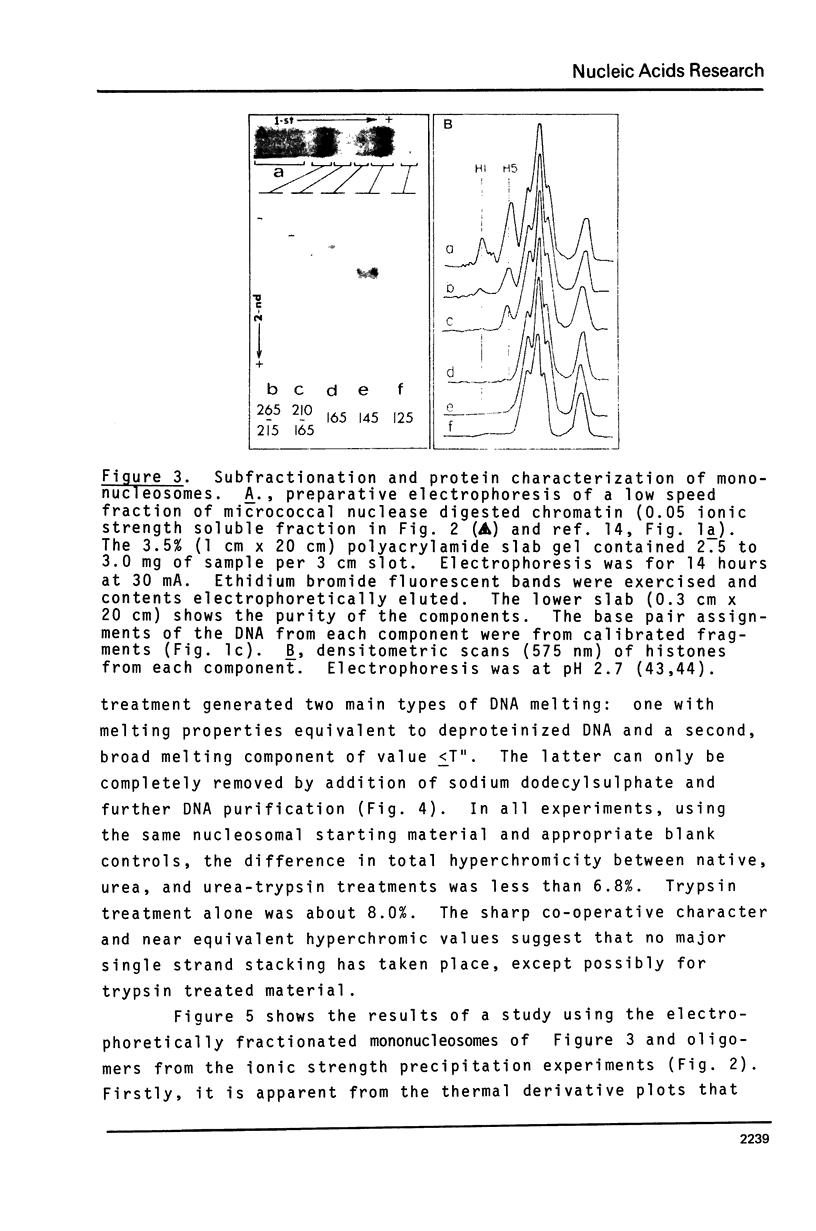
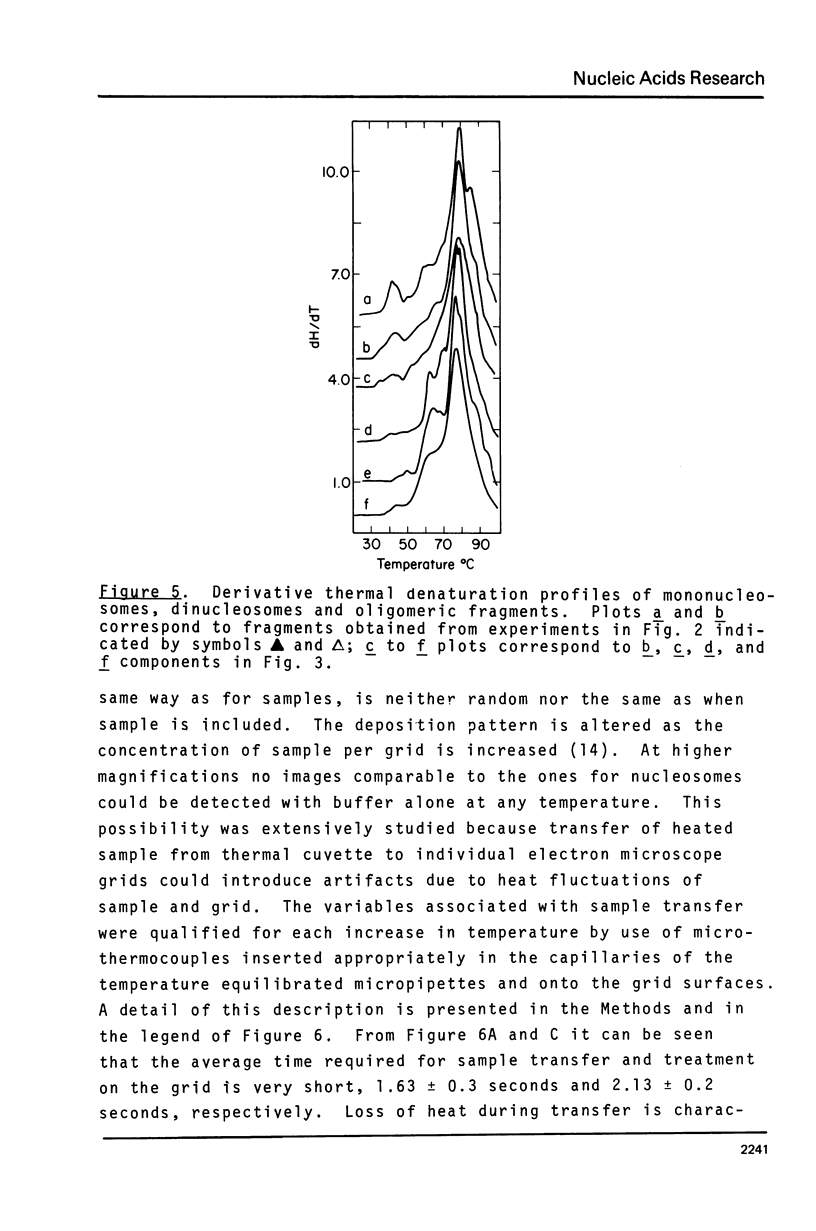
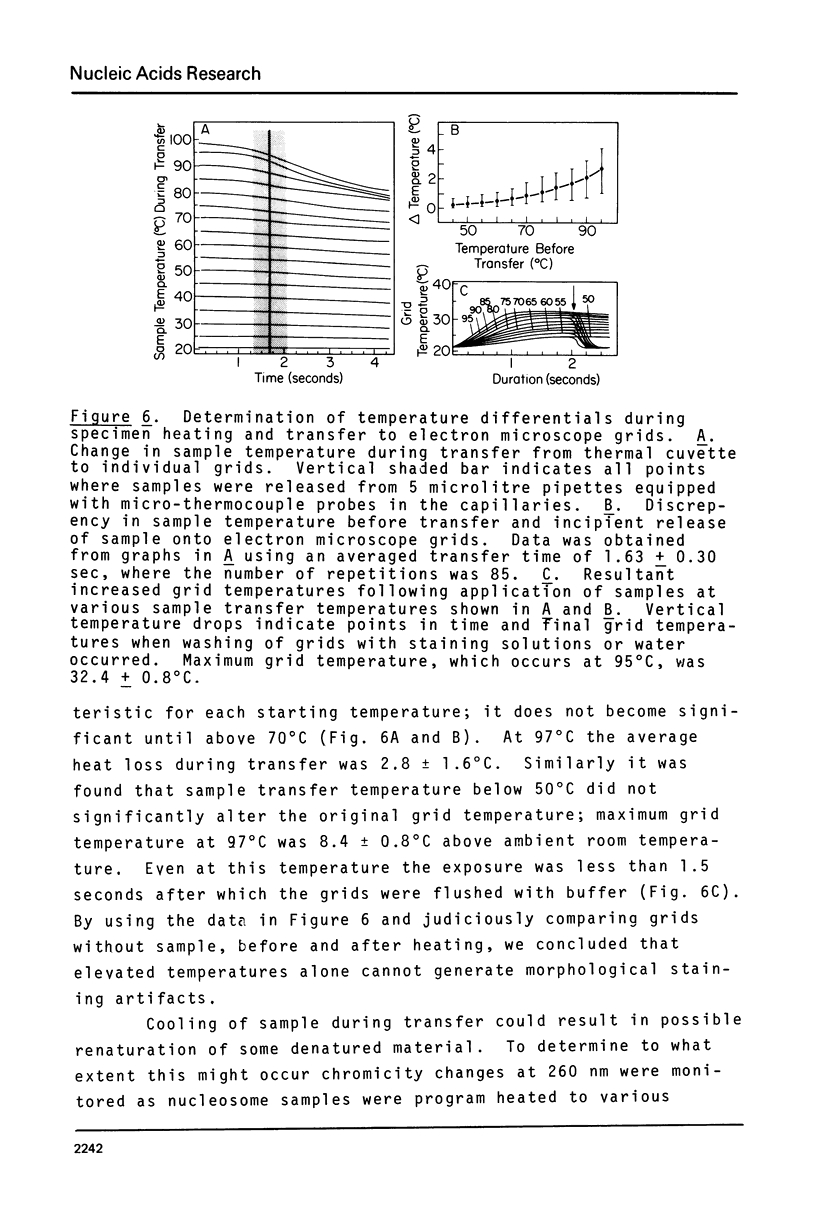
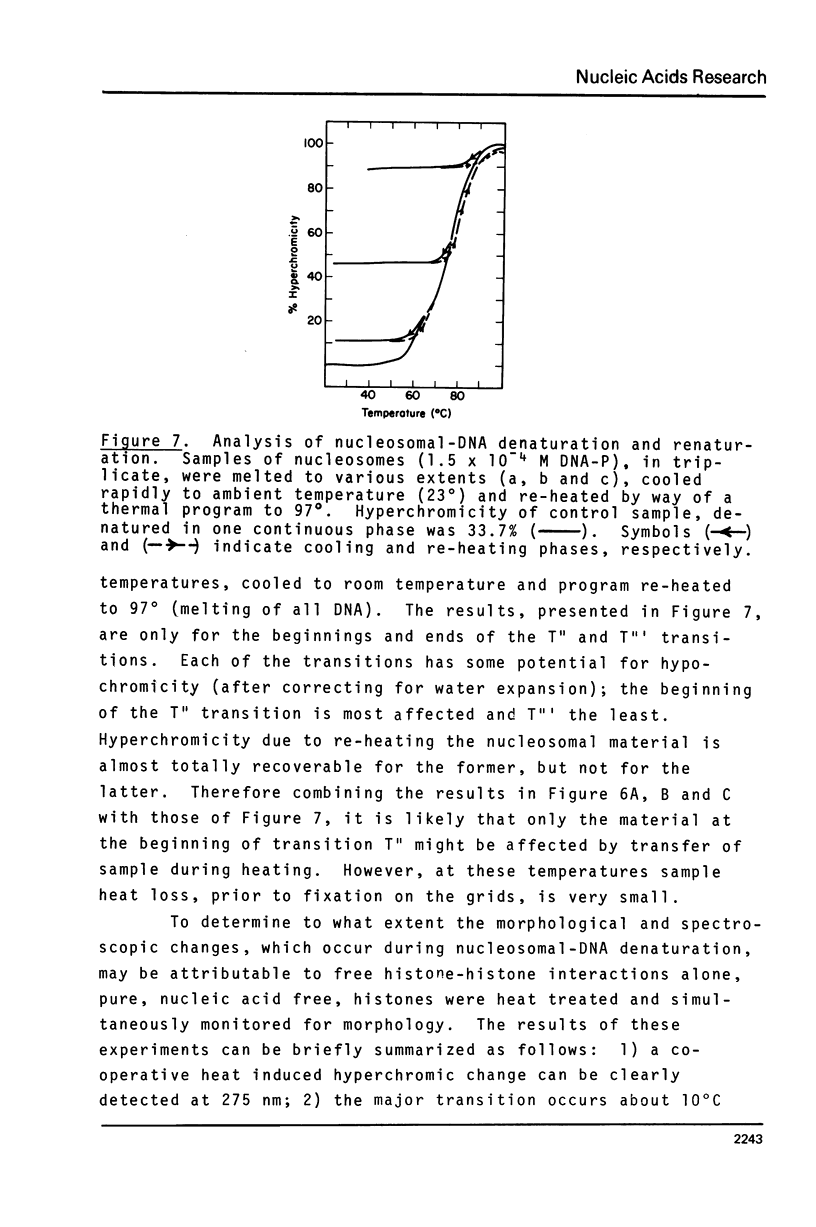
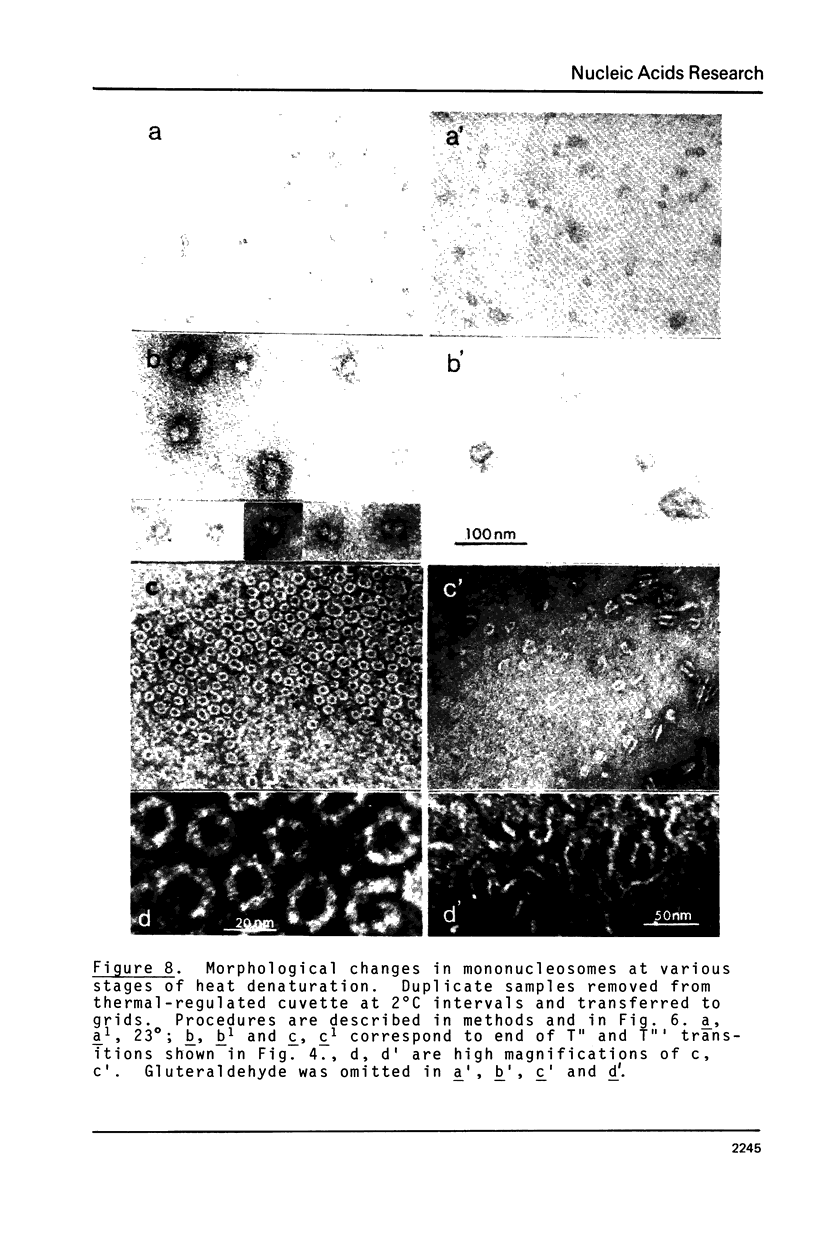
Mononucleosomes prepared from goose erythrocyte nuclei exhibited limited heterogeneity with respect to number of electrophoretic components, histones and DNA composition. The components differ slightly in ionic strength induced self-association. Thermal denaturation of each component gave only two dominant, highly cooperative, melting transitions, T" and T"'. Urea and trypsin were used to establish the differential lability of these two transitions. Comparison of the morphologies of the mononucleosomes at various stages throughout the melting profile indicated that the 13.3 +/- 1.5 nm diameter mononucleosomes start to disrupt only in the latter half of transition T" and do not unfold until after reaching T"'. The resultant, open ended (17.4 +/- 2.2 nm diameter) toroids are still largely negatively staining and much more uniform in shape if fixed simultaneously with gluteraldehyde.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansevin A. T., Hnilica L. S., Spelsberg T. C., Kehm S. L. Structure studies on chromatin and nucleohistones. Thermal denaturation profiles recorded in the presence of urea. Biochemistry. 1971 Dec 7;10(25):4793–4803. doi: 10.1021/bi00801a030. [DOI] [PubMed] [Google Scholar]
- Bakayev V. V., Bakayeva T. G., Varshavsky A. J. Nucleosomes and subnucleosomes: heterogeneity and composition. Cell. 1977 Jul;11(3):619–629. doi: 10.1016/0092-8674(77)90079-4. [DOI] [PubMed] [Google Scholar]
- Bakayev V. V., Melnickov A. A., Osicka V. D., Varshausky A. J. Studies on chromatin. II. Isolation and characterization of chromatin subunits. Nucleic Acids Res. 1975 Aug;2(8):1401–1419. doi: 10.1093/nar/2.8.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasch K., Seligy V. L., Setterfield G. Effects of low salt concentration on structural organization and template activity of chromatin in chicken erythrocyte nuclei. Exp Cell Res. 1971 Mar;65(1):61–72. doi: 10.1016/s0014-4827(71)80050-2. [DOI] [PubMed] [Google Scholar]
- Camerini-Otero R. D., Felsenfeld G. Supercoiling energy and nucleosome formation: the role of the arginine-rich histone kernel. Nucleic Acids Res. 1977;4(5):1159–1181. doi: 10.1093/nar/4.5.1159-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A. M., Cotter R. I. Subunit associations among chromatin particles. Nucleic Acids Res. 1977 Nov;4(11):3877–3886. doi: 10.1093/nar/4.11.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter R. I., Lilley D. M. The conformation of DNA and protein within chromatin subunits. FEBS Lett. 1977 Oct 1;82(1):63–68. doi: 10.1016/0014-5793(77)80886-7. [DOI] [PubMed] [Google Scholar]
- Dahlberg A. E., Dingman C. W., Peacock A. C. Electrophoretic characterization of bacterial polyribosomes in agarose-acrylamide composite gels. J Mol Biol. 1969 Apr 14;41(1):139–147. doi: 10.1016/0022-2836(69)90131-4. [DOI] [PubMed] [Google Scholar]
- Defer N., Kitzis A., Kruh J., Brahms S., Brahms J. Effect of non-histone proteins on thermal transition of chromatin and of DNA. Nucleic Acids Res. 1977 Jul;4(7):2293–2306. doi: 10.1093/nar/4.7.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin. Nature. 1978 Jan 12;271(5641):115–122. doi: 10.1038/271115a0. [DOI] [PubMed] [Google Scholar]
- Finch J. T., Lutter L. C., Rhodes D., Brown R. S., Rushton B., Levitt M., Klug A. Structure of nucleosome core particles of chromatin. Nature. 1977 Sep 1;269(5623):29–36. doi: 10.1038/269029a0. [DOI] [PubMed] [Google Scholar]
- Finch J. T., Noll M., Kornberg R. D. Electron microscopy of defined lengths of chromatin. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3320–3322. doi: 10.1073/pnas.72.9.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett R. A. A physical study of the stability of the native nucleohistone conformation to salt dissociation and heating. Biochemistry. 1971 Jun 8;10(12):2227–2230. doi: 10.1021/bi00788a007. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D. Structure of chromatin. Annu Rev Biochem. 1977;46:931–954. doi: 10.1146/annurev.bi.46.070177.004435. [DOI] [PubMed] [Google Scholar]
- Kovacic R. T., van Holde K. E. Sedimentation of homogeneous double-strand DNA molecules. Biochemistry. 1977 Apr 5;16(7):1490–1498. doi: 10.1021/bi00626a038. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langmore J. P., Wooley J. C. Chromatin architecture: investigation of a subunit of chromatin by dark field electron microscopy. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2691–2695. doi: 10.1073/pnas.72.7.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J. J., Chan D. C., Piette L. H. Conformational state of DNA in chromatin subunits. Circular dichroism, melting, and ethidium bromide binding analysis. Nucleic Acids Res. 1976 Nov;3(11):2879–2893. doi: 10.1093/nar/3.11.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P. N. A thermal denaturation study of chromatin and nuclease-produced chromatin fragments. Can J Biochem. 1977 Jul;55(7):736–746. doi: 10.1139/o77-106. [DOI] [PubMed] [Google Scholar]
- Li H. J., Chang C., Weiskopf M. Helix-coil transition in nucleoprotein-chromatin structure. Biochemistry. 1973 Apr 24;12(9):1763–1772. doi: 10.1021/bi00733a016. [DOI] [PubMed] [Google Scholar]
- Li H. J., Hu A. W., Maciewicz R. A., Cohen P., Santella R. M., Chang C. Structural transition in chromatin induced by ions in solution. Nucleic Acids Res. 1977 Nov;4(11):3839–3854. doi: 10.1093/nar/4.11.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley D. M., Tatchell K. Chromatin core particle unfolding induced by tryptic cleavage of histones. Nucleic Acids Res. 1977 Jun;4(6):2039–2055. doi: 10.1093/nar/4.6.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr D., Corden J., Tatchell K., Kovacic R. T., Van Holde K. E. Comparative subunit structure of HeLa, yeast, and chicken erythrocyte chromatin. Proc Natl Acad Sci U S A. 1977 Jan;74(1):79–83. doi: 10.1073/pnas.74.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurquin P. F., Seligy V. L. Distribution of intercalative dye binding sites in chromatin. Chem Biol Interact. 1976 Apr;13(1):27–45. doi: 10.1016/0009-2797(76)90011-9. [DOI] [PubMed] [Google Scholar]
- Mandel R., Fasman G. D. Chromatin and nucleosome structure. Nucleic Acids Res. 1976 Aug;3(8):1839–1855. doi: 10.1093/nar/3.8.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markov G. G., Ivanov I. G. Hydroxyapatite column chromatography in procedures for isolation of purified DNA. Anal Biochem. 1974 Jun;59(2):555–563. doi: 10.1016/0003-2697(74)90309-1. [DOI] [PubMed] [Google Scholar]
- Miller P., Kendall F., Nicolini C. Thermal denaturation of sheared and unsheared chromatin by absorption and circular dichroism measurements. Nucleic Acids Res. 1976 Aug;3(8):1875–1881. doi: 10.1093/nar/3.8.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olins A. L., Carlson R. D., Olins D. E. Visualization of chromatin substructure: upsilon bodies. J Cell Biol. 1975 Mar;64(3):528–537. doi: 10.1083/jcb.64.3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olins A. L., Carlson R. D., Wright E. B., Olins D. E. Chromatin nu bodies: isolation, subfractionation and physical characterization. Nucleic Acids Res. 1976 Dec;3(12):3271–3291. doi: 10.1093/nar/3.12.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olins A. L., Olins D. E. Spheroid chromatin units (v bodies). Science. 1974 Jan 25;183(4122):330–332. doi: 10.1126/science.183.4122.330. [DOI] [PubMed] [Google Scholar]
- Olins D. E., Bryan P. N., Harrington R. E., Hill W. E., Olins A. L. Conformational states of chromatin nu bodies induced by urea. Nucleic Acids Res. 1977 Jun;4(6):1911–1931. doi: 10.1093/nar/4.6.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olins D. E., Olins A. L. Physical studies of isolated eucaryotic nuclei. J Cell Biol. 1972 Jun;53(3):715–736. doi: 10.1083/jcb.53.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudet P., Gross-Bellard M., Chambon P. Electron microscopic and biochemical evidence that chromatin structure is a repeating unit. Cell. 1975 Apr;4(4):281–300. doi: 10.1016/0092-8674(75)90149-x. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. The heterogeneity of histones. I. A quantitative analysis of calf histones in very long polyacrylamide gels. Biochemistry. 1969 Oct;8(10):3972–3979. doi: 10.1021/bi00838a013. [DOI] [PubMed] [Google Scholar]
- Pardon J. F., Worcester D. L., Wooley J. C., Cotter R. I., Lilley D. M., Richards R. M. The structure of the chromatin core particle in solution. Nucleic Acids Res. 1977 Sep;4(9):3199–3214. doi: 10.1093/nar/4.9.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacow I., Cabasso L., Li H. J. Histone redistribution and conformational effect on chromatin induced by formaldehyde. Biochemistry. 1976 Oct 19;15(21):4559–4565. doi: 10.1021/bi00666a002. [DOI] [PubMed] [Google Scholar]
- Poon N. H., Seligy V. L. Comparative bright field microscopy of isolated nucleosomes, ribosomes and histone aggregates. Exp Cell Res. 1978 Apr;113(1):95–110. doi: 10.1016/0014-4827(78)90091-5. [DOI] [PubMed] [Google Scholar]
- Sahasrabuddhe C. G., Van Holde K. E. The effect of trypsin on nuclease-resistant chromatin fragments. J Biol Chem. 1974 Jan 10;249(1):152–156. [PubMed] [Google Scholar]
- Sanders M. M., Hsu J. T. Fractionation of purified nucleosomes on the basis of aggregation properties. Biochemistry. 1977 Apr 19;16(8):1690–1695. doi: 10.1021/bi00627a026. [DOI] [PubMed] [Google Scholar]
- Seligy V. L., Miyagi M. Comparison of template-property changes after salt extraction of avian erythrocyte and liver chromatin. Eur J Biochem. 1974 Jul 15;46(2):259–269. doi: 10.1111/j.1432-1033.1974.tb03618.x. [DOI] [PubMed] [Google Scholar]
- Seligy V., Miyagi M. Studies of template activity of chromatin isolated from metabolically active and inactive cells. Exp Cell Res. 1969 Nov;58(1):27–34. doi: 10.1016/0014-4827(69)90113-x. [DOI] [PubMed] [Google Scholar]
- Shelton K. R., Neelin J. M. Nuclear residual proteins from goose erythroid cells and liver. Biochemistry. 1971 Jun 8;10(12):2342–2348. doi: 10.1021/bi00788a026. [DOI] [PubMed] [Google Scholar]
- Sollner-Webb B., Felsenfeld G. A comparison of the digestion of nuclei and chromatin by staphylococcal nuclease. Biochemistry. 1975 Jul;14(13):2915–2920. doi: 10.1021/bi00684a019. [DOI] [PubMed] [Google Scholar]
- Staynov D. Z. Thermal denaturation profiles and the structure of chromatin. Nature. 1976 Dec 9;264(5586):522–525. doi: 10.1038/264522a0. [DOI] [PubMed] [Google Scholar]
- Stein A., Bina-Stein M., Simpson R. T. Crosslinked histone octamer as a model of the nucleosome core. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2780–2784. doi: 10.1073/pnas.74.7.2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin R. S., Seligy V. L. Characterization of chromatin-bound erythrocyte histone V (f2c). Synthesis, acetylation, and phosphorylation. J Biol Chem. 1975 Jan 25;250(2):358–364. [PubMed] [Google Scholar]
- Todd R. D., Garrard W. T. Two-dimensional electrophoretic analysis of polynucleosomes. J Biol Chem. 1977 Jul 10;252(13):4729–4738. [PubMed] [Google Scholar]
- Varshavsky A. J., Bakayev V. V., Chumackov P. M., Georgiev G. P. Minichromosome of simian virus 40: presence of histone HI. Nucleic Acids Res. 1976 Aug;3(8):2101–2113. doi: 10.1093/nar/3.8.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengerov Y. Y., Popenko V. I. Changes in chromatin structure induced by EDTA treatment and partial removal of histone H1. Nucleic Acids Res. 1977 Sep;4(9):3017–3027. doi: 10.1093/nar/4.9.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Van Lente F. Dissection of chromosome structure with trypsin and nucleases. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4249–4253. doi: 10.1073/pnas.71.10.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Worcel A., Alberts B. A model for chromatin based upon two symmetrically paired half-nucleosomes. Cell. 1976 Nov;9(3):409–417. doi: 10.1016/0092-8674(76)90085-4. [DOI] [PubMed] [Google Scholar]
- Weischet W. O., Tatchell K., Van Holde K. E., Klump H. Thermal denaturation of nucleosomal core particles. Nucleic Acids Res. 1978 Jan;5(1):139–160. doi: 10.1093/nar/5.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock J. P., Jr, Simpson R. T. Preparation and physical characterization of a homogeneous population of monomeric nucleosomes from HeLa cells. Nucleic Acids Res. 1976 Sep;3(9):2255–2266. doi: 10.1093/nar/3.9.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm F. X., de Murcia G. M., Champagne M. H., Daune M. P. Conformational changes of histones and DNA during the thermal denaturation of nucleoprotein. Eur J Biochem. 1974 Jun 15;45(2):431–443. doi: 10.1111/j.1432-1033.1974.tb03567.x. [DOI] [PubMed] [Google Scholar]
- Woodcock C. L., Frado L. L. Thermal denaturation of subchromosomal particles. Biochem Biophys Res Commun. 1975 Sep 2;66(1):403–410. doi: 10.1016/s0006-291x(75)80342-1. [DOI] [PubMed] [Google Scholar]
- Woodcock C. L. Reconstitution of chromatin subunits. Science. 1977 Mar 25;195(4284):1350–1352. doi: 10.1126/science.841333. [DOI] [PubMed] [Google Scholar]
- Woodcock C. L., Safer J. P., Stanchfield J. E. Structural repeating units in chromatin. I. Evidence for their general occurrence. Exp Cell Res. 1976 Jan;97:101–110. doi: 10.1016/0014-4827(76)90659-5. [DOI] [PubMed] [Google Scholar]
- van Bruggen E. F., Arnberg A. C., van Holde K. E., Sahasrabuddhe C. G., Shaw B. R. Electron microscopy of chromatin subunit particles. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1365–1370. doi: 10.1016/0006-291x(74)90348-9. [DOI] [PubMed] [Google Scholar]