Abstract
An isolate of Klebsiella oxytoca carrying a novel IMP metallo-β-lactamase was discovered in Madrid, Spain. The blaIMP-28 gene is part of a chromosomally located class I integron. The IMP-28 kcat/Km values for ampicillin, ceftazidime, and cefepime and, to a lesser extent, imipenem and meropenem, are clearly lower than those of IMP-1. The His306Gln mutation may induce important modifications of the L3 loop and thus of substrate accessibility and hydrolysis and be the main reason for this behavior.
TEXT
The emergence of metallo-β-lactamases (MBL) in members of the family Enterobacteriaceae is a problem of major concern for clinicians worldwide (11). These enzymes can hydrolyze most β-lactams, including carbapenems, and are not susceptible to conventional β-lactamase inhibitors (2).
The IMP family has at least 33 unique IMP variants (http://www.lahey.org/Studies), which may differ widely in regard to the primary sequence and biochemical activity. However, some allelic variants with the following mutations are associated with decreased overall activity (particularly against penicillins), i.e., Ser62 in IMP-12 (3), Ser196 in IMP-3 (6) and IMP-6 (17), and Gly242 in IMP-18 (1).
Here we describe the genetic context and kinetic parameters of the new MBL IMP-28, which was first described in a Klebsiella oxytoca isolate from Spain, and in addition, we consider the possible cause of its poor overall activity.
K. oxytoca HGUGM21530 was isolated from a lip wound patient seropositive for human immunodeficiency virus diagnosed with progressive multifocal leukoencephalopathy in the Gregorio Marañon Hospital (Madrid, Spain) in 2009.
Pulsed-field gel electrophoresis (PFGE) with S1 nuclease digestion of whole-genome DNA (S1-PFGE) and PCR-based replicon typing (PBRT) were used to characterize plasmids as described previously (4). The S1-PFGE-I gel was transferred and hybridized with IMP and Inc A/C probes (the only amplicon obtained by PBRT). The results showed one band of 340 kb that hybridized only with the A/C probe. PFGE with I-CeuI digestion of whole-genome DNA, as described by Liu et al. (9), was used to determine whether the blaIMP-28 gene was located in the chromosome. The PFGE-I-CeuI gel was transferred and hybridized with 16S rRNA and IMP probes. The results showed one band that hybridized with both the 16S rRNA and IMP probes. These data suggest that the blaIMP-28 gene is located in the chromosome (data not shown).
The genetic context of the blaIMP-28 gene was elucidated by PCR and sequencing. The blaIMP-28 gene is located in a class I integron, designated In767 (http://integrall.bio.ua.pt/), that displays more structural differences from (1, 5, 7, 14, 15, 17) than similarities to (12, 18) the other integrons published for IMP-encoding genes in the last decade. The structure consists of two aminoglycoside resistance genes, aacA44 and aadA13, just downstream of blaIMP-28. The aacA44 gene codes for a newly described aminoglycoside-(6′)-acetyltransferase variant showing 86% sequence identity with AacA4.
The blaIMP-1 and blaIMP-28 genes were cloned into plasmid pBGS18-pCTX under the control of the promoter of the gene for the CTX-M-14 β-lactamase and then transformed into Escherichia coli strain TG1. The blaIMP-28 gene was obtained from K. oxytoca HGUGM21530 by PCR and cloned into plasmid pBGS18 harboring the bla CTXM-14 promoter, which was previously used in similar studies of β-lactamase expression (10). The primers used for cloning were 5′AAAAGGTACCATGAGCAAGTTATTTGTATTCTTTATG (forward) and 5′ AAAAGAATTCTTAGTTACTTGGTTTTGATGGTTTTTTA (reverse). The blaIMP-1 gene was obtained by PCR from plasmid pET-28a(+) with blaIMP-1 as an insert (obtained from Y. Ishii [Toho University School of Medicine, Tokyo, Japan]) and cloned into the same plasmid as the blaIMP-28 gene. The primers used for cloning in this case were 5′AAAGATCCATGAGCAAGTTATCTGTA (forward) and 5′AAAGAATTCTTAGTTGCTTGGTTTTGA(reverse). Microbiological analysis showed show a 4-fold minimum decrease in the MICs of all of the antibiotics, except cefotaxime and aztreonam, for bacteria expressing IMP-28 relative to those for bacteria expressing IMP-1 (Table 1). These data suggest that the IMP-28 enzyme displays lower activity than IMP-1. In order to confirm this point, we purified both enzymes and obtained the corresponding kinetic data.
Table 1.
MICs of ampicillin, oxyiminocephalosporins, carbapenems, and other antibiotics for E. coli TG1 and the bacterial clinical isolate expressing the IMP-1 and IMP-28 β-lactamases
| Antibiotic | MIC (μg/ml)a for: |
|||
|---|---|---|---|---|
| K. oxytoca HGUGM21530 (IMP-28) | E. coli TG1 PBGS18-pCTX IMP-28 | E. coli TG1 PBGS18-pCTX IMP-1 | E. coli TG1 PBGS18-pCTX | |
| Ampicillin | 32 | 64 | 512 | 2 |
| Cefoxitin | 256 | 256 | 1,028 | 1 |
| Cefotaxime | 64 | 256 | 256 | 0.06 |
| Ceftazidime | 64 | 128 | 512 | 0.06 |
| Cefepime | 2 | 32 | 128 | <0.12 |
| Aztreonam | <0.25 | <0.25 | <0.25 | <0.25 |
| Imipenem | 1 | 0.5 | 2 | 0.12 |
| Meropenem | 1 | 4 | 16 | <0.03 |
The results were confirmed in three independent experiments.
To purify IMP-28, the blaIMP-1 gene was cloned into the pGEX-6P-1 expression vector with primers 5′AAAAGAATTCAGCGGGGAGGCCCCGC (forward) and 5′AAAAGTCGACTCACTCGGCCAACTGACTCAG (reverse). The construct was transformed into E. coli M15 and produced a fusion protein consisting of glutathione S-transferase (GST) and the IMP-28 enzyme without its signal peptide. The β-lactamase was purified to homogeneity according to the manufacturer′s instructions for the GST gene fusion system (Amersham Pharmacia Biotech, Europe, GmbH). After the cleavage of GST from IMP-28, the purified (≥99% pure) protein appeared as a single band on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (data not shown).
Hydrolysis of the antibiotics by the IMP-28 β-lactamase was monitored by recording the variation in absorbance resulting from the opening of the β-lactam ring. The kinetic parameters of nitrocefin were determined from the initial rates by Hanes-Woolf linearization of the Henri-Michaelis-Menten equation. For the other antibiotics, the Km value was measured as the Ki in a competition experiment with nitrocefin as the reporter substrate (16). The kcat values were then obtained by monitoring the hydrolysis of the antibiotic at a concentration >10 times the Km.
The results revealed a systematic decrease in the kcat values of IMP-28 relative to those of IMP-1. This decrease was not very significant with nitrocefin and cefotaxime and was largest for ampicillin (>1,000), for which IMP-1 displays the highest kcat value. Comparison of the Km values of IMP-1 and IMP-28 did not reveal any significant modifications; the greatest difference was a 6-fold increase in the Km of IMP-28 for cefepime. The catalytic efficiency of IMP-28 was relatively poor against the other antibiotics tested, especially ampicillin, ceftazidime, and cefepime, and much lower than that of IMP-1 against these antibiotics (Table 2). Although the reduced Km contributed to some extent, the most important factor in this behavior was the significantly lower kcat. The overall kinetic data were consistent with a general decrease in the MICs when the enzymes were expressed in E. coli TG-1. Therefore, the data confirmed that IMP-28 has a lower hydrolytic capacity than IMP-1. To rule out a loss of activity linked with this lower activity, stability studies by thermal denaturation were performed. The overall data showed the two enzymes to be similarly stable (data not shown).
Table 2.
Kinetic data for the pure IMP-28 and IMP-1 β-lactamasesa
| Antibiotic |
kcat (s−1) |
Km (μM) |
kcat/Km (μM−1 s−1) |
|||
|---|---|---|---|---|---|---|
| IMP-1 | IMP-28 | IMP-1 | IMP-28 | IMP-1 | IMP-28 | |
| Nitrocefin | 63 ± 10 | 35.86 ± 12 | 27 ± 3 | 17.6 ± 4 | 2.3 | 2.03 |
| Ampicillin | 950 ± 50 | 0.649 ± 0.14 | 200 ± 25 | 359 ± 172 | 4.8 | 1.8 10−3 |
| Cefoxitin | 16 ± 1 | 0.75 ± 0.07 | 8 ± 1 | 7 ± 0.3 | 2 | 0.1 |
| Cefotaxime | 1.3 ± 5 | 0.98 ± 0.042 | 4 ± 0.5 | 9.4 ± 0.5 | 0.35 | 0.104 |
| Ceftazidime | 8 ± 1 | 0.35 ± 0.046 | 44 ± 3 | 112 ± 10 | 0.18 | 3 10−3 |
| Cefepime | 7 ± 0.5 | 0.145 ± 0.01 | 11 ± 1 | 72 ± 9 | 0.66 | 2 10−3 |
| Imipenem | 46 ± 3 | 8.66 ± 0.17 | 39 ± 4 | 90 ± 21 | 1.2 | 0.096 |
| Meropenem | 50 ± 5 | 3.05 ± 0.1 | 10 ± 2 | 14.4 ± 0.4 | 5 | 0.21 |
Data are means ± standard deviations (where applicable) and are from Laraki et al. (8).
It is difficult to assign any mutation to this general low activity since IMP-28 differs in 6 amino acids from IMP-5, its most similar counterpart, and in 15 residues from IMP-1 (Fig. 1). An alignment of the amino acid sequences of representative IMP β-lactamases, including those with lower activity toward beta-lactams, revealed that there are no modifications in the catalytic residues of IMP-28. Moreover, IMP-28 does not contain the mutations that some groups have associated with decreased overall activity (particularly against penicillins). We also observed three unique replacements in IMP-28, namely, Arg47Lys, Gly174Ser, and His306Gln, none of which was repeated in any other enzyme of this group.
Fig 1.
Alignment of the amino acid sequences of some representative members of the IMP β-lactamase family (IMP-1, GenBank accession number, S71932; IMP-3, AB010417; IMP-5, 290912; IMP-6, AB040994; IMP-12, AJ420864; IMP-18, AY780674; IMP28, JQ407409; IMP-29, HQ438058). Asterisks indicate strictly conserved amino acids. The catalytic residues are shown in blue. Amino acids of IMP-28 differing from those of IMP-1 are shown in red. Residues of IMP enzymes known to affect substrate hydrolysis (relative to IMP-1) are highlighted in yellow. Alignment was performed with the CLUSTAL W program of EMBL-EBL.
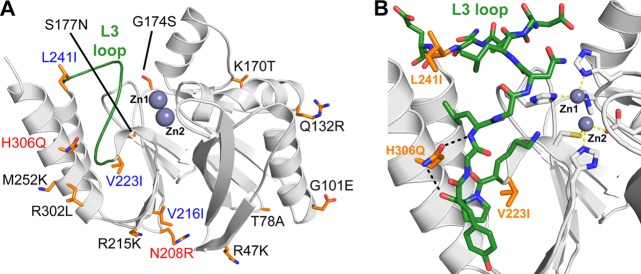
The impact of the 15 amino acids modified in IMP-28 relative to IMP-1 was therefore assessed by examining the various IMP-1 structures available. Among the 15 mutations differentiating IMP-28 from IMP-1, the 10 involving surface residues and the homologous Val216Ile mutation are not expected to affect substrate hydrolysis (Fig. 2A). The Asn208Arg mutation, which we identified as potentially being involved in protein dynamics, is also unlikely to play a major role because it would probably have affected the hydrolysis of the various substrates more uniformly. The remaining 3 amino acid differences between IMP-28 and IMP-1 are related to the L3 loop, which defines one side of the MBL active site and is known to be important for efficient hydrolysis (13). The Val223Ile and Leu241Ile differences found at both ends of this loop are not specific to the IMP-28–IMP-1 pair, unlike the His306Gln mutation.
Fig 2.
(A) Molecular representation of the structure of IMP-1 (Protein Data Bank code 1DDK). The 15 residues that are different in IMP-28 are shown as orange sticks, surface residues are black, internal hydrophobic residues are blue, and polar internal residues are red. (B) Enlarged view of the L3 loop of IMP-1 (green).
The His306Gln mutation is of the greatest interest. In IMP-1, the glutamine side chain is involved in two hydrogen bonds with residues of the L3 loop, which defines one side of the active-site cleft (Fig. 2B). Therefore, replacement of this amino acid with a shorter bulkier histidine may induce significant modifications of the L3 loop and thus of substrate accessibility and hydrolysis. In summary, a new IMP variant has been characterized that shows low overall activity, probably due to a His306Gln modification. Laboratory studies are under way to clarify this point.
Nucleotide sequence accession numbers.
The nucleotide sequence of the class 1 integron harboring blaIMP-28 in K. oxytoca strain HGUGM21530 has been deposited in the GenBank database under accession number JQ407409.
ACKNOWLEDGMENTS
This work was financially supported by REIPI, Spanish Network for Research in Infectious Diseases (Instituto de Salud Carlos III, RD06/0008), Fondo de Investigaciones Sanitarias (PI081368, PS09/00687, PS09/0125), and grants from Xunta de Galicia (07CSA050916PR) and EU FP-7-HEALTH-2011 (278232) Magic Bullet to G.B. F.K. is a research associate of the Fonds de la Recherche Scientifique (F.R.S.-FNRS, Brussels, Belgium). A.B. is supported by the Ministry of Economy and Competitiveness, under program Juan de la Cierva.
Footnotes
Published ahead of print 5 June 2012
REFERENCES
- 1. Borgianni L, et al. 2011. Genetic context and biochemical characterization of the IMP-18 metallo-β-lactamase identified in a Pseudomonas aeruginosa isolate from the United States. Antimicrob. Agents Chemother. 55:140–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bush K. 2001. New β-lactamases in gram-negative bacteria: diversity and impact on the selection of antimicrobial therapy. Clin. Infect. Dis. 32:1085–1089 [DOI] [PubMed] [Google Scholar]
- 3. Docquier JD, et al. 2003. IMP-12, a new plasmid-encoded metallo-β-lactamase from a Pseudomonas putida clinical isolate. Antimicrob. Agents Chemother. 47:1522–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. García A, et al. 2007. Acquisition and diffusion of bla CTX-M-9 gene by R478-IncHI2 derivative plasmids. FEMS Microbiol. Lett. 271:71–77 [DOI] [PubMed] [Google Scholar]
- 5. Garza-Ramos U, et al. 2008. Metallo-β-lactamase gene blaIMP-15 in a class 1 integron, In95, from Pseudomonas aeruginosa clinical isolates from a hospital in Mexico. Antimicrob. Agents Chemother. 52:2943–2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iyobe S, et al. 2000. Amino acid substitutions in a variant of IMP-1 metallo-β-lactamase. Antimicrob. Agents Chemother. 44:2023–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Laraki N, et al. 1999. Structure of In31, a blaIMP-containing Pseudomonas aeruginosa integron phyletically related to In5, which carries an unusual array of gene cassettes. Antimicrob. Agents Chemother. 43:890–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laraki N, et al. 1999. Biochemical characterization of the Pseudomonas aeruginosa 101/1477 metallo-β-lactamase IMP-1 produced by Escherichia coli. Antimicrob. Agents Chemother. 43:902–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu SL, Hessel A, Sanderson KE. 1993. Genomic mapping with I-Ceu I, an intron-encoded endonuclease specific for genes for ribosomal RNA, in Salmonella spp., Escherichia coli, and other bacteria. Proc. Natl. Acad. Sci. U. S. A. 90:6874–6878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mallo S, et al. 2010. A tripeptide deletion in the R2 loop of the class C β-lactamase enzyme FOX-4 impairs cefoxitin hydrolysis and slightly increases susceptibility to β-lactamase inhibitors. J. Antimicrob. Chemother. 65:1187–1194 [DOI] [PubMed] [Google Scholar]
- 11. Maltezou HC. 2009. Metallo-β-lactamases in Gram-negative bacteria: introducing the era of pan-resistance? Int. J. Antimicrob. Agents 33:405.e1-7. [DOI] [PubMed] [Google Scholar]
- 12. Mendes RE, et al. 2007. Characterization of an integron carrying blaIMP-1 and a new aminoglycoside resistance gene, aac(6′)-31, and its dissemination among genetically unrelated clinical isolates in a Brazilian hospital. Antimicrob. Agents Chemother. 51:2611–2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Merino M, et al. 2010. Roles of changes in the L3 loop of the active site in the evolution of enzymatic activity of VIM-type metallo-β-lactamases. J. Antimicrob. Chemother. 65:1950–1954 [DOI] [PubMed] [Google Scholar]
- 14. Neuwirth C, Siebor E, Robin F, Bonnet R. 2007. First occurrence of an IMP metallo-β-lactamase in Aeromonas caviae: IMP-19 in an isolate from France. Antimicrob. Agents Chemother. 51:4486–4488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pellegrini C, et al. 2009. Identification of blaIMP-22 in Pseudomonas spp. in urban wastewater and nosocomial environments: biochemical characterization of a new IMP metallo-enzyme variant and its genetic location. J. Antimicrob. Chemother. 63:901–908 [DOI] [PubMed] [Google Scholar]
- 16. Pérez-Llarena FJ, et al. 2008. Structure-function studies of arginine at position 276 in CTX-M β-lactamases. J. Antimicrob. Chemother. 61:792–797 [DOI] [PubMed] [Google Scholar]
- 17. Yano H, et al. 2001. Plasmid-encoded metallo-β-lactamase (IMP-6) conferring resistance to carbapenems, especially meropenem. Antimicrob. Agents Chemother. 45:1343–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao WH, Chen G, Ito R, Hu ZQ. 2009. Relevance of resistance levels to carbapenems and integron-borne blaIMP-1, blaIMP-7, blaIMP10 and blaVIM-2 in clinical isolates of Pseudomonas aeruginosa. J. Med. Microbiol. 58:1080–1085 [DOI] [PubMed] [Google Scholar]