Abstract
Respiratory virus infections, including infections with rhinoviruses (RVs), are related to exacerbations of chronic obstructive pulmonary disease (COPD). A new quinolone antibiotic, levofloxacin (LVFX), has been used to treat bacterial infections that cause COPD exacerbations as well as bacterial infections that are secondary to viral infection in COPD patients. However, the inhibitory effects of LVFX on RV infection and RV infection-induced airway inflammation have not been studied. We examined the effects of LVFX on type 14 rhinovirus (RV14) (a major human RV) infection of human tracheal epithelial cells pretreated with LVFX. LVFX pretreatment reduced the RV14 titer, the level of cytokines in the supernatant, the amount of RV14 RNA in the cells after RV14 infection, and the cells' susceptibility to RV14 infection. LVFX pretreatment decreased the mRNA level of intercellular adhesion molecule 1 (ICAM-1), a receptor for RV14, in the cells and the concentration of the soluble form of ICAM-1 in the supernatant before RV14 infection. LVFX pretreatment also decreased the number and the fluorescence intensity of the acidic endosomes from which RV14 RNA enters the cytoplasm. LVFX pretreatment inhibited the activation of nuclear factor κB proteins, including p50 and p65, in nuclear extracts. LVFX pretreatment did not reduce the titers of RV2 (a minor human RV) but reduced the titers of RV15 (a major human RV). These results suggest that LVFX inhibits major-group rhinovirus infections in part by reducing ICAM-1 expression levels and the number of acidic endosomes. LVFX may also modulate airway inflammation in rhinoviral infections.
INTRODUCTION
Rhinoviruses (RVs) are the main cause of the common cold, and they are responsible for the most common acute infectious illness in humans (41). In addition, RVs are associated with exacerbations of inflammatory chronic pulmonary diseases, such as chronic obstructive pulmonary disease (COPD) (30). New quinolone antibiotics, such as levofloxacin (LVFX), have clinical benefits in the treatment of COPD exacerbations, including a longer infection-free period and a reduction in the number of hospitalizations after treatment compared with treatment with other antibiotics (7, 28, 34, 42). Several reasons have been suggested for the clinical effects of quinolone antibiotics, including a high serum concentration of the drug that far exceeds the MIC (10), a broader antibiotic spectrum (4), and anti-inflammatory properties (40). However, the inhibitory effects of new quinolone antibiotics on RV infections and on RV infection-induced airway inflammation have not been studied.
Several mechanisms for the RV-induced exacerbation of COPD have been proposed, including virus-induced mucus hypersecretion, airway inflammation (30), and smooth muscle contraction. RV infection induces the production of cytokines and monokines, including interleukin-1 (IL-1), IL-6, and IL-8 (33, 48). These cytokines and monokines have proinflammatory effects (1), and they may also be involved in the pathogenesis of RV infections and RV infection-induced exacerbations of COPD. LVFX pretreatment reduces lipopolysaccharide (LPS)-induced IL-1β production in a murine macrophage-like cell line (AW264.7 cells) (14) and reduces levels of IL-6 and IL-8 production in a human lung epithelial cell line (40). However, the inhibitory effects of new quinolone antibiotics on RV infection-induced airway inflammation have not been well studied.
Type 14 rhinovirus (RV14) (a major human RV) enters the cytoplasm of infected cells after binding to the receptor known as intercellular adhesion molecule 1 (ICAM-1) (6, 11). The entry of the RNA from this group of RVs into the cytoplasm of infected cells has been suggested to be mediated by a destabilization of the cell membrane due to ICAM-1 binding. Furthermore, the entry of the RNA into the cytoplasm is mediated by endosomal acidification when the virions enter the cell via endosomes before they enter the cytoplasm (6). Glucocorticoids (37), the macrolide antibiotics bafilomycin (25, 35) and erythromycin (36), the proton pump inhibitor lansoprazole (29), and the β2 agonist procaterol (43) inhibit RV infection by reducing the ICAM-1 expression level or increasing the endosomal pH. One of the new quinolone antibiotics, ciprofloxacin, inhibits the expression of ICAM-1 by monocytes (19). However, the inhibitory effects of new quinolone antibiotics on RV infection of human airway epithelial cells are still unclear.
We studied the effects of LVFX on RV infection of primary cultures of human airway epithelial cells. We also examined the effects of LVFX on ICAM-1 production and on the endosomal pH to clarify the mechanisms responsible for the inhibition of RV infection.
MATERIALS AND METHODS
Human tracheal epithelial cell culture.
Human tracheal surface epithelial cells were isolated and cultured as described previously (43). The cells were plated at 5 × 105 viable cells/ml in plastic tubes with round bottoms (16 mm in diameter and 125 mm in length; Becton Dickinson, Franklin Lakes, NJ) that were coated with human placental collagen. The plastic tubes were fixed in an inclined stainless-steel tube rack (30 cm wide, 10 cm high, and 10 cm deep) (Te-Her Tube Rack Inclinable RF-6; Hirasawa Works Co. Ltd., Tokyo, Japan), which was placed in a humid incubator. The tubes were kept stationary, and the cells were immersed in 1 ml of Dulbecco's modified Eagle's medium (DMEM)–Ham's F-12 medium (50%/50%, vol/vol) containing 2% Ultroser G (USG; Pall BioSepra, Cergy-Saint-Christophe, France). The cells were then cultured at 37°C in 5% CO2–95% air in an incubator.
To enhance RV14 release from the cells, the tubes containing the cultured cells were subjected to rolling (41, 43). As described below, the tubes were rolled during epithelial cell culture (43) to study the effects of LVFX on the release of RV14 and cytokines. We also assessed RV14 RNA replication after RV14 infection and cellular susceptibility to RV14 infection. In contrast, acidic endosomes could be observed in the cells living on coverslips in stationary petri dishes. Therefore, the cells used for the measurement of acidic endosomes were cultured under stationary conditions. Furthermore, to study the effects of LVFX on nuclear factor κB (NF-κB) activation prior to RV14 infection, cells were cultured in tubes under stationary conditions (43).
To examine the effects of LVFX on the adhesion and growth of human tracheal epithelial cells, the cells were placed into tubes and cultured in medium containing 2% USG supplemented with LVFX (3 μg/ml) or vehicle (water). The concentration of LVFX was chosen according to the reasons described below (see “Treatment with LVFX”). Cells were counted 24 h after plating and when the cells made confluent sheets 5 to 7 days after plating. We measured the time required for the cells to generate confluent sheets. We also measured the concentration of lactate dehydrogenase (LDH) in the medium 3 days after the treatment of confluent cell sheets with LVFX (3 μg/ml).
The tracheas from which cells were derived for culturing were obtained after death from 33 patients (mean age, 71 ± 3 years; 13 females and 20 males). None of the patients had been diagnosed with bronchial asthma, whereas three of the patients had COPD. None of the patients were being treated with LVFX at the time of death. Of the 33 patients, 13 were ex-smokers and 20 had never smoked. This study was approved by the Tohoku University Ethics Committee.
Culture of human embryonic fibroblast cells.
Human embryonic fibroblast cells (HFL-III cells) (RCB0523; Riken Bio Resource Center Cell Bank, Tsukuba, Japan) were cultured in flasks (25-cm2 surface area; Becton Dickinson) and then plated onto plastic dishes (96 wells; Becton Dickinson) or into plastic tubes with round bottoms. The cells were then cultured at 37°C in 5% CO2–95% air (43).
Viral stocks.
Stocks of RV14 (a major-group RV) and RV2 (a minor-group RV) (6, 11, 12) were prepared from a patient with a common cold by infecting human embryonic fibroblast cells as previously described (22, 34). Stocks of RV15 (a major-group RV) (39) were also prepared as previously described (18). We used RV stocks that had been passaged 3 to 5 times.
Detection and titration of viruses.
RVs (RV2, RV14, or RV15) in the supernatant (cell culture medium) were detected and titrated by endpoint methods (8), by infecting replicate confluent human embryonic fibroblast cells in plastic 96-well plates (Becton Dickinson) with serial 10-fold dilutions of virus-containing supernatants, as previously described (43). The presence of the typical cytopathic effects of RVs in all replicate cell cultures was monitored for 7 days (168 h) (43). On the basis of these data, the TCID50 (50% tissue culture infective dose) was calculated as previously described (22). The rates of RV release into the supernatant are expressed as TCID50 units/ml/24 h (43).
Quantification of rhinoviral RNA.
To quantify RV14 RNA and rRNA (18S rRNA) expression in human tracheal epithelial cells after RV14 infection, a two-step real-time quantitative reverse transcription (RT)-PCR using the TaqMan technique (Roche Molecular Diagnostic Systems) was performed by using TaqMan Gene Expression Master Mix (Applied Biosystems, Bedford, CA) and methods described by previously by Nolan et al. (21), as previously reported (43).
In the first step, cDNA was reverse transcribed from RV14 RNA by using the QuantiTect reverse transcription kit (Qiagen) and RV reverse primer 5′-CGGACACCCAAAGTAGTCGGT-3′.
In the second step, real-time PCR was performed by using this cDNA and TaqMan Gene Expression Master Mix. The cDNA sample (2 μl) was mixed with TaqMan Gene Expression Master Mix (10 μl), a forward primer (5′-GCACTTCTGTTTCCCAGGAGC-3′) (0.5 μl), a reverse primer (5′-CGGACACCCAAAGTAGTCGGT-3′) (0.5 μl), the TaqMan RV14 probe (5′–6-carboxyfluorescein [FAM]–CCTTTAACCGTTATCCGCCA–6-carboxytetramethylrhodamine [TAMRA]–3′) (0.5 μl), and RNase-free water (6.5 μl).
To quantify the rRNA, the conversion of rRNA to cDNA and real-time PCR were performed by using the same two-step process described above. To obtain quantitative data, the minimum number of PCR cycles required to detect the fluorescent signal was defined as the cycle threshold (CT) of RV14 RNA and rRNA from the cells, and quantitative data were obtained as described previously (43).
Viral infection of epithelial cells.
The infection of human tracheal epithelial cells with RVs (RV2, RV14, or RV15) or vehicle (minimum essential medium [MEM] plus 2% ultralow-γ-globulin calf serum) was performed by using previously described methods (34, 43). A stock solution of RVs (100 μl in each tube [1.0 × 104 TCID50 units/100 μl, or 5.0 × 10−2 TCID50 units/cell]) was added to the human tracheal epithelial cells in the tubes (34, 43), except where other virus doses are indicated. After a 1-h incubation at 33°C, RV14 stocks were aspirated, and the cells were rinsed with phosphate-buffered saline (PBS) and then fed with fresh medium and cultured at 33°C with rolling.
Treatment with LVFX.
The mean peak concentration of LVFX in the alveolar epithelial lining fluid was reported previously to be 3.4 μg/ml at 1 h after the oral ingestion of 500 mg LVFX (47). Therefore, to examine the effects of LVFX, cultured human tracheal epithelial cells were treated with either LVFX (3 μg/ml [8.1 × 10−6 M]; supplied by Daiichi-Sankyo Co. Ltd., Tokyo, Japan) or vehicle (0.1% double-distilled water). The cells were treated with LVFX or vehicle beginning 3 days (72 h) before infection with RVs until the end of the experimental period after RV infection (except when other concentrations or times are listed), as described previously in reports using other agents (36, 43).
To examine the effects of LVFX on RV14 infection when the cells were treated with LVFX just after RV14 infection, the cells were treated with vehicle (water) before infection with RV14. After a 1-h exposure to RV14, the cells were rinsed with PBS and then fed with fresh medium supplemented with LVFX (3 μg/ml [8.1 × 10−6 M]) and cultured at 33°C with rolling.
Likewise, to examine the time-dependent effects of LVFX on RV14 titers, the cells were pretreated with LVFX (3 μg/ml) for time periods ranging from 0 to 3 days (72 h) before RV14 infection.
To examine the concentration-dependent effects of LVFX on RV14 infection, cells were treated with LVFX at concentrations ranging from 0.01 μg/ml to 10 μg/ml.
To examine the effects of LVFX on ICAM-1 mRNA expression in the cells and the concentration of the soluble form of ICAM-1 (sICAM-1) in the supernatant, the cells were pretreated with LVFX (3 μg/ml [8.1 × 10−6 M]) or vehicle (water) for 3 days (72 h) before RV14 infection. The supernatants were collected, and RNA was extracted from cells just before RV14 infection.
Collection of supernatants for measurements.
For the cells cultured in the tubes, we measured the time course of viral release using previously described methods (43). To measure the release of RVs (RV2, RV14, or RV15) during the first 24 h, we used three separate cultures from each trachea. We collected the supernatants at 1 h, 12 h, or 24 h after RV infection. We also collected supernatants at 3 days (72 h), 5 days (120 h), and 7 days (168 h) after infection. At 1 day (24 h), 3 days (72 h), and 5 days (120 h) after infection, supernatants were collected, cells were rinsed with PBS, the medium was replaced with fresh medium, and the cell culture was continued.
Likewise, to examine the effects of LVFX on the secretion of IL-1β, IL-6, and IL-8, supernatants were collected immediately before infection and then again at 1 day (24 h), 3 days (72 h), and 5 days (120 h) after RV14 infection.
Effects of LVFX on susceptibility to rhinovirus infection.
The effects of LVFX on the cellular susceptibility to RV14 infection were evaluated as previously described (43). Epithelial cells were pretreated with LVFX (3 μg/ml) or vehicle for 3 days (72 h) before infection. The epithelial cells were exposed to serial 10-fold dilutions of RV14 at doses ranging from 101 to 105 TCID50 units/ml in medium containing LVFX or vehicle for 1 h at 33°C. After exposure to RV14, fresh medium with no LVFX was added. The cells in the tubes were then cultured at 33°C with rolling.
We collected the supernatants at 1 day (24 h) and 3 days (72 h) after RV14 infection and measured the RV14 titers in the supernatants with the human embryonic fibroblast cell assay described above to assess whether infection occurred at each dose (101, 102, 103, 104, and 105 TCID50 units/ml) of RV14 (43).
Measurement of ICAM-1 expression.
The level of ICAM-1 mRNA was examined by using two-step real-time RT-PCR analysis according to the methods described above (see “Quantification of rhinoviral RNA”), with a forward primer designed for ICAM-1 (43). The concentration of sICAM-1 in the supernatants was measured with an enzyme immunoassay (EIA) (43).
Measurement of changes in acidic endosomes.
The distribution and the fluorescence intensity of acidic endosomes in the cells were measured, as previously described, with LysoSensor DND-189 dye (Molecular Probes, Eugene, OR) (35). Live-cell imaging was performed by observing cells on coverslips in petri dishes with a fluorescence microscope (Olympus IX70; Olympus Co. Ltd., Tokyo, Japan). The fluorescence intensity was calculated by using a fluorescence image analyzer system (Lumina Vision; Mitani Co. Ltd., Fukui, Japan) equipped with a fluorescence microscope. The fluorescence intensity of the acidic endosomes in 100 human tracheal epithelial cells was measured, and the mean value of the fluorescence intensity was expressed as a percentage of the control value compared with the fluorescence intensity of the cells before any treatment.
We studied the effects of a long period of treatment with LVFX (3 μg/ml for 72 h) on acidic endosomes because the cells were pretreated with LVFX or vehicle (water) for 3 days (72 h) before RV14 infection.
Measurement of cytokine production.
We measured IL-1β, IL-6, and IL-8 levels in supernatants with specific enzyme-linked immunosorbent assays (ELISAs) (43) in duplicate human tracheal epithelial cells in plastic tubes at all time points mentioned above. The concentrations of IL-1β, IL-6, and IL-8 in the supernatants of the cells were measured before and after infection with RV14 or exposure to culture medium for RV14 stocks (MEM supplemented with 2% ultralow-γ-globulin calf serum) in the presence of LVFX. We also measured the concentrations of IL-1β, IL-6, and IL-8 in the supernatants of the cells that were exposed to UV-inactivated RV14 or the vehicle of RV14 stocks.
NF-κB assay.
Nuclear extracts from human tracheal epithelial cells were prepared by using a TransFactor extraction kit (BD Bioscience/Clontech, Mountain View, CA). The presence of p50, p65, and c-Rel subunits was assayed by using a TransFactor Family NF-κB colorimetric kit (BD Bioscience/Clontech) according to the manufacturer's instructions, as previously described (43). The results were expressed in terms of the optical density (OD), which provides quantitative levels of the NF-κB subunits (43). The cells were treated with LVFX (3 μg/ml) or vehicle for 3 days. To adjust the data for cell numbers, we counted the number of cells that were cultured in the tubes. We found that there were only small differences in cell numbers in each tube from the same tracheas (data not shown). We also counted cells to monitor the cell number in the NF-κB experiment using five different tracheas. We found that the cell number was between 1.9 × 106 and 2.3 × 106 cells per tube for five different tracheas. To adjust the data for cell numbers, we calculated the OD according to 1.9 × 106 cells.
Statistical analysis.
The results are expressed as the means ± standard errors of the means (SEM). Statistical analysis was performed by using a one-way analysis of variance (ANOVA). A subsequent post hoc analysis was performed by using Bonferroni's method. For all analyses, P values of <0.05 were assumed to be significant. The term “n” refers to the number of donors (tracheas) from which cultured epithelial cells were obtained.
RESULTS
Effects of LVFX on rhinoviral infection of human tracheal epithelial cells.
The exposure of confluent human tracheal epithelial cell monolayers to RV14 (5.0 × 10−2 TCID50 units/cell) consistently led to infection. No virus was detected at 1 h after infection; however, RV14 was detected in the supernatants (culture medium) at 12 h, and the viral content progressively increased between 1 and 12 h after infection (Fig. 1A). Evidence of continuous viral production was obtained by demonstrating that each of the supernatants collected from 12 h to 24 h (1 day), 1 day (24 h) to 3 days (72 h), 3 days (72 h) to 5 days (120 h), or 5 days (120 h) to 7 days (168 h) after infection contained significant levels of RV14 (Fig. 1A). The viral titers in supernatants increased significantly with time for the first 3 days (72 h) (P < 0.05 by ANOVA). Furthermore, in the tracheal cells from subjects whose cells were infected with RV14, the supernatants collected from 1 day (24 h) to 3 days (72 h) after infection contained consistent levels of RV14 (4.49 ± 0.15 log TCID50 units/ml/24 h; n = 33).
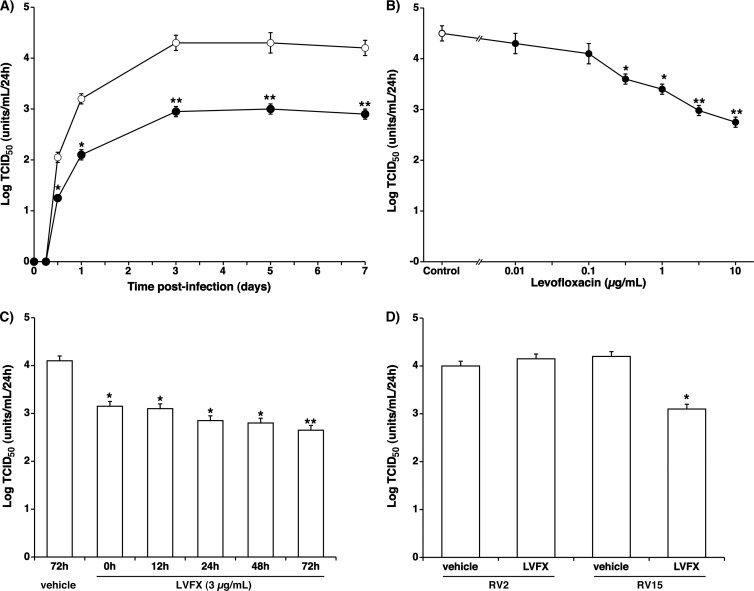
Fig 1.
(A) Time course of viral release into the supernatants of human tracheal epithelial cells obtained at different times after exposure to 5.0 × 10−2 TCID50 units/cell of RV14 in the presence of LVFX (3 μg/ml) (closed circles) or vehicle (0.1% double-distilled water) (open circles). The epithelial cells isolated from each donor were treated with LVFX or vehicle beginning 3 days before infection until the end of the experimental period. To examine whether the supernatants contained a significant amount of RV14, human embryonic fibroblast cells were observed for evidence of cytopathic changes for 7 days (168 h) after the exposure of the fibroblasts to the supernatants. The rates of change in the RV14 concentrations in the supernatants are expressed as TCID50 units/ml/24 h. The results are reported as the means ± SEM from five different tracheas (two ex-smokers and three nonsmokers). Significant differences compared to treatment with the vehicle alone are indicated (*, P < 0.05; **, P < 0.01). (B) Concentration-dependent effects of LVFX on viral release into supernatants collected between 1 day (24 h) and 3 days (72 h) after infection. The cells were treated with LVFX (closed circles) or vehicle (control) (open circle) beginning 3 days (72 h) before RV14 infection until the end of the experimental period. The epithelial cells isolated from each donor were treated with LVFX or vehicle. To examine whether the supernatants contained a significant amount of RV14, human embryonic fibroblasts were observed for evidence of cytopathic changes for 7 days (168 h) after the exposure of the fibroblasts to the supernatants. The rates of change in the RV14 concentrations in the supernatants are expressed as TCID50 units/ml/24 h. The results are reported as the means ± SEM from 3 (0.5 μg/ml of LVFX), 5 (0.01, 0.1, 1, 3, and 10 μg/ml of LVFX), or 8 (control) different tracheas. Significant differences compared to treatment with the vehicle alone (control) are indicated (*, P < 0.05; **, P < 0.01). (C) RV14 titers in the supernatants of cells pretreated with LVFX (3 μg/ml) for times ranging from 0 days (just after) to 3 days (72 h) and RV14 titers in the supernatants of the cells treated with vehicle (water) for 3 days (72 h). The results are reported as the means ± SEM from 3 different tracheas (two ex-smokers and one nonsmoker). Significant differences compared to treatment with the vehicle alone are indicated (*, P < 0.05; **, P < 0.01). (D) Effects of LVFX (3 μg/ml) on titers of RV2 and RV15 in the supernatants collected between 1 day (24 h) and 3 days (72 h) after infection. The cells were treated with LVFX or vehicle (water) beginning 3 days (72 h) before RV infection until the end of the experimental period. The epithelial cells isolated from each donor were treated with LVFX or vehicle. The RV2 and RV15 titers in the supernatants are expressed as TCID50 units/ml/24 h. The results are reported as the means ± SEM from 3 different tracheas. Significant differences compared to treatment with the vehicle alone (vehicle) are indicated (*, P < 0.05). LVFX did not reduce RV2 titers.
The pretreatment of cells with LVFX (3 μg/ml [8.1 × 10−6 M]) resulted in significantly lower viral titers of RV14 in the supernatants from 12 h after infection (Fig. 1A) and reduced RV14 titers in the supernatants in a concentration-dependent manner (Fig. 1B). The pretreatment of the cells with LVFX reduced viral titers of RV14 in the supernatants at concentrations of 0.5 μg/ml or higher (Fig. 1B).
The inhibitory effects of LVFX on the RV14 titers were time dependent. The maximum inhibitory effect was obtained when the cells were pretreated with LVFX for 3 days (72 h) (Fig. 1C). Significant inhibitory effects were observed even when the cells were treated with LVFX (3 μg/ml) just after RV14 infection (Fig. 1C).
The pretreatment of the cells with LVFX (3 μg/ml) did not reduce the viral titers of RV2 in the supernatants (Fig. 1D). In contrast, the pretreatment of the cells with LVFX (3 μg/ml) reduced viral titers of RV15 in the supernatants (Fig. 1D).
RV14 titers in the supernatants collected from the cells of the 13 ex-smokers from 1 day (24 h) to 3 days (72 h) after infection did not differ from those of the 20 patients who had never smoked (4.49 ± 0.13 log TCID50 units/ml/24 h and 4.51 ± 0.15 log TCID50 units/ml/24 h, respectively; P > 0.02). Likewise, the RV14 titers in the supernatants from the 3 patients with COPD did not differ from those of the 30 patients without COPD (data not shown). No virus was detected in the supernatants after infection with UV-inactivated RV14 (data not shown).
To measure the effects of LVFX on cell attachment, human tracheal epithelial cells were placed into tubes containing medium supplemented with LVFX (3 μg/ml) or vehicle (water). The number of cells that attached to the culture vessels was then counted 24 h later. The number of cells treated with LVFX was the same as the number of cells treated with vehicle (1.51 × 105 ± 0.1 × 105 cells treated with LVFX versus 1.49 × 105 ± 0.1 × 105 cells treated with vehicle; n = 3) after initially placing 5 × 105 cells into each tube. Pretreatment with LVFX (3 μg/ml) for 3 days (72 h) did not change cell viability (99% ± 1% for LVFX treatment versus 98% ± 1% for vehicle treatment; n = 5; P > 0.50), as assessed by a trypan blue exclusion assay. Furthermore, until 7 days (168 h) after the start of the cell culture, LVFX- and vehicle-treated cells made confluent cell sheets in the same amount of time following their placement into tubes (data not shown). The cell numbers in the confluent sheets cultured in medium supplemented with LVFX (3 μg/ml) did not differ from the cell counts in the unsupplemented medium (2.1 × 106 ± 0.3 × 106 cells/tube with LVFX treatment versus 2.2 × 106 ± 0.3 × 106 cells/tube for vehicle treatment; n = 5; P > 0.50). Pretreatment with LVFX (3 μg/ml) for 3 days (72 h) did not alter the lactate dehydrogenase (LDH) concentration (29 ± 2 IU/liter/24 h for LVFX treatment versus 31 ± 3 IU/liter/24 h IU/ml for vehicle treatment; n = 5; P > 0.50) in the supernatants collected 3 days (72 h) after LVFX treatment.
Effects of LVFX on viral RNA replication.
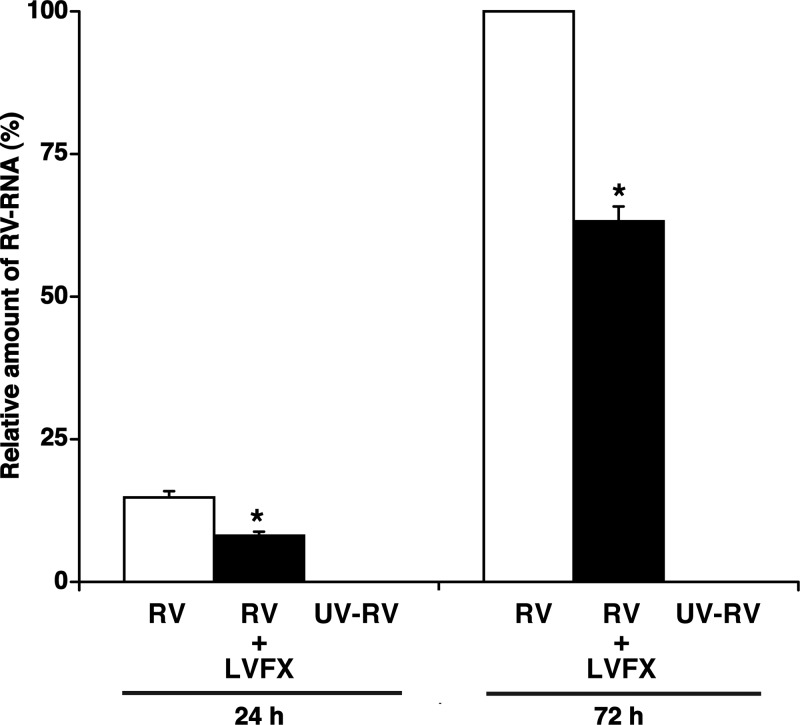
Further evidence of the inhibitory effects of LVFX on RV14 RNA replication in human tracheal epithelial cells was provided by real-time RT-PCR analysis. RNA extraction was performed at 1 day (24 h), 3 days (72 h), and 5 days (120 h) after RV14 infection. RV14 RNA was consistently observed in the cells from 1 day (24 h) after infection onwards, and the levels increased with time after infection (Fig. 2). In preliminary experiments, the maximum level of RV14 RNA replication was observed at 3 days (72 h) after infection (data at 120 h not shown), whereas RV14 RNA was not observed in the cells prior to infection (data not shown). Pretreatment with LVFX (3 μg/ml) resulted in a lower level of RV14 RNA at 1 day (24 h) and 3 days (72 h) after infection (Fig. 2). Detectable amounts of RV14 RNA were not observed in the cells after infection with UV-inactivated RV14 (Fig. 2).
Fig 2.
Replication of viral RNA in human tracheal epithelial cells at 1 day (24 h) or 3 days (72 h) after infection with RV14 in the presence of LVFX (3 μg/ml) (RV + LVFX) or vehicle (RV) or after infection with UV-inactivated RV14 in the presence of vehicle of LVFX (UV-RV), as detected by real-time quantitative RT-PCR. The epithelial cells isolated from each donor were treated with LVFX or vehicle. The results are expressed as the relative amounts of RNA expression (percent) compared with that of maximal RV14 RNA levels on day 3 (72 h) in the cells treated with vehicle, and the results are reported as the means ± SEM from five samples (two ex-smokers and three nonsmokers) (RV and RV + LVFX) or 3 different samples (two ex-smokers and one nonsmoker) (UV-RV). Significant differences compared to treatment with the vehicle alone (RV) at each time point are indicated (*, P < 0.05). RV14 RNA was not detected at significant levels after UV-RV infection.
Effects of LVFX on susceptibility to rhinovirus infection.
The pretreatment of the cells with LVFX (3 μg/ml) decreased the susceptibility of the cells to infection by RV14. When viral release in supernatants collected 3 days (72 h) after RV14 infection was measured, the minimum dose of RV14 necessary to cause infection of cells treated with LVFX (3.2 ± 0.3 log TCID50 units/ml; n = 5) was significantly higher than that of the cells treated with the vehicle alone (2.1 ± 0.2 log TCID50 units/ml; n = 5; P < 0.05).
Effects of LVFX on expression of ICAM-1.
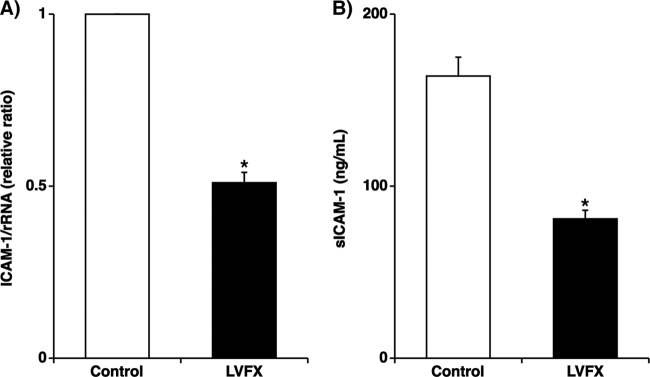
LVFX treatment (3 μg/ml for 72 h) reduced baseline ICAM-1 mRNA expression levels in the cells by approximately 50% compared with the levels in the cells treated with the vehicle alone prior to RV14 infection (Fig. 3A). Furthermore, the concentrations of sICAM-1 in the supernatants of cells treated with LVFX (3 μg/ml) were significantly lower than those in the supernatants of cells treated with the vehicle alone prior to RV14 infection (Fig. 3B).
Fig 3.
(A) Expression of ICAM-1 mRNA before RV14 infection in human tracheal epithelial cells treated with LVFX (3 μg/ml for 72 h) or vehicle (control), detected by real-time quantitative RT-PCR. The epithelial cells isolated from each donor were treated with LVFX or vehicle. ICAM-1 mRNA levels were normalized to the constitutive expression level of rRNA. The expression level of ICAM-1 mRNA in the cells treated with vehicle was set to 1.0. The results are reported as the means ± SEM from five different tracheas (two ex-smokers and three nonsmokers). Significant differences compared to treatment with the vehicle alone are indicated (*, P < 0.05). (B) Concentration of sICAM-1 in supernatants before RV14 infection of human tracheal epithelial cells treated with LVFX (3 μg/ml for 72 h) or vehicle (control), as detected by an enzyme immunoassay. sICAM-1 concentrations in the supernatants are expressed in ng/ml. The results are reported as the means ± SEM from five different tracheas (two ex-smokers and three nonsmokers). Significant differences compared to control values are indicated (*, P < 0.05).
Effects of LVFX on acidification of endosomes.
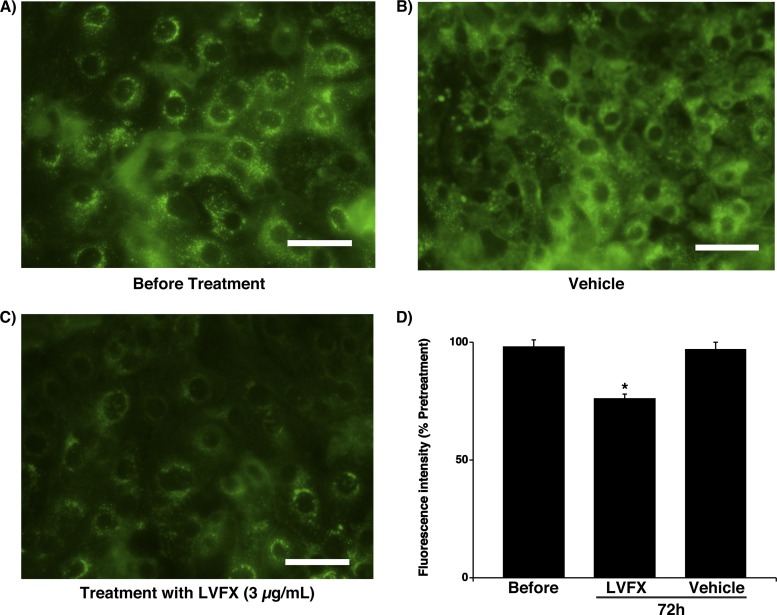
Acidic endosomes in human tracheal epithelial cells were stained green with LysoSensor DND-189 (Fig. 4A to C) as described previously (43). Treatment with vehicle for 3 days (72 h) did not change the number of fluorescent acidic endosomes in the cells (Fig. 4B) or the fluorescence intensity of the acidic endosomes compared with that of the cells prior to any treatment (Fig. 4D). In contrast, treatment with LVFX (3 μg/ml for 72 h) reduced the number of acidic endosomes showing green fluorescence (Fig. 4C) and also reduced the fluorescence intensity of the acidic endosomes compared with measurements of cells treated with vehicle and of cells prior to any treatment (Fig. 4D).
Fig 4.
(A to C) Changes in the distribution of acidic endosomes (green fluorescence) in human tracheal epithelial cells before treatment with LVFX (A) and 3 days (72 h) after treatment with LVFX (3 μg/ml) (C) or vehicle (B). Data are representative of data from five different experiments (two ex-smokers and three nonsmokers). Bar = 100 μm. (D) Fluorescence intensity of acidic endosomes 3 days (72 h) after treatment with LVFX (3 μg/ml) or vehicle and before treatment (Before). The results are reported as the fluorescence intensity divided by the fluorescence intensity before treatment (expressed as a percentage) and are reported as the means ± SEM from five samples (two ex-smokers and three nonsmokers). Significant differences compared to values before treatment are indicated (*, P < 0.05).
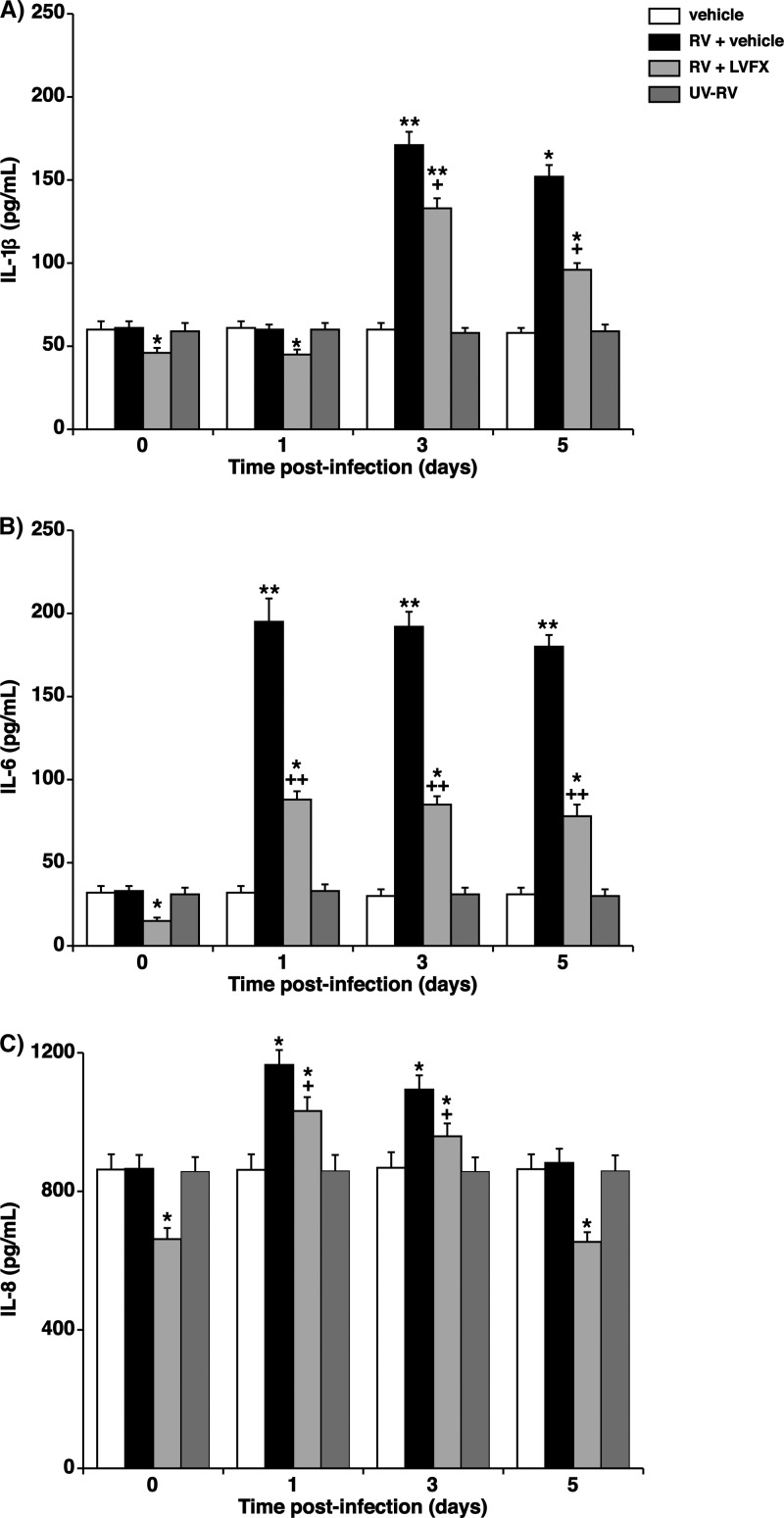
Effects of LVFX on cytokine production.
LVFX (3 μg/ml) pretreatment reduced the baseline levels of secretion of IL-1β, IL-6, and IL-8 for 24 h before RV14 infection compared with the secretion levels for cells treated with the vehicle alone (Fig. 5). RV14 infection increased the levels of secretion of IL-1β, IL-6, and IL-8. The maximum secretion level was observed at 1 day (24 h) after RV14 infection for IL-6 and IL-8 and peaked at 3 days (72 h) after infection for IL-1β. LVFX (3 μg/ml) also reduced the RV14 infection-induced secretion of IL-1β, IL-6, and IL-8 compared with the secretion levels in the cells treated with the vehicle alone (Fig. 5). Exposure to culture medium used for RV14 stocks (vehicle of the RV14 stock [MEM supplemented with 2% ultralow-γ-globulin calf serum]) and infection with UV-inactivated RV14 did not change the concentrations of IL-1β, IL-6, and IL-8 in the supernatants (Fig. 5).
Fig 5.
(A to C) Altered time course for cytokine release into supernatants of human tracheal epithelial cells before (time zero) and after infection with RV14 in the presence of LVFX (3 μg/ml) or the vehicle of LVFX (water). The epithelial cells isolated from each donor were treated with LVFX or vehicle. The concentrations of cytokines in the supernatants are expressed in pg/ml. The results are reported as the means ± SEM from eight different tracheas (four ex-smokers and four nonsmokers with RV14 infection). Significant differences compared to values before RV14 infection (time zero) in the presence of vehicle are indicated (*, P < 0.05; **, P < 0.01). Significant differences compared to RV14 infection alone (RV + vehicle) at each time point after infection are indicated (+, P < 0.05; ++, (P < 0.01). Exposure to the culture medium used for RV14 stocks (vehicle) and infection with UV-inactivated RV14 (UV-RV) did not change the concentrations of cytokines in the supernatants; the results are reported as the means ± SEM from 3 different tracheas (two ex-smokers and one nonsmoker).
The secretion levels of IL-1β, IL-6, and IL-8 in the supernatants of the cells from the four ex-smokers did not differ from the levels secreted by cells from the four patients who had never smoked (data not shown). Likewise, the levels of secretion of IL-1β, IL-6, and IL-8 in the supernatants of the cells from three patients with COPD did not differ from the levels secreted by cells from the 30 patients without COPD (data not shown).
Effects on NF-κB.
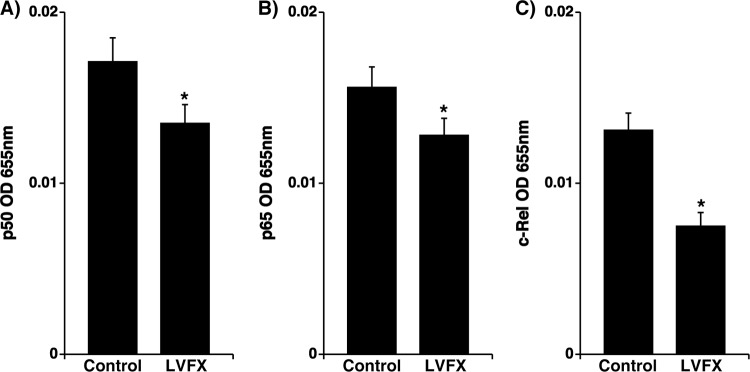
LVFX treatment (3 μg/ml for 72 h) significantly reduced the levels of the p50, p65, and c-Rel subunits of NF-κB in the nuclear extracts of cells cultured under stationary conditions prior to RV14 infection compared with the NF-κB levels in the cells treated with the vehicle alone (Fig. 6).
Fig 6.
Amounts of p50 (A), p65 (B), and c-Rel (C) in nuclear extracts of human tracheal epithelial cells treated with LVFX (3 μg/ml) or vehicle (control) for 3 days (72 h) prior to RV14 infection. The results are expressed as optical density (OD) units and are reported as the means ± SEM for five different tracheas (two ex-smokers and three nonsmokers). Significant differences compared to control values prior to RV14 infection are indicated (*, P < 0.05).
DISCUSSION
In the present study, we have shown that the new quinolone antibiotic levofloxacin (LVFX) reduced the titers of a major-group rhinovirus (RV), RV14, in supernatants and reduced the RNA replication of the virus in primary cultures of human tracheal epithelial cells. Pretreatment with LVFX reduced the mRNA and protein expression levels of ICAM-1, the receptor for RV14 (6, 11), prior to RV14 infection. The minimum dose of RV14 necessary to cause infection of cells treated with LVFX was significantly higher than that necessary to cause infection of cells treated with the vehicle alone. The pretreatment of the cells with LVFX also reduced the titers of RV15, one of the major-group RVs (39), but did not reduce the titers of RV2, a minor-group RV (12). These findings suggest that LVFX might inhibit infection by major-group RVs, including RV14 and RV15, in part through reducing the level of production of its receptor ICAM-1.
Major human RVs, including RV14 and RV15, enter the cytoplasm of infected cells after binding to the receptor known as ICAM-1 (6, 11, 39). The entry of the RNA from this group of RVs into the cytoplasm of infected cells has been suggested to be mediated by the destabilization of the cell membrane due to ICAM-1 binding. Furthermore, the entry of the RNA into the cytoplasm is mediated by endosomal acidification, which occurs when the virions enter the cell via endosomes before they enter the cytoplasm (6). In contrast, a minor-group RV, RV2, enters the cytoplasm of infected cells after binding to its receptor, a low-density lipoprotein (LDL) receptor (12). The entry of RV2 is also mediated by endosomal acidification in cells (26). Therefore, the inhibition of ICAM-1 expression and the reduction in the number of acidic endosomes might mean that fewer whole virions enter the cytoplasm. However, in this study, LVFX significantly reduced the ICAM-1 expression level, whereas the magnitude of the reduction of the fluorescence intensity from acidic endosomes was low. Therefore, the reduction of the ICAM-1 expression level might mainly contribute to the inhibition of infection by major-group RVs, including RV14 and RV15, induced by LVFX in this study. Although we did not examine the effects of LVFX on the expression of the LDL receptor, the small effects of LVFX on acidic endosomes might be associated with the finding in this study that LVFX did not inhibit infection by RV2.
In the present study, LVFX reduced ICAM-1 expression levels in primary cultures of human tracheal epithelial cells. The reduction of ICAM-1 expression levels resulting from treatment with ciprofloxacin was previously reported by Mori et al. for monocytes (19). The inhibitory effects of LVFX on ICAM-1 expression by human tracheal epithelial cells might be associated with the inhibitory effects of LVFX on RV14 infection. This phenomenon was previously reported in terms of the inhibitory effects of various agents, including dexamethasone, erythromycin, the proton pump inhibitor lansoprazole, and the β2 agonist procaterol (29, 36, 37, 43).
Human embryonic fibroblast cells did not show any morphological changes that demonstrate the presence of RV14 when the supernatants collected 1 h after infection were added to fibroblasts. In contrast, the supernatants collected 12 h after infection produced morphological changes in the fibroblasts, indicating the presence of RVs (8, 22). These findings suggest that the supernatants collected 12 h after infection contained significant amounts of RV14 virions that had been newly produced after infection, as reported previously (43).
Furthermore, in the tracheal cells from all subjects infected with RV14, the supernatant fluids collected from 1 day (24 h) to 3 days (72 h) after infection contained significant levels of RV14. These findings suggest that the human tracheal epithelial cells from all subjects were consistently infected with RV14.
Lachowicz-Scroggins et al. previously demonstrated by immunohistochemistry that RV16 was detected in some, but not all, of the cultured cells after infection with 105 TCID50 units/ml of RV16 (15), which was the concentration of RV14 used in this study. These data suggest the existence of cells in the cell sheets that were not infected during their exposure to RV16 stocks but instead could have been infected with RV16 that was replicated and released from adjacent cells. As we describe in Materials and Methods, after exposure to RV14, RV14 stocks were aspirated and removed from the cells, the cells were rinsed with PBS, and fresh medium supplemented with LVFX or vehicle (water) was added to the tubes. Likewise, to measure the time course of RV14 titers, supernatants were collected at 1 day (24 h), 3 days (72 h), and 5 days (120 h) after infection. Cells in the tubes were rinsed with PBS, fresh medium supplemented with LVFX or vehicle was added, and the cell culture was continued. Therefore, supernatants might contain RV14 that was released by a second round of replication during the 7 days of the experiments in addition to that released after exposure to RV14 stocks. Because the cells were treated with LVFX after RV14 infection in this study, LVFX might also inhibit a second round of replication of RV14. Furthermore, RV14 virions released into the supernatants were sufficient to cause infection of the cells because RV14 titers in the supernatants were higher than those needed to cause infection in this study. Therefore, these findings suggest that the RV14 present in the supernatants might also include RV14 virions that were released by a second round of replication in this study.
The mean peak concentration of LVFX in the alveolar epithelial lining fluid was reported previously to be 3.4 μg/ml at 1 h after the oral ingestion of 500 mg LVFX (47). The pretreatment of cells with LVFX reduced the viral titers of RV14 in the supernatants at concentrations of 0.5 μg/ml or higher. The inhibitory effects of LVFX on RV14 titers were time dependent, whereas significant inhibitory effects were observed even when the cells were treated with LVFX (3 μg/ml) immediately after RV14 infection. These findings suggest a clinically relevant possibility that LVFX may inhibit RV infection when patients receive LVFX after RV infection.
In the present study, treatment with LVFX reduced various cell functions, including ICAM-1 expression, endosomal acidification, the production of cytokines, and NF-κB expression. However, the treatment of cells with LVFX did not change cell attachment, the growth and viability of the cells, or LDH concentrations in the supernatants. These findings were consistent with previous reports that ofloxacin, a fluoroquinolone, does not affect the number of cultured primary human renal proximal tubular epithelial cells (31). Shimoda and Kato demonstrated previously that LVFX does not exacerbate retinal degeneration induced by phototoxicity in mouse eyes (32). Thus, LVFX might not affect cell functions such as attachment and growth, and there was not any apparent cytotoxicity caused by LVFX at the concentrations used.
As we reported previously, RV14 infection increased ICAM-1 expression levels in cultured human tracheal epithelial cells (29, 36, 38). We also previously demonstrated that anti-IL-1β antibodies inhibited RV14-induced ICAM-1 expression (29, 38) and that treatment with IL-1β (200 pg/ml) increased ICAM-1 expression levels in human tracheal epithelial cells by an immunohistochemical analysis (38). In the present study, RV14 infection increased the concentration of IL-1β (200 pg/ml) in the supernatants. Regarding the effects of ICAM-1 on inflammation, ICAM-1 plays a vital role in the recruitment and migration of immune effector cells to sites of local inflammation in patients with COPD (27). These findings suggest that RV14 infection-induced cytokine levels may be sufficient to modulate inflammation. The inhibitory effects of LVFX on ICAM-1 shown in this study may also be associated with the inhibition of airway inflammation and the subsequent exacerbation of COPD after RV infection (30) and with clinical benefits in the treatment of COPD exacerbations (29).
RVs are associated with the exacerbation of COPD (30). Neutrophilic inflammation, which takes place during the exacerbation of COPD, was suggested previously to be associated with a variety of mediators, including IL-6, after RV infection (30). Ciprofloxacin reduces the induction of tumor necrosis factor in rabbit alveolar macrophages in response to a lipopolysaccharide (LPS) derived from Pseudomonas aeruginosa (23). These data suggest that new quinolones may modulate the inflammatory response seen in infections caused by Gram-negative bacilli. In the present study, LVFX reduced the RV14 infection-induced production of IL-1β, IL-6, and IL-8. The inhibitory effects of LVFX on IL-1β production are consistent with previous findings for LPS-stimulated peripheral blood mononuclear cells (46), although IL-8 production was affected little by LVFX. Trovafloxacin reduces the production of IL-6 and IL-1β by human monocytes in response to LPS stimulation (13). LVFX reduces levels of LPS-induced IL-1β production in a murine macrophage-like cell line (14) and reduces levels of IL-6 and IL-8 production in human bronchial epithelial cell lines (40). Similar to the inhibitory effects of glucocorticoids (37) and the β2 agonist procaterol (43), LVFX may also modulate the airway inflammation induced by RV14 infections.
Endosomal pH was suggested previously to be regulated by the vacuolar H+-ATPases (17) and ion transport across Na+/H+ exchangers (16, 20). The vacuolar H+-ATPase inhibitor bafilomycin and the Na+/H+ exchanger inhibitors 5-(N-ethyl-N-isopropyl) amiloride (EIPA) and N″-[3-(hydroxymethyl)-5-(1H-pyrrol-1-yl)benzoyl]guanidine methanesulfonate (FR168888) were shown previously to increase endosomal pH and inhibit RV14 infection of cultured human tracheal epithelial cells (35). In the present study, LVFX increased the endosomal pH; however, it is unknown if LVFX inhibits vacuolar H+-ATPases or Na+/H+ exchangers. The physiological functions, other than the antimicrobial effects, of new quinolone antibiotics, including LVFX, in airway epithelial cells have not been well studied. However, H+-ATPases are functional in the bacterium Streptococcus pneumoniae and are associated with the mechanisms of quinolone resistance (9). These findings suggest the possibility that LVFX may have an effect on H+-ATPases in airway epithelial cells.
NF-κB activation increases the expression levels of the ICAM-1 gene and genes encoding various proinflammatory cytokines (24, 48). In the present study, LVFX reduced the expression level of ICAM-1 prior to RV14 infection and reduced the secretion of proinflammatory cytokines in supernatants before and after RV14 infection. Moxifloxacin was shown previously to decrease NF-κB activation in a human bronchial epithelial cell line (5). The reduction in the expression level of ICAM-1 and the secretion of interleukins by human tracheal epithelial cells in response to LVFX may be associated with a decrease in NF-κB activity.
We previously reported that macrolide antibiotics and the mucolytic agent l-carbocisteine inhibit respiratory syncytial virus (RSV) infection by reducing levels of its receptors, activated RhoA (isoform A of the Ras-homologous [Rho] family) and/or ICAM-1 (2, 3). We also reported previously that a macrolide antibiotic, clarithromycin, and l-carbocisteine inhibit seasonal influenza virus infection by reducing the expression level of its receptor SAα2,6Gal and by reducing the number of acidic endosomes in human tracheal epithelial cells (44, 45). In this study, we demonstrated that LVFX reduces the ICAM-1 expression level and slightly but significantly reduces the number of acidic endosomes. These findings suggest the possibility that LVFX may also inhibit infection by other viruses such as RSV and influenza virus. Recent reports have demonstrated that the fluoroquinolones LVFX and ofloxacin inhibit human polyomavirus BK (BKV) replication in primary human kidney cells (31). Further experiments are needed to study this possibility.
In summary, this is the first report to show that the new quinolone antibiotic LVFX reduces the release of RV14 into human tracheal epithelial cell supernatants, reduces the replication of RV14 RNA in these cells, and decreases the susceptibility of these cells to RV14 infection. These effects may occur, in part, through a reduction in the expression level of ICAM-1, the receptor for RV14, and a reduction in the number of acidic endosomes, through which RV14 RNA enters the cytoplasm. The pretreatment of cells with LVFX reduced the baseline and RV14 infection-induced release of IL-1β, IL-6, and IL-8 into the supernatant. LVFX may therefore inhibit infection by RV14 and modulate inflammatory responses in the airways after RV infection.
Footnotes
Published ahead of print 14 May 2012
REFERENCES
- 1. Akira S, Hirano T, Taga T, Kishimoto T. 1990. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF). FASEB J. 4:2860–2867 [PubMed] [Google Scholar]
- 2. Asada M, et al. 2012. L-Carbocisteine inhibits respiratory syncytial virus infection in human tracheal epithelial cells. Respir. Physiol. Neurobiol. 180:112–118 [DOI] [PubMed] [Google Scholar]
- 3. Asada M, et al. 2009. Macrolide antibiotics inhibit respiratory syncytial virus infection in human airway epithelial cells. Antiviral Res. 83:191–200 [DOI] [PubMed] [Google Scholar]
- 4. Blasi F. 2004. Atypical pathogens and respiratory tract infections. Eur. Respir. J. 24:171–181 [DOI] [PubMed] [Google Scholar]
- 5. Blau H, Klein K, Shalit I, Halperin D, Fabian I. 2007. Moxifloxacin but not ciprofloxacin or azithromycin selectively inhibits IL-8, IL-6, ERK1/2, JNK, and NF-κB activation in a cystic fibrosis epithelial cell line. Am. J. Physiol. 292:L343–L352 [DOI] [PubMed] [Google Scholar]
- 6. Casasnovas JM, Springer TA. 1994. Pathway of rhinovirus disruption by soluble intercellular adhesion molecule 1 (ICAM-1): an intermediate in which ICAM-1 is bound and RNA is released. J. Virol. 68:5882–5889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chodosh S, et al. 1998. Efficacy of oral ciprofloxacin vs. clarithromycin for treatment of acute bacterial exacerbations of chronic bronchitis. Clin. Infect. Dis. 27:730–738 [DOI] [PubMed] [Google Scholar]
- 8. Condit RC. 2006. Principles of virology, p 25–57 In Knipe DM, et al. (ed), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 9. de la Campa AG, Garcia E, Fenoll A, Munoz R. 1997. Molecular bases of three characteristic phenotypes of pneumococcus: optochin-sensitivity, coumarin-sensitivity, and quinolone-resistance. Microb. Drug Resist. 3:177–193 [DOI] [PubMed] [Google Scholar]
- 10. Gotfried MH, Danziger LH, Rodvold KA. 2001. Steady-state plasma and intrapulmonary concentrations of levofloxacin and ciprofloxacin in healthy adult subjects. Chest 119:1114–1122 [DOI] [PubMed] [Google Scholar]
- 11. Greve JM, et al. 1989. The major human rhinovirus receptor is ICAM-1. Cell 56:839–847 [DOI] [PubMed] [Google Scholar]
- 12. Hofer F, et al. 1994. Members of the low density lipoprotein receptor family mediate cell entry of a minor-group common cold virus. Proc. Natl. Acad. Sci. U. S. A. 91:1839–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khan AA, Slifer TR, Remington JS. 1998. Effect of trovafloxacin on production of cytokines by human monocytes. Antimicrob. Agents Chemother. 42:1713–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kitazawa T, et al. 2007. Biphasic regulation of levofloxacin on lipopolysaccharide-induced IL-1β production. Life Sci. 80:1572–1577 [DOI] [PubMed] [Google Scholar]
- 15. Lachowicz-Scroggins ME, Boushey HA, Finkbeiner WE, Widdicombe JH. 2010. Interleukin-13-induced mucous metaplasia increases susceptibility of human airway epithelium to rhinovirus infection. Am. J. Respir. Cell Mol. Biol. 43:652–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Marshansky V, Vinay P. 1996. Proton gradient formation in early endosomes from proximal tubes. Biochim. Biophys. Acta 1284:171–180 [DOI] [PubMed] [Google Scholar]
- 17. Mellman I, Fuchs R, Helenius A. 1986. Acidification of the endocytic and exocytic pathways. Annu. Rev. Biochem. 55:663–700 [DOI] [PubMed] [Google Scholar]
- 18. Mizuta K, et al. 2010. Phylogenetic and cluster analysis of human rhinovirus species A (HRV-A) isolated from children with acute respiratory infections in Yamagata, Japan. Virus Res. 147:265–274 [DOI] [PubMed] [Google Scholar]
- 19. Mori S, et al. 2010. Ciprofloxacin inhibits advanced glycation end products-induced adhesion molecule expression on human monocytes. Br. J. Pharmacol. 161:229–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nass R, Rao R. 1998. Novel localization of a Na+/H+ exchanger in a late endosomal compartment of yeast. Implications for vacuole biogenesis. J. Biol. Chem. 273:21054–21060 [DOI] [PubMed] [Google Scholar]
- 21. Nolan T, Hands RE, Bustin SA. 2006. Quantification of mRNA using real-time RT-PCR. Nat. Protoc. 1:1559–1582 [DOI] [PubMed] [Google Scholar]
- 22. Numazaki Y, et al. 1987. A microplate methods for isolation of viruses from infants and children with acute respiratory infections. Microbiol. Immunol. 31:1085–1095 [DOI] [PubMed] [Google Scholar]
- 23. Nwariaku FE, McIntyre KL, Sikes PJ, Mileski WJ. 1997. The effect of antimicrobial agents on the induction of tumour necrosis factor by alveolar macrophages in vitro in response to endotoxin. J. Antimicrob. Chemother. 39:265–267 [DOI] [PubMed] [Google Scholar]
- 24. Papi A, Johnston SL. 1999. Respiratory epithelial cell expression of vascular cell adhesion molecule-1 and its up-regulation by rhinovirus infection via NF-κB and GATA transcription factors. J. Biol. Chem. 274:30041–30051 [DOI] [PubMed] [Google Scholar]
- 25. Pérez L, Carrasco L. 1993. Entry of poliovirus into cells does not require a low-pH step. J. Virol. 67:4543–4548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prchla E, Kuechler E, Blaas D, Fuchs R. 1994. Uncoating of human rhinovirus serotype 2 from late endosomes. J. Virol. 68:3713–3723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Riise GC, Larsson S, Lofdahl CG, Andersson BA. 1994. Circulating cell adhesion molecules in bronchial lavage and serum in COPD patients with chronic bronchitis. Eur. Respir. J. 7:1673–1677 [DOI] [PubMed] [Google Scholar]
- 28. Ruiz-Gonzalez A, et al. 2007. Open-label, randomized comparison trial of long-term outcomes of levofloxacin versus standard antibiotic therapy in acute exacerbations of chronic obstructive pulmonary disease. Respirology 12:117–121 [DOI] [PubMed] [Google Scholar]
- 29. Sasaki T, et al. 2005. The proton pump inhibitor lansoprazole inhibits rhinovirus infection in cultured human tracheal epithelial cells. Eur. J. Pharmacol. 509:201–210 [DOI] [PubMed] [Google Scholar]
- 30. Seemungal T, Harper-Owen R, Bhowmik A, Jeffries DJ, Wedzicha JA. 2000. Detection of rhinovirus in induced sputum at exacerbation of chronic obstructive pulmonary disease. Eur. Respir. J. 16:677–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sharma BN, Li R, Bernhoff E, Gutteberg TJ, Rinaldo CH. 2011. Fluoroquinolones inhibit human polyomavirus BK (BKV) replication in primary human kidney cells. Antiviral Res. 92:115–123 [DOI] [PubMed] [Google Scholar]
- 32. Shimoda K, Kato M. 1999. Apoptotic photoreceptor cell death induced by quinolone phototoxicity in mice. Toxicol. Lett. 105:9–15 [DOI] [PubMed] [Google Scholar]
- 33. Subauste MC, Jacoby DB, Richards SM, Proud D. 1995. Infection of a human respiratory epithelial cell line with rhinovirus. Induction of cytokine release and modulation of susceptibility to infection by cytokine exposure. J. Clin. Invest. 96:549–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Suzuki T, et al. 2001. Type 2 rhinovirus infection of cultured human tracheal epithelial cells: role of low density lipoprotein receptor. Am. J. Physiol. 280:L409–L420 [DOI] [PubMed] [Google Scholar]
- 35. Suzuki T, et al. 2001. Bafilomycin A1 inhibits rhinovirus infection in human airway epithelium: effects on endosome and ICAM-1. Am. J. Physiol. 280:L1115–L1127 [DOI] [PubMed] [Google Scholar]
- 36. Suzuki T, et al. 2002. Erythromycin inhibits rhinovirus infection in cultured human tracheal epithelial cells. Am. J. Respir. Crit. Care Med. 165:1113–1118 [DOI] [PubMed] [Google Scholar]
- 37. Suzuki T, et al. 2000. Effects of dexamethasone on rhinovirus infection in cultured human tracheal epithelial cells. Am. J. Physiol. 278:L560–L571 [DOI] [PubMed] [Google Scholar]
- 38. Terajima M, et al. 1997. Rhinovirus infection of primary cultures of human tracheal epithelium: role of ICAM-1 and IL-1β. Am. J. Physiol. 273:L749–L759 [DOI] [PubMed] [Google Scholar]
- 39. Tomassini JE, et al. 1989. cDNA cloning reveals that the major group rhinovirus receptor on HeLa cells is intercellular adhesion molecule 1. Proc. Natl. Acad. Sci. U. S. A. 86:4907–4911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tsivkovskii R, et al. 2011. Levofloxacin reduces inflammatory cytokine levels in human bronchial epithelia cells: implications for aerosol MP-376 (levofloxacin solution for inhalation) treatment of chronic pulmonary infections. FEMS Immunol. Med. Microbiol. 61:141–146 [DOI] [PubMed] [Google Scholar]
- 41. Turner RB, Couch RB. 2006. Rhinoviruses, p 895–909 In Knipe DM, et al. (ed), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 42. Wilson R, et al. 2004. Short-term and long-term outcomes of moxifloxacin compared to standard antibiotic treatment in acute exacerbations of chronic bronchitis. Chest 125:953–964 [DOI] [PubMed] [Google Scholar]
- 43. Yamaya M, et al. 2011. Procaterol inhibits rhinovirus infection in primary cultures of human tracheal epithelial cells. Eur. J. Pharmacol. 650:431–444 [DOI] [PubMed] [Google Scholar]
- 44. Yamaya M, et al. 2010. Inhibitory effects of carbocisteine on type A seasonal influenza virus infection in human airway epithelial cells. Am. J. Physiol. 299:L160–L168 [DOI] [PubMed] [Google Scholar]
- 45. Yamaya M, et al. 2010. Clarithromycin inhibits type A seasonal influenza virus infection in human airway epithelial cells. J. Pharmacol. Exp. Ther. 333:81–90 [DOI] [PubMed] [Google Scholar]
- 46. Yoshimura T, et al. 1996. Immunomodulatory action of levofloxacin on cytokine production by human peripheral blood mononuclear cells. Chemotherapy 42:459–464 [DOI] [PubMed] [Google Scholar]
- 47. Zhang J, et al. 2010. Permeability and concentration of levofloxacin in epithelial lining fluid in patients with lower respiratory tract infections. J. Clin. Pharmacol. 50:922–928 [DOI] [PubMed] [Google Scholar]
- 48. Zhu Z, et al. 1996. Rhinovirus stimulation of interleukin-6 in vivo and in vitro. Evidence for nuclear factor κB-dependent transcriptional activation. J. Clin. Invest. 97:421–430 [DOI] [PMC free article] [PubMed] [Google Scholar]