Abstract
We examined the effect of three clinically used antimicrobials on Streptococcus mutans UA159 biofilm detachment under flow conditions. Sodium fluoride (NaF) and chlorhexidine at MIC levels promoted biofilm detachment and inhibited detachment when concentrations were higher than the MIC and reduced detached-cell viability only at high concentrations. Ampicillin at all concentrations tested inhibited detachment and reduced the percentage of viable biofilm-detached cells. All the three antimicrobial treatments reduced biofilm live/dead cell ratios.
TEXT
Microbial biofilms have been associated with many chronic infections in humans (2, 4). Regardless of location, biofilms release cells into the surrounding environment (14), contributing to bacterial survival, colonization of new sites, and disease transmission (8, 13, 24). It has been reported that environment conditions such as nutrient and oxygen tension affect the biofilm detachment of various species (1, 7, 14, 25, 27). Reattachment of Neisseria subflava and Aggregatibacter actinomycetemcomitans biofilm-detached cells has been previously noted (12). Several studies have indicated that the extent of Candida albicans biofilm detachment and detached-cell viability are dependent on the types of antimicrobials employed (26) and that detached cells gain enhanced adherence abilities (25). Given the breadth of the detrimental effects caused by biofilms and biofilm-detached cells, there have been significant efforts to develop and find agents controlling biofilm detachment and decreasing pathogenicity and viability of biofilm-detached cells (11, 15).
Streptococcus mutans is one of the principal cariogenic dental biofilm inhabitants (16), which are controlled mainly through treatment using broad-spectrum antibiotics or nonspecific mechanical removal. NaF, ampicillin, and chlorhexidine are three different types of clinically used antimicrobials (2a, 3, 22). We hypothesized that the three antimicrobials affect the detachment of S. mutans biofilm. And we investigated the effects of NaF, ampicillin, and chlorhexidine on S. mutans biofilm detachment extent and detached-cell viability as well as biofilm structure alterations.
S. mutans UA159 was anaerobically grown in brain heart infusion (BHI) medium (Forma Scientific, Inc., Marietta, OH) (10% H2, 5% CO2, and 85% N2) at 37°C overnight. Cells were harvested by centrifugation (5,000 rpm) at 4°C and resuspended in BHI–1% sucrose to a concentration of 105 CFU/ml (19). Twenty-five milliliters of S. mutans suspension was poured into a 100-mm-diameter petri dish containing four polystyrene (PLS) blocks (VWR Scientific) (22 mm by 30 mm by 1 mm) and maintained for 48 h to develop biofilms. The medium was replaced by fresh medium at 24 h. Biofilm-colonized PLS blocks were washed twice with phosphate-buffered saline (PBS; 50 mM, pH 6.8) and transferred to a flask connected to a peristaltic pump to allow continuous flow (20). Fresh BHI–1% sucrose with NaF (250 μg/ml, 500 μg/ml, 1,000 μg/ml, or 2,000 μg/ml), ampicillin (0.04 μg/ml, 0.08 μg/ml, 0.16 μg/ml, or 0.32 μg/ml), or chlorhexidine (0.31 μg/ml, 0.63 μg/ml, 1 μg/ml, or 2 μg/ml) was continuously pumped into the flask at a constant flow rate (0.5 ml/min). The control contained BHI–1% sucrose without antimicrobials. The flowthrough was collected at 1 h, 3 h, and 6 h, and the detached cells were collected by centrifugation as detailed previously.
Studies have described the detached cells as having several virulence traits distinct from those of planktonic cells (20). Whether the S. mutans biofilm-detached cells inherit antimicrobial resistance from multidrug-resistant biofilm cells has not been investigated. The MIC and minimum bactericidal concentration (MBC) of NaF, ampicillin, and chlorhexidine against S. mutans UA159 planktonic and biofilm-detached cells were determined (17). NaF inhibited in vitro growth of S. mutans UA159 planktonic cells (MIC = 600 μg/ml) in BHI medium and had an MBC of 2,500 μg/ml. Compared to planktonic cells, biofilm-detached cells were two times more resistant to NaF and no bactericidal concentration was detected at an MBC of up to 2,500 μg/ml. The two cell populations showed identical ampicillin MICs and MBCs. Reduced efficacy of chlorhexidine against detached cells was observed, with an MIC of 1.25 μg/ml and an MBC of 2.5 μg/ml (Table 1). This multidrug resistance characteristic may contribute to cell survival under adverse environmental conditions and colonization of new susceptible sites.
Table 1.
Antimicrobial effect of NaF, ampicillin, and chlorhexidine treatment of S. mutans UA159 planktonic and biofilm-detached cells
| Agent | Planktonic cells |
Biofilm-detached cells |
||
|---|---|---|---|---|
| MIC (μg/ml) | MBC (μg/ml) | MIC (μg/ml) | MBC (μg/ml) | |
| NaF | 600 | 2,500 | 1,200 | >2,500 |
| Ampicillin | 0.16 | 0.32 | 0.16 | 0.32 |
| Chlorhexidine | 0.63 | 2.00 | 1.25 | 2.50 |
Next, we investigated the effect of the three antimicrobials on the extent of biofilm detachment and detached-cell viability by measuring the absorbance of flowthrough at 550 nm and counting of the culturable bacteria on a BHI agar plate (28). The results were expressed as percentages of detachment and viable cells compared to untreated biofilm measurements. NaF treatment at 2,000 μg/ml inhibited biofilm detachment regardless of treatment time (P < 0.05) (Fig. 1A) and killed 66.80% ± 7.40% of the detached cells after 3 h (Table 2). NaF (1,000 μg/ml) also showed an inhibitory effect after 3 h and 6 h (65.56% ± 8.87% and 73.01% ± 6.0%; P < 0.05) (Fig. 1A) but had little effect on cell viability (Table 2). At all concentrations tested, ampicillin inhibited biofilm detachment and reduced detached-cell viability. Ampicillin treatment at 0.04 μg/ml and 0.32 μg/ml reduced the detachment to 51.86% ± 3.7% within 1 h and to 30.74% ± 2.8% after 6 h, respectively (P < 0.05) (Fig. 1B). Overall reductions in detached-cell viability of greater than 80% were observed after exposure to ampicillin (0.16 μg/ml and 0.32 μg/ml) (Table 2). The effects of chlorhexidine on biofilm detachment differed depending on treatment time and concentration. Chlorhexidine treatment at sub-MIC levels for 3 h and 6 h increased detachment from 125.13% ± 3.40% to 136.6% ± 2.23% (P < 0.05), whereas 1 h of treatment showed no effect (P > 0.05). Chlorhexidine treatment at 1 μg/ml and 2 μg/ml showed a detachment-inhibitory effect (P < 0.05) (Fig. 1C), chlorhexidine at 2 μg/ml decreased the percentage of viable detached cells to 8.00% ± 0.30%, and cells were virtually all killed after 6 h (Table 2). The decreased detachment percentage determined in our study may have resulted from the self-protection mechanism regulated by biofilm cells (6, 21, 23).
Fig 1.
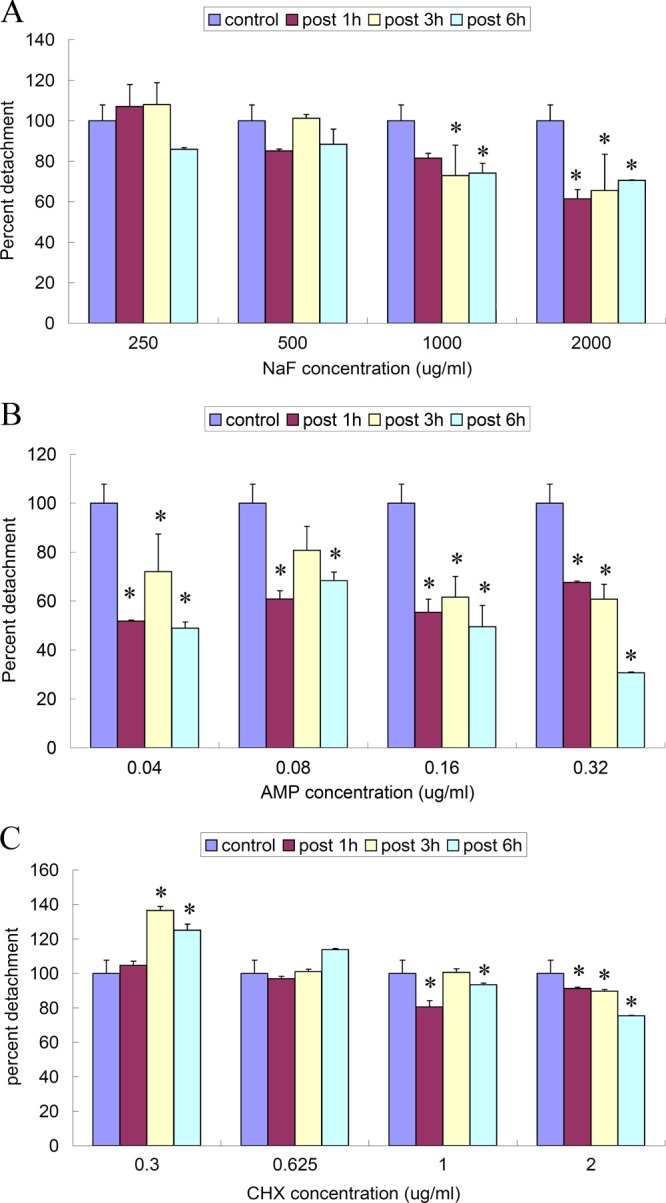
Effect of NaF, ampicillin, and chlorhexidine treatment on the extent of biofilm detachment. Biofilm developed on PLS blocks was further treated with different concentrations of NaF, ampicillin, and chlorhexidine. The optical density at 550 nm (OD550) of flowthrough with biofilm-detached cells at various time points after treatment was measured. Results are expressed as percentages compared to OD550 measurements of untreated biofilms formed in parallel, which were considered 100% (0.50 after 1 h, 0.47 after 3 h, and 0.53 after 3 h). *, significant difference compared to control group (P < 0.05). (A) NaF at 1,000 μg/ml and 2,000 μg/ml inhibited biofilm detachment. (B) Ampicillin (AMP) at all concentrations tested significantly inhibited biofilm detachment (P < 0.05). (C) Chlorhexidine (CHX) at sub-MIC levels promoted biofilm detachment but otherwise inhibited detachment.
Table 2.
Viability of S. mutans UA159 biofilm-detached cells during NaF, ampicillin, and chlorhexidine treatment
| Agent | Concn (μg/ml) | % viable detached cells after indicated treatmenta |
||
|---|---|---|---|---|
| 1 h | 3 h | 6 h | ||
| NaF | 250 | 95.21 ± 0.32 | 95.44 ± 1.02 | 94.71 ± 0.63 |
| 500 | 94.83 ± 0.17 | 94.30 ± 0.40 | 92.72 ± 0.30 | |
| 1,000 | 68.74 ± 4.21 | 66.86 ± 7.44 | 64.23 ± 7.70 | |
| 2,000 | 62.54 ± 2.33 | 66.80 ± 8.70 | 65.32 ± 3.94 | |
| Ampicillin | 0.04 | 64.86 ± 7.23 | 68.51 ± 3.80 | 63.46 ± 5.22 |
| 0.08 | 52.03 ± 13.50 | 46.33 ± 7.84 | 40.84 ± 5.80 | |
| 0.16 | 11.74 ± 5.32 | 17.40 ± 5.42 | 6.93 ± 2.61 | |
| 0.32 | 1.65 ± 2.47 | 0.23 ± 1.34 | 0.00 ± 0.00 | |
| Chlorhexidine | 0.3 | 80.26 ± 6.44 | 83.45 ± 6.10 | 74.82 ± 4.90 |
| 0.625 | 73.53 ± 4.80 | 68.74 ± 2.63 | 52.15 ± 2.17 | |
| 1 | 34.80 ± 2.93 | 36.15 ± 0.47 | 23.54 ± 1.80 | |
| 2 | 8.00 ± 0.33 | 7.41 ± 3.25 | 7.32 ± 0.10 | |
Results represent percentages of viable cells compared to control data (6.93 × 106 CFU/ml after 1 h, 7.22 × 106 CFU/ml after 3 h, and 1.37 × 107 CFU/ml after 6 h) and are expressed as means and standard deviations.
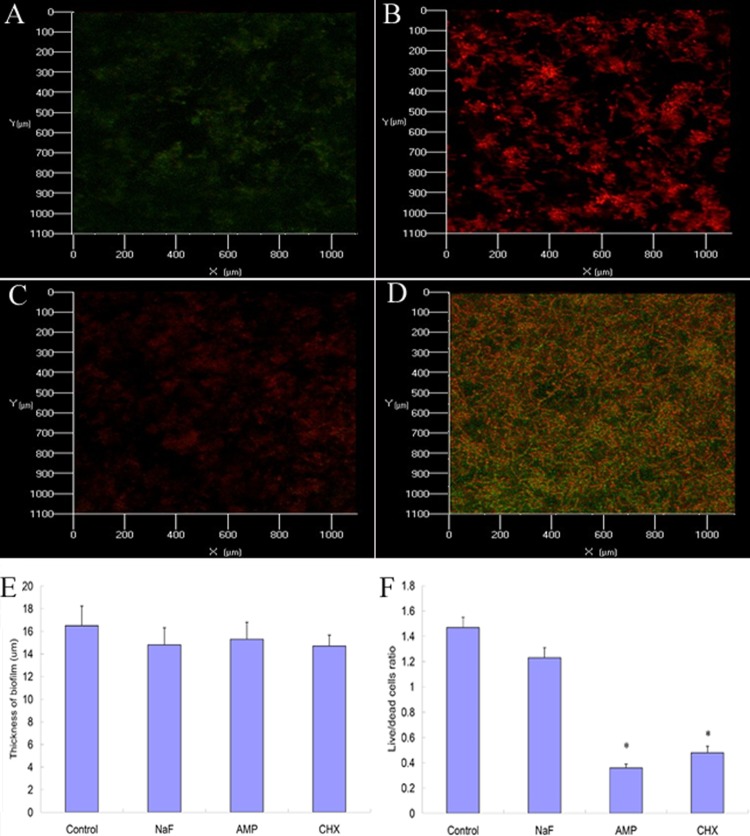
In addition, the effect of the antimicrobials on S. mutans biofilm structures was assessed using confocal laser scanning microscopy (CLSM) (15, 17). Biofilm thickness and live/dead cell ratios were calculated using COMSTAT software. Since the prerequisite for successful antimicrobial treatments is that bacteria within biofilms are exposed to an adequate concentration of antimicrobials (10), we selected the highest concentrations tested for the CLSM sample. Untreated biofilms showed an elaborated architecture with a thickness of 16.85 ± 0.75 um (Fig. 2D). Upon treatment with NaF, ampicillin, and chlorhexidine, S. mutans cells were sporadically scattered on the substrate (Fig. 2A to C), while vertical sectioning revealed no thickness change (P > 0.05) (Fig. 2E). Ampicillin and chlorhexidine treatment reduced live/dead cell ratios from 1.47 ± 0.08 to 0.36 ± 0.03 and 0.48 ± 0.05 (P < 0.05), while NaF treatment had no significant effect on the live/dead cell ratio (Fig. 2F). The structure alteration may partly explain the reduction in the live/dead cell ratio (5).
Fig 2.
Biofilm structures analyzed by CLSM before and after NaF, ampicillin, and chlorhexidine treatment for 1 h. The biofilms were stained by Syto 9 and propidium iodide (PI) and examined with CLSM. *, significant difference compared to control group (P < 0.05). (A) Biofilm treated with NaF (2,000 μg/ml). (B) Biofilm treated with ampicillin (0.32 μg/ml). (C) Biofilm treated with chlorhexidine (2 μg/ml). (D) Control biofilm. (E) There was no significant difference in biofilm thickness between treated and control biofilms. (F) After ampicillin and chlorhexidine treatment, the live/dead cell ratio of biofilm decreased compared to the ratio seen with untreated biofilm (P < 0.05).
In summary, antimicrobial treatments affect S. mutans UA159 biofilm detachment and detached-cell viability. The results emphasize the importance of considering concentration, treatment time, and antimicrobial types when using antimicrobials to control S. mutans UA159 biofilm-associated infections.
ACKNOWLEDGMENT
This study was supported by the specialized Research Fund for the Doctoral Program of Higher Education of China (no. 20100171120102).
Footnotes
Published ahead of print 4 June 2012
REFERENCES
- 1. Allison DG, Begona R, SanJose C, Jaspe A, Gilbert P. 1998. Extracellular products as mediators of the formation and detachment of Pseudomonas fluorescens biofilms. FEMS Microbiol. Lett. 167:179–184 [DOI] [PubMed] [Google Scholar]
- 2. Archer NK, et al. 2011. Staphylococcus aureus biofilms: properties, regulation, and roles in human disease. Virulence 2:445–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a. Bradshaw DJ, Marsh PD, Hodgson RJ, Visser JM. 2002. Effects of glucose and fluoride on competition and metabolism within in vitro dental bacterial communities and biofilms. Caries Res. 36:81–86 [DOI] [PubMed] [Google Scholar]
- 3. Bryskier A. 2005. Antimicrobial agents: antibacterials and antifungals. ASM Press, Washington, DC [Google Scholar]
- 4. Chandra J, et al. 2001. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J. Bacteriol. 183:5385–5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. El-Azizi M, Rao S, Kanchanapoom T, Khardori N. 2005. In vitro activity of vancomycin, quinupristin/dalfopristin, and linezolid against intact and disrupted biofilms of staphylococci. Ann. Clin. Microbiol. Antimicrob. 4:2 doi:10.1186/1476-0711-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Friedman L, Kolter R. 2004. Two genetic loci produce distinct carbohydrate-rich structural components of the Pseudomonas aeruginosa biofilm matrix. J. Bacteriol. 186:4457–4465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fux CA, Wilson S, Stoodley P. 2004. Detachment characteristics and oxacillin resistance of Staphylococcus aureus biofilm emboli in an in vitro catheter infection model. J. Bacteriol. 186:4486–4491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gjermansen M, Ragas P, Sternberg C, Molin S, Tolker-Nielsen T. 2005. Characterization of starvation-induced dispersion in Pseudomonas putida biofilms. Environ. Microbiol. 7:894–906 [DOI] [PubMed] [Google Scholar]
- 9. Reference deleted.
- 10. Hope CK, Wilson M. 2004. Analysis of the effects of chlorhexidine on oral biofilm vitality and structure based on viability profiling and an indicator of membrane integrity. Antimicrob. Agents Chemother. 48:1461–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Izano EA, Wang H, Ragunath C, Ramasubbu N, Kaplan JB. 2007. Detachment and killing of Aggregatibacter actinomycetemcomitans biofilms by dispersin B and SDS. J. Dent. Res. 86:618–622 [DOI] [PubMed] [Google Scholar]
- 12. Kaplan JB, Fine DH. 2002. Biofilm dispersal of Neisseria subflava and other phylogenetically diverse oral bacteria. Appl. Environ. Microbiol. 68:4943–4950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaplan JB. 2010. Biofilm dispersal: mechanisms, clinical implications, and potential therapeutic uses. J. Dent. Res. 89:205–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Karatan E, Watnick P. 2009. Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol. Mol. Biol. Rev. 73:310–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu C, Worthington RJ, Melander C, Wu H. 2011. A new small molecule specifically inhibits the cariogenic bacterium Streptococcus mutans in multispecies biofilms. Antimicrob. Agents Chemother. 55:2679–2687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Loesche WJ. 1986. Role of Streptococcus mutans in human dental decay. Microbiol. Rev. 50:353–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maeyama R, Mizunoe Y, Anderson JM, Tanaka M, Matsuda T. 2004. Confocal imaging of biofilm formation process using fluoroprobed Escherichia coli and fluoro-stained exopolysaccharide. J. Biomed. Mater. Res. A 70:274–282 [DOI] [PubMed] [Google Scholar]
- 18. Reference deleted.
- 19. Pereira-Cenci T, et al. 2008. The effect of Streptococcus mutans and Candida glabrata on Candida albicans biofilms formed on different surfaces. Arch. Oral Biol. 53:755–764 [DOI] [PubMed] [Google Scholar]
- 20. Rollet C, Gal L, Guzzo J. 2009. Biofilm-detached cells, a transition from a sessile to a planktonic phenotype: a comparative study of adhesion and physiological characteristics in Pseudomonas aeruginosa. FEMS Microbiol. Lett. 290:135–142 [DOI] [PubMed] [Google Scholar]
- 21. Shapiro JA. 1998. Thinking about bacterial populations as multicellular organisms. Annu. Rev. Microbiol. 52:81–104 [DOI] [PubMed] [Google Scholar]
- 22. Shen Y, Stojicic S, Haapasalo M. 2011. Antimicrobial efficacy of chlorhexidine against bacteria in biofilms at different stages of development. J. Endod. 37:657–661 [DOI] [PubMed] [Google Scholar]
- 23. Smith AW. 2005. Biofilms and antibiotic therapy: is there a role for combating bacterial resistance by the use of novel drug delivery systems? Adv. Drug Deliv. Rev. 57:1539–1550 [DOI] [PubMed] [Google Scholar]
- 24. Tolker-Nielsen T, et al. 2000. Development and dynamics of Pseudomonas sp. biofilms. J. Bacteriol. 182:6482–6489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Uppuluri P, et al. 2010. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog. 6:e1000828 doi:10.1371/journal.ppat.1000828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Uppuluri P, Srinivasan A, Ramasubramanian A, Lopez-Ribot JL. 2011. Effects of fluconazole, amphotericin B, and caspofungin on Candida albicans biofilms under conditions of flow and on biofilm dispersion. Antimicrob. Agents Chemother. 55:3591–3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wrangstadh M, Conway PL, Kjelleberg S. 1986. The production and release of an extracellular polysaccharide during starvation of a marine Pseudomonas sp. and the effect thereof on adhesion. Arch. Microbiol. 145:220–227 [DOI] [PubMed] [Google Scholar]
- 28. Xu X, Zhou XD, Wu CD. 2011. The tea catechin epigallocatechin gallate suppresses cariogenic virulence factors of Streptococcus mutans. Antimicrob. Agents Chemother. 55:1229–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]