LETTER
We briefly report on a patient with infective endocarditis (IE) due to Candida glabrata that was treated with a double dosage of anidulafungin. A 65-year-old woman was admitted on 28 May 2011 with fever and weight loss. Important comorbidities were hepatitis C virus (HCV)-related cirrhosis, pancytopenia due to spleen enlargement and previous episodes of autoimmune hemolytic anemia and thrombocytopenia, multiple cardiosurgeries during a 20-year period (in 1980, aortic and mitral valve substitution, in 2001, prosthetic mitral valve implanted for IE, and in 2005, bioprosthetic tricuspid valve implant and intravascular electrocatheters). In October 2010, the patient had a femoral fracture which needed a prosthetic implant; in December 2010, C. glabrata candidemia was treated with intravenous and oral voriconazole by the attending physician for 21 days in another hospital. No voriconazole plasma concentration study was performed at that time.
During the current admission, on 28 May 2011, transesophageal echocardiography (TEE) showed a vegetation 8 mm in length on the anterior medial corner and a vegetation of 9 by 13 mm on the posterolateral corner of the bioprosthetic tricuspid valve; empirical intravenous (i.v.) treatment was started by the cardiologists with 60 mg of gentamicin two times a day, 300 mg of rifampin three times a day, and 350 mg of vancomycin two times a day. All drawn blood cultures were positive for C. glabrata, and liposomal amphotericin B was administered and then switched for infusion intolerance to 100 mg of anidulafungin i.v. daily for 5 days and then 200 mg daily, according to the 2009 IDSA guidelines (5). Laboratory parameters were as follows: procalcitonin, 22.3 ng/ml; C-reactive protein, 38 mg/dl; fibrinogen, 297 mg/dl; hemoglobin, 9.7 g/dl; white blood cells, 2.27 × 109/liter; platelets, 51 × 109/liter; aspartate transaminase, 76 UI/liter; alanine transaminase, 46 UI/liter; gamma-glutamyl transferase, 246 UI/liter; and albumin, 2.7 g/dl.
In vitro C. glabrata sensitivities were determined with Sensitre YeastOne (Trek Diagnostic, Inc.) with the following results: amphotericin, 0.5 mg/liter; fluconazole, 8 mg/liter; caspofungin, 0.125 mg/liter; anidulafungin, 0.125 mg/liter; voriconazole, 0.125 mg/liter; and itraconazole, 0.5 mg/liter. Blood cultures were negative 72 h after the beginning of antifungal treatment. Anidulafungin at 200 mg daily was given for 2 weeks before cardiosurgery (3 to 17 June) and for 4 weeks thereafter, including 17 days in the intensive care unit due to a right lung hemorrhagic lesion which needed a further surgical approach.
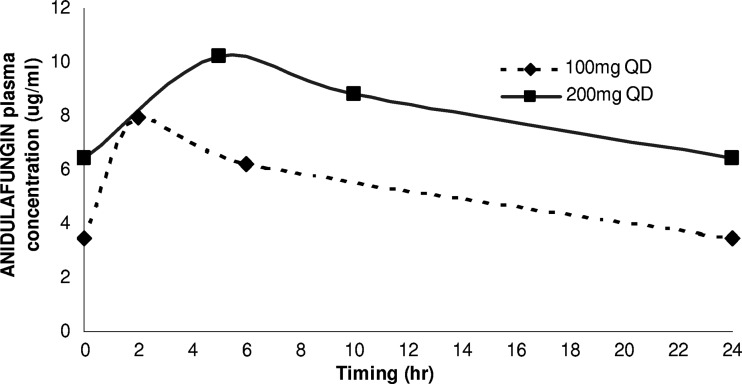
The plasma concentrations of anidulafungin were studied with both dosages and are illustrated in Fig. 1. Anidulafungin plasma concentrations were determined by a validated ultra-performance liquid chromatography–tandem mass spectrometry method (UPLC–MS-MS) (2). Chromatographic separation was performed using an Acquity UPLC HSS T3, 1.8-μm, 2.1- by 150-mm column (Waters, Milford, CT). The assay response was linear over the concentration range of 0.059 to 15 mg/liter with a lower limit of quantification of 0.059 mg/liter.
Fig 1.
Anidulafungin plasma concentration for standard (100 mg QD [once a day]) and double dosage (200 mg QD) intravenous administration.
The results of the monitoring of plasma concentrations are illustrated in Fig. 1. The maximum and minimum concentrations of drug in serum (Cmax and Cmin), Cmax/MIC ratio, and area under the concentration-time curve at 24 h (AUC24)/MIC ratio were 7.93 mg/liter versus 10.19 mg/liter, 3.46 mg/liter versus 6.41 mg/liter, 63.4 versus 81.5, and 1,012.0 versus 1,563.2 for the standard dosage and double dosage of anidulafungin, respectively. AUC values were as follows: 126.50 mg · h/liter versus 195.40 mg · h/liter. No side effects were observed; in particular, there were no infusional side effects nor further increase of the hepatic enzymes. The patient was discharged to rehabilitation on July 19, where treatment was continued with voriconazole. On October 2011, a new TEE showed no signs of IE.
According to the 2009 IDSA guidelines, echinocandins are preferred as the initial therapy for candidemia and should be administered for 2 weeks after the first negative blood culture (5). Anidulafungin has potent in vitro activity against sessile Candida cells within biofilms, and studies have clearly shown that the rate of Candida glabrata biofilm production is equal to that of Candida parapsilosis, typically isolated in central venous catheter-related candidemia (6, 7). The same guideline recommended with a BIII level of evidence a double daily dosage of an echinocandin for treatment of Candida IE, with evidence mostly based on case reports with caspofungin—data are limited on the safety and efficacy of double-dosage anidulafungin (5).
It has in fact been proposed that Candida IE may follow candidemia after as many as 240 days (153 days in our case), thus requiring appropriate treatment of candidemia, as well as precocious diagnostic and follow-up considerations for IE (3, 4), especially when prosthetic valves are present. Conventional treatment for IE by Candida spp. may be hampered by renal toxicity or failure or leukopenia, making a combination therapy with lipid formulation of amphotericin B and flucytosine difficult. Several studies showed how medical management of candidemia in patients with prosthetic valves probably needs prolonged courses of intravenous antifungals owing to the possibility of developing IE.
Specific literature breakpoints for anidulafungin for C. glabrata bloodstream infections and IE have not been clearly defined. According to EUCAST, the following parameters are probably predictive of efficacy: Cmax, 7 mg/liter; Cmin, 3 mg/liter; and AUC24, 110 mg · h/liter. Values for Cmax/MIC and AUC24/MIC ratios have not been established. In our case, pharmacokinetic parameters were achieved with both dosages of anidulafungin, which, according to Andes et al., seems to have a more linear kinetic profile of fungicidal activity against Candida spp., suggesting that the fungicidal activity may improve with increasing dosages (1). The effectiveness of 200 mg of anidulafungin daily is shown in our report by the early achievement of negative blood cultures without any side effect, even if the patient had serious comorbidities which may have altered pharmacokinetic parameters. However, we should remember that the definitive cure of such patients requires surgical and medical treatment.
In conclusion, we report the pharmacokinetic parameters of 200 mg of anidulafungin in a case of C. glabrata IE, successfully treated with surgery and a 6-week intravenous antifungal treatment followed by prolonged voriconazole administration. No side effects were recorded during the 6-week course, and there was no relapse by 11 months after the cardiosurgery.
Footnotes
Published ahead of print 29 May 2012
Contributor Information
S. Raviolo, Department of Infectious Diseases, University of Turin, Amedeo di Savoia Hospital, Turin, Italy
P. Centofanti, Ospedale S. Giovanni Battista—Molinette, Cardiosurgery Unit, University of Turin, Italy
D. Pasero, Ospedale S. Giovanni Battista—Molinette, Anesthesia and Critical Care Medicine Unit, University of Turin, Italy
M. Rinaldi, Ospedale S. Giovanni Battista—Molinette, Cardiosurgery Unit, University of Turin, Italy
G. Di Perri, Department of Infectious Diseases, University of Turin, Amedeo di Savoia Hospital, Turin, Italy
REFERENCES
- 1. Andes D, et al. 2010. In vivo comparison of the pharmacodynamic targets for echinocandin drugs against Candida species. Antimicrob. Agents Chemother. 54:2497–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Decosterd LA, et al. 2010. Multiplex ultra-performance liquid chromatography-tandem mass spectrometry method for simultaneous quantification in human plasma of fluconazole, itraconazole, hydroxyitraconazole, posaconazole, voriconazole, voriconazole-N-oxide, anidulafungin and caspofungin. Antimicrob. Agents Chemother. 54:5303–5315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Falcone M, et al. 2009. Candida infective endocarditis: report of 15 cases from a prospective multicenter study. Medicine 86:160–168 [DOI] [PubMed] [Google Scholar]
- 4. Nasser RM, Melgar GR, Longworth DL, Gordon SM. 1997. Incidence and risk factors of developing fungale prosthetic valve endocarditis after nosocomial candidemia. Am. J. Med. 103:25–32 [DOI] [PubMed] [Google Scholar]
- 5. Pappas PG, et al. 2009. Clinical practice guidelines for the management of candidiasis: update by the Infectious Disease Society of America. 2009. Clin. Infect. Dis. 48:503–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tumbarello M, et al. 2007. Biofilm production by Candida species and inadequate antifungal therapy as predictor of mortality in patients with candidemia. J. Clin. Microbiol. 45:1843–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Venditti M. 2009. Clinical aspects of invasive candidiasis: endocarditis and other localized infections. Drugs 69(Suppl 1):39–43 [DOI] [PubMed] [Google Scholar]