Abstract
Punta Toro virus (PTV; Bunyaviridae, Phlebovirus) is related to Rift Valley fever virus (RVFV), a pathogenic agent which causes severe disease in humans and livestock primarily in the sub-Saharan region of Africa. The recent range expansion of RVFV and the potential for its intentional release into naïve populations pose a significant threat to public health and agriculture. Studies modeling disease in rodents and nonhuman primates have shown that PTV and RVFV are highly sensitive to the antiviral effects of alpha interferon (IFN-α), an important component of the innate antiviral host response. While recombinant IFN-α has high therapeutic value, its utility for the treatment of neglected tropical diseases is hindered by its short in vivo half-life and costly production of longer-lasting pegylated IFNs. Here, we demonstrate extended preexposure protection against lethal PTV challenge following a single intranasal administration of DEF201, which is a replication-deficient human adenovirus type 5 vector engineered to constitutively express consensus IFN-α (cIFN-α) from transduced host cells. DEF201 was also efficacious when administered within 24 h as a postexposure countermeasure. Serum concentrations of cIFN-α could be detected as early as 8 h following treatment and persisted for more than 1 week. The prolonged antiphlebovirus prophylactic effect, low production costs, and ease of administration make DEF201 a promising agent for intervention during natural disease outbreaks and for countering possible bioterrorist acts.
INTRODUCTION
Rift Valley fever virus (RVFV; Bunyaviridae, Phlebovirus) has been the cause of numerous devastating epizootics throughout sub-Saharan Africa and, more recently, the Arabian Peninsula (3). It is a mosquito-borne virus that causes significant losses in livestock characterized by dramatic “abortion storms” resulting in near-complete mortality in newborn animals (5). RVFV transmission to humans occurs through the bites of infected mosquitoes or contact with tissue from infected animals. Because the virus is also infectious by the airborne route, it poses a potential bioterrorism threat, which is amplified by the fact that mosquitoes native to the United States can readily transmit RVFV and serve as vectors (24). Presently, there are no FDA-approved vaccines or antivirals to prevent or treat RVFV infection, which underscores the urgent need to develop new antiviral therapies.
Several reports suggest that RVFV is sensitive to the effects of alpha interferon (IFN-α) (15, 16, 18), a potent cytokine essential to the control of viral replication and dissemination (20). Studies employing the closely related Punta Toro virus (PTV), a less biohazardous, more accessible model for RVFV infection, have also demonstrated sensitivity toward agents that elicit type I IFN responses (8, 22). In addition, a recent report described the antiphlebovirus activity of human consensus IFN-α (cIFN-α) in cell culture and its prophylactic and therapeutic efficacy in hamsters challenged with PTV (10). Because recombinant IFN protein therapeutics are costly to manufacture and administer due to the requirement of frequent injections to maintain therapeutic levels and the high cost of pegylated IFNs, we conducted experiments to evaluate a strategy that involves the expression of cIFN-α from cells transduced by a replication-incompetent adenoviral vector, DEF201. This strategy has shown promise in yellow fever virus and arenavirus infection models (7, 12). Similarly, a DEF201 construct expressing mouse IFN-α has been successfully used to prevent and treat severe acute respiratory syndrome coronavirus (SARS-CoV), vaccinia, and Ebola virus infections in mouse models (13, 23, 25). The present study expands the spectrum of viral infections that can be effectively countered with a single dose of DEF201, examines the kinetics of cIFN-α expression following treatment, and demonstrates the long-lasting antiviral effects of DEF201 toward the prevention of phleboviral disease in a hamster model of RVFV infection.
MATERIALS AND METHODS
Ethics statement.
All animal procedures complied with USDA guidelines and were conducted at the AAALAC-accredited Laboratory Animal Research Center at Utah State University under protocol 1229, approved by the Utah State University Institutional Animal Care and Use Committee.
Animals.
Female golden Syrian hamsters were obtained from Charles River Laboratories (Wilmington, MA) and acclimated for a minimum of 6 days prior to experimentation. They were fed standard hamster chow and tap water ad libitum. Animals were 7 to 9 weeks old at the time of virus challenge.
Viruses.
PTV, Adames strain, was provided by Dominique Pifat of the U.S. Army Medical Research Institute for Infectious Diseases, Fort Detrick (Frederick, MD). The virus used was from a clarified hamster liver homogenate stock that was prepared following 4 passages of the original virus stock through LLC-MK2 rhesus monkey kidney cells and 1 passage in hamsters. DEF201 and the empty vector (EV) control virus were provided by Defyrus, Inc. (Toronto, ON, Canada).
Liver, spleen, and serum virus titers.
Virus titers were assayed using an infectious cell culture assay as described previously (11). Briefly, a specific volume of tissue homogenate or serum was serially diluted and added to triplicate wells of Vero 76 (African green monkey kidney) cell monolayers in 96-well microplates. The viral cytopathic effect (CPE) was determined 7 to 8 days post-virus inoculation, and the 50% endpoints were calculated using the Reed-Muench method (19). The assay detection ranges were 2.8 to 9.5 log10 50% cell culture infectious doses (CCID50)/g of tissue and 1.8 to 8.5 log10 CCID50/ml of serum. In samples presenting with undetectable virus titers, the lower limits of detection were assigned. Conversely, in cases wherein virus exceeded the detection range, the upper limits of detection were used.
Serum ALT determinations.
Detection of alanine aminotransferase (ALT) in serum is an indirect method for evaluating liver disease. Serum ALT concentrations were measured using the ALT (serum glutamic pyruvic transaminase [SGPT]) reagent set purchased from Pointe Scientific, Inc. (Lincoln Park, MI) per the manufacturer's recommendations. The reagent volumes were adjusted for analysis on 96-well microplates.
Hamster efficacy studies.
Hamsters were weighed on the morning of initial treatment or PTV challenge and grouped so that the average weight per group across the entire experiment varied by less than 5 g. Hamsters (n = 15 to 25/group) were anesthetized with isoflurane prior to treatment and received a single dose of 108, 107, or 106 PFU of DEF201, the EV control virus, or phosphate-buffered saline (PBS) vehicle by intranasal (i.n.) instillation (0.1 ml/nostril) at the indicated times relative to i.n. or subcutaneous (s.c.) challenge with PTV. In the initial experiment, ribavirin (ICN Pharmaceuticals, Inc., Costa Mesa, CA) was given once daily by intraperitoneal (i.p.) injection (0.1 ml) for 6 days beginning 4 h prior to PTV challenge. Up to five animals per group were sacrificed for evaluation of viral titers and liver disease on day 4 of infection, and the rest were observed for morbidity and mortality. Sham-infected animals (n = 6 to 8 per experiment) were included as normal controls. In several experiments, the surviving animals (including 5 naïve sham-infected controls) were rechallenged 4 weeks after the initial infection and observations continued out to 49 days relative to the time of the original PTV challenge.
In the initial experiment, animals anesthetized with isoflurane were challenged by i.n. instillation of 0.2 ml containing 5 × 103 PFU of PTV. Subsequent i.n. challenges were increased to 1.5 × 104 PFU to achieve higher mortality, viral titers, and liver disease in the placebo-treated animals. For the s.c. PTV challenge, 50 PFU was used as described previously (10). The 50% lethal dose by s.c. challenge is approximately 500-fold less compared to i.n. challenge. However, the natural history of disease does not differ substantially between the challenge routes (data not shown). Presumably, once the hepatotropic PTV goes systemic, it targets the liver and produces similar disease.
Longitudinal expression of cIFN-α.
Hamsters were sorted so that the average weight per group (n = 3) across the entire experiment varied by less than 5 g. Single dose i.n. treatments with 107 or 108 PFU of DEF201, 108 PFU of EV, or PBS placebo were administered on day 0 of the experiment. Serum was obtained through retro-orbital blood sampling of all animals at 2, 8, 24, and 48 h posttreatment, with additional samples collected at 4, 8, 16, and 32 days. Following each sample collection, 0.25 ml of Ringer's solution was injected subcutaneously for fluid replacement. All animals were observed for signs of illness and weighed individually every 3 days starting on day 0. Serum concentrations of cIFN-α were measured using human IFN-α enzyme-linked immunosorbent assay (ELISA) reagents from PBL (Piscataway, NJ) as specified by the manufacturer. The limit of detection for our analysis was 60 pg/ml.
Statistical analysis.
Kaplan-Meier survival plots and all statistical evaluations were done using Prism (GraphPad Software, CA). The Mantel-Cox log rank test was used for survival analysis. For comparing differences in viral titers and ALT concentrations, one-way analysis of variance (ANOVA) with Newman-Keuls posttest or the Kruskal-Wallis (two-tailed) test with the Dunn's posttest was performed based on Gaussian distribution of the data.
RESULTS
Prophylactic use of DEF201 to prevent PTV infection and disease.
The use of DEF201 was first examined in a prophylactic setting through i.n. dosing of hamsters with various PFU amounts 24 h prior to i.n. challenge with 5 × 103 PFU of PTV. The goal of this experiment was to evaluate the DEF201 technology in the hamster PTV respiratory route infection model of acute phleboviral disease, with the purpose of identifying the most appropriate dose for future studies and to demonstrate IFN-based specificity by including the EV construct for comparison. Remarkably, all tested doses of 106 to 108 PFU of DEF201 given prophylactically protected 100% of challenged animals, whereas 9 of 10 hamsters treated with the EV control succumbed to the infection. Although a lower-than-expected level of mortality was observed in the placebo group (55%), the protection afforded by DEF201 treatment was statistically significant (Fig. 1A). The positive-control ribavirin treatment resulted in hamsters succumbing during the later stages of the experiment with fewer survivors than normally seen. It is likely that once-a-day dosing, rather than twice daily, contributed to reduced ribavirin efficacy.
Fig 1.
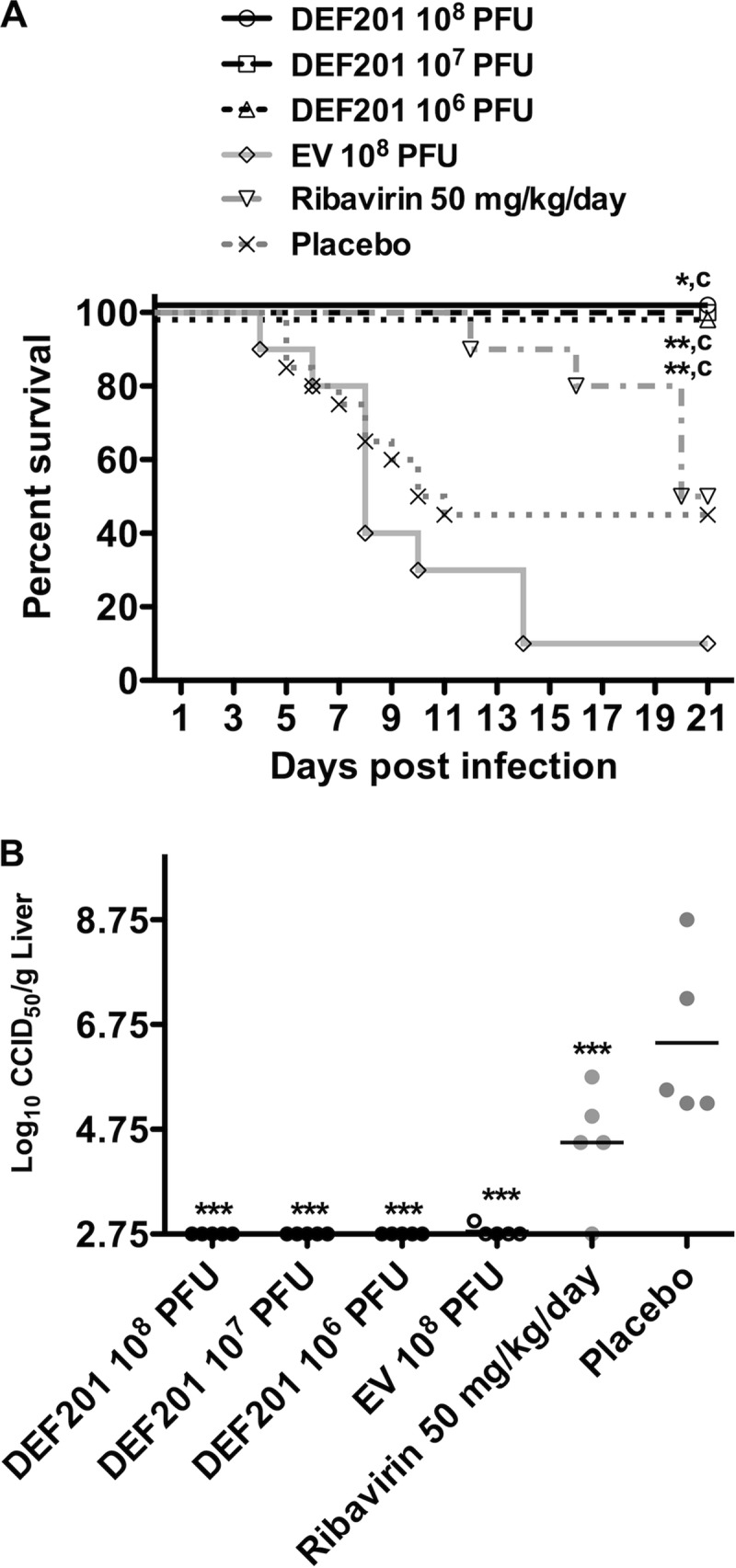
Prophylactic DEF201 protects PTV-infected hamsters from mortality and prevents viral replication in the liver. Animals were treated with a single i.n. instillation of the indicated dose of DEF201, the EV control virus, or PBS placebo 24 h prior to i.n. PTV infection with 5 × 103 PFU. Ribavirin treatment was administered i.p. once daily for 6 days starting 4 h prior to PTV infection. The effect of treatments on survival (n = 8 to 10 per group for DEF201, EV, and ribavirin; n = 20 for the placebo group) (A) and liver virus titers measured on day 4 of infection (B) are shown. *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared to results for PBS vehicle placebo-treated animals. c, P < 0.001 compared to results for EV-treated animals.
Due to the slower development of disease in the i.n. PTV challenge model, day-4 serum virus titers and ALT had not advanced sufficiently in the placebo group to allow for robust comparison with the DEF201 treatment groups (data not shown). In contrast, liver virus loads were well developed (5 to 8.5 log10 CCID50/g of tissue) in the placebo-treated animals, while those treated with DEF201 had undetectable levels of PTV (Fig. 1B). Notably, the EV control also dramatically reduced viral burden in the liver to the same degree as the DEF201 treatment, suggesting that the immune response triggered by the adenoviral vector alone was sufficient to delay viral replication; however, 90% of hamsters receiving EV still succumbed to the disease. Ribavirin significantly reduced liver virus titers by approximately 2 logs compared to the placebo but was less effective compared to the groups treated with DEF201 or EV (Fig. 1B). Consequently, ribavirin was not included in subsequent studies. Collectively, the data were very encouraging and demonstrated a robust protective effect associated with DEF201 preexposure prophylaxis. The EV construct appeared to delay the onset of disease, in that it effectively reduced virus titer development measured on day 4 postinfection, but did not protect the animals from mortality.
Longitudinal analysis of systemic cIFN-α following DEF201 treatment.
DEF201 treatment of hamsters 7 days prior to challenge with yellow fever virus and 14 days before Pichinde arenavirus (PICV) infection offered significant protection specific to the expression of cIFN since the EV control virus was not efficacious (7, 12). However, the magnitude and duration of cIFN-α levels present in the plasma following DEF201 administration were not investigated. To assist in the design of an experiment to evaluate the prophylactic treatment window for DEF201 in the PTV infection model, cIFN-α concentrations were measured in hamsters following a single i.n. dose. Because the ELISA used was designed for detection of human IFN-α, the measured values were specific to cIFN-α produced as a result of expression from the DEF201 construct in transduced hamster cells.
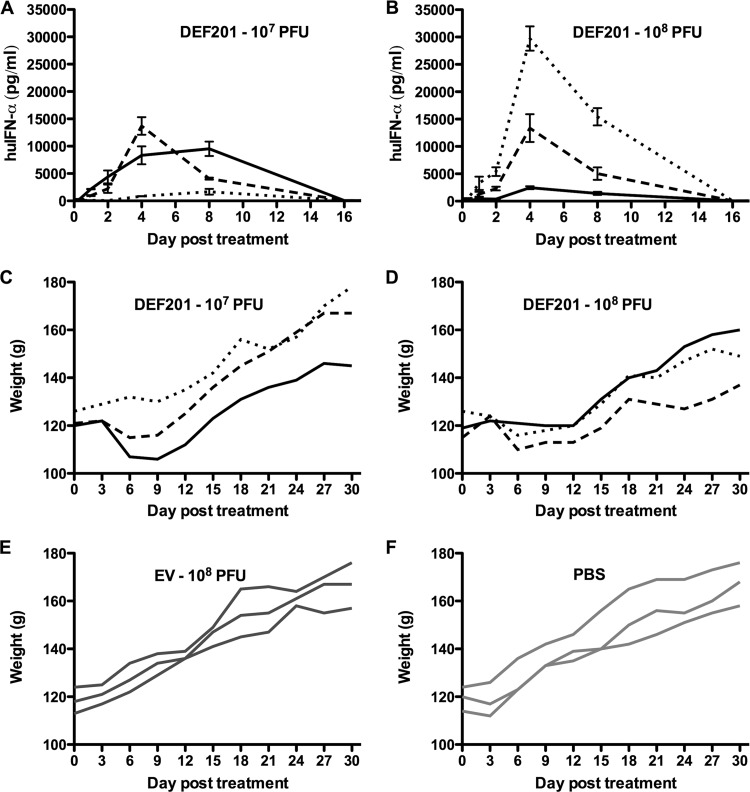
As shown in Fig. 2A and B, cIFN-α was detected in all DEF201-treated hamsters but not in the animals treated with the EV control virus or PBS placebo (data not shown). A measurable increase in cIFN-α concentration was detected as early as 8 h posttreatment in 1 of 3 animals treated with the 107 PFU dose of DEF201 (Fig. 2A) and in 2 of 3 animals treated with the 108 PFU dose (Fig. 2B). Regardless of the magnitude of cIFN-α detected on days 4 and 8, protein could not be detected at 16 days (Fig. 2A and B) or later (data not shown). Notably, there was considerable variability across the low- and high-dose DEF201 treatment groups ranging from peak levels of 1,600 to 30,000 pg/ml (Fig. 2A and B). Very high systemic concentrations of cIFN-α exceeding 5,000 pg/ml were evident in 4 of 6 DEF201-treated animals. Weight loss from day 3 through day 9 correlated with the expression of cIFN-α (Fig. 2C to F). Finally, peak cIFN-α expression levels were present on day 4 in the hamsters treated with high-dose DEF201 (Fig. 2B), while 2 of 3 animals in the low-dose group had higher concentrations at day 8 (Fig. 2A). The variation in cIFN-α serum concentrations, and production that likely continued from the construct well into the second week following i.n. administration, may explain the significant but reduced efficacy reported for hamsters pretreated 14 days prior to challenge with PICV (7).
Fig 2.
Longitudinal analysis of systemic cIFN-α in hamsters treated with DEF201. Hamsters (n = 3 per group) were treated with the indicated doses of DEF201, the EV control virus, or PBS. (A, B) Serum concentrations of cIFN-α were determined by ELISA (mean and standard deviation of replicate wells). (C to F) Body weights were measured every 3 days. Individual solid or hashed lines in each graph represent data obtained from individual animals. The values for the low-dose (A, C) and high-dose (B, D) DEF201-treated hamsters are linked (similarly formatted lines represent the same animal) to show correlation between high cIFN-α concentrations and weight loss. cIFN-α protein was undetectable in hamsters treated with the negative-control EV virus or PBS (data not shown).
Extended prophylaxis conferred by DEF201.
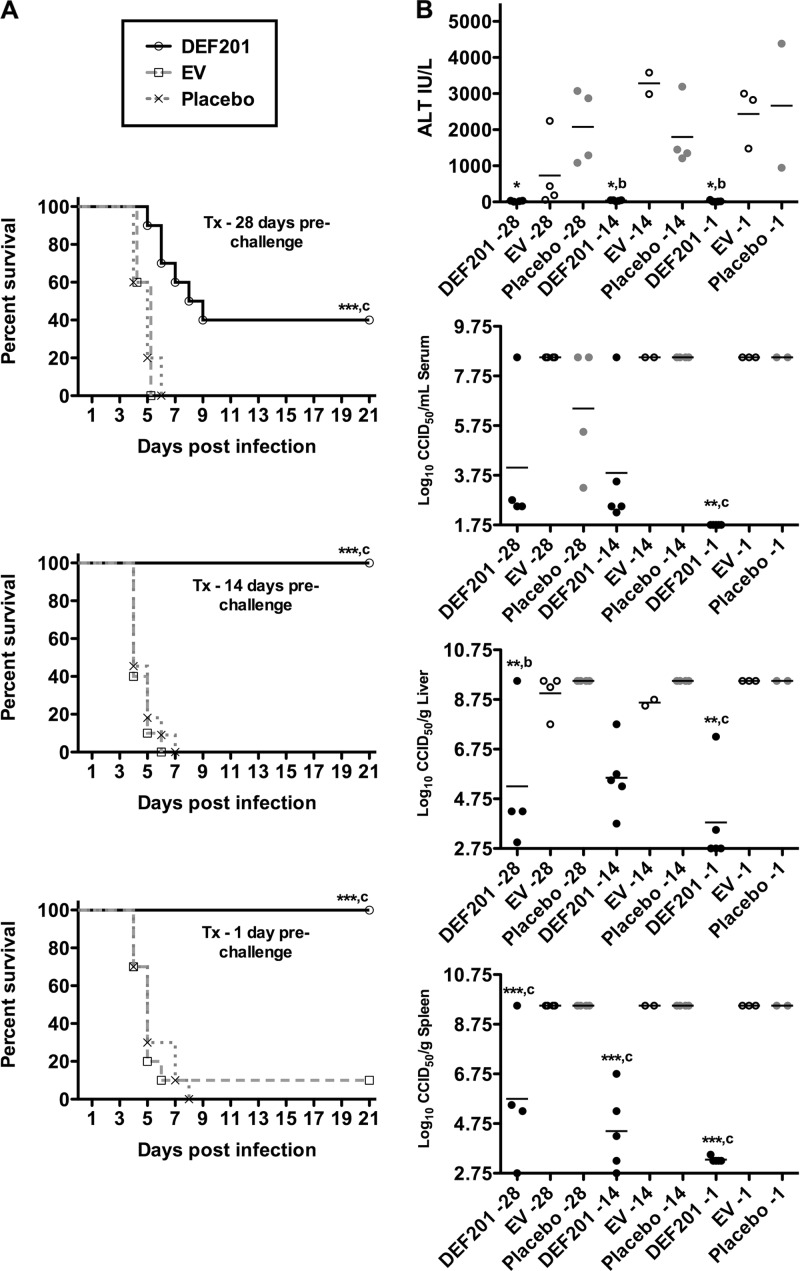
Previous data indicated that PTV infection was more sensitive to the effects of i.p. treatment with recombinant cIFN-α protein compared to PICV infection (6, 10). Thus, despite not being able to detect cIFN-α in the sera of hamsters 2 weeks beyond DEF201 treatment, we decided to investigate the efficacy of pretreatment out to 28 days prior to challenge with 1.5 × 104 PFU of PTV. Based on the previous two studies, we selected a dose of 107 PFU of DEF201 for the extended prophylaxis evaluation. Remarkably, when pretreated with a single i.n. dose of DEF201 4 weeks prior to i.n. challenge with PTV, significant protection was observed compared to hamsters treated with the EV control or PBS placebo (Fig. 3A, top panel). The challenge was 100% lethal in both the EV and placebo control groups, whereas 40% of the hamsters treated with DEF201 survived the infection, and those that did not survive, on average, succumbed 2 days later (DEF201, 6.8 ± 1.5; EV, 4.6 ± 0.5; placebo, 4.8 ± 0.8). When hamsters were exposed to DEF201 14 days and 1 day prior to challenge, the latter serving as a positive control, 100% efficacy was observed (Fig. 3A, bottom panels). In contrast, 39 of 40 (98%) animals in the EV and placebo control groups succumbed to the infection.
Fig 3.
DEF201 extended preexposure prophylaxis protects hamsters from lethal PTV challenge. Animals were treated i.n. with a single dose of 107 PFU of DEF201, the EV control virus, or PBS placebo 28, 14, or 1 day(s) prior to i.n. PTV infection with 1.5 × 104 PFU. The effect of the prophylactic treatments on survival (n = 10 to 11 animals per group) (A) and other disease parameters assessed on day 4 of infection (due to death prior to time of sacrifice, most groups had fewer than 5 animals per group) (B) are shown. *, P < 0.05; **, P < 0.01; ***, P < 0.001 compared to results for respective PBS vehicle placebo-treated animals. b, P < 0.01; c, P < 0.001 compared to results for respective EV-treated animals. Tx, treatment.
In addition to survival, liver disease (ALT) and serum, liver, and spleen virus titers were assessed as additional parameters to measure the extended prophylactic efficacy of DEF201. As shown in Fig. 3B, DEF201 pretreatment had a dramatic impact on all parameters, even when dosed 28 days prior to infection with PTV. With the exception of the ALT data for the day −28-treated animals, the effect was specific to DEF201, as the values for animals treated with the EV control were generally very similar to those for animals treated with the placebo. This observed reduction in serum ALT concentration was not evident in the EV 14- or 1-day pretreatment groups (Fig. 3B, top panel). Nevertheless, the day −28 EV ALT data did not statistically differ from the placebo group data. Due to the death of several animals in the EV and placebo groups prior to the time of sacrifice, mean viral titers and ALT levels are likely an underestimate of the disease severity, since the sickest animals in the affected groups were not included in the analysis.
Postexposure DEF201 prophylaxis and protection against subsequent PTV infection.
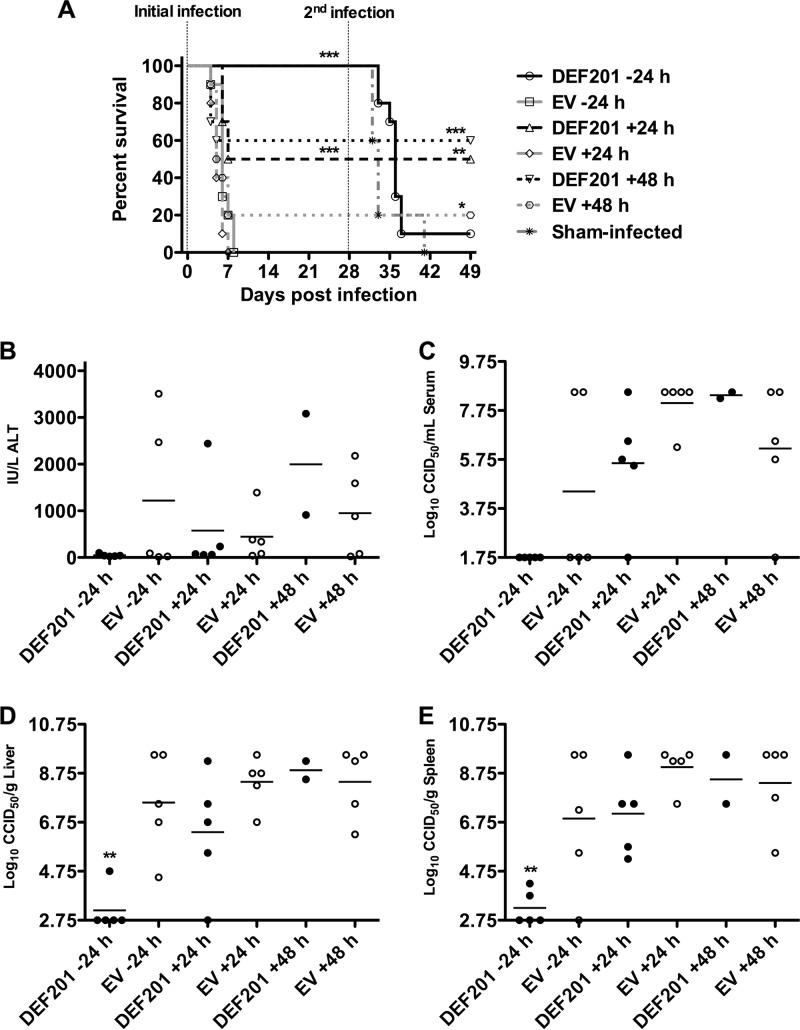
We have previously reported on effective post-PTV challenge treatment of hamsters with purified cIFN-α protein (10). Therefore, we next evaluated the therapeutic efficacy of 107 PFU of DEF201 in reducing disease burden and limiting mortality associated with both i.n. and s.c. PTV challenge. For comparison, 107 PFU of the EV control virus was evaluated in parallel as the negative control. When PTV (1.5 × 104 PFU) was inoculated by the i.n. route, statistically significant protection was achieved when dosing DEF201 within 24 h of challenge (Fig. 4A). Although the effectiveness of the treatment was not diminished when given at 48 h postinfection, several hamsters in the respective EV control group survived the challenge, thereby making the difference less dramatic. As expected, complete protection was observed with DEF201 administered 24 h prechallenge, which was included as a positive control. To assess longer-term protective immunity, we challenged survivors (including the sham-infected animals from the initial infection) to a second PTV infection. All the surviving animals that were treated therapeutically were immune to rechallenge when inoculated with the same lethal i.n. dose of PTV (Fig. 4A). In contrast, 9 of the 10 animals in the −24-h group succumbed to the second virus challenge.
Fig 4.
Postexposure DEF201 treatment protects hamsters challenged with PTV by the i.n. route. Animals were treated once i.n. with a dose of 107 PFU of DEF201, the EV control virus, or PBS placebo −24 h, +24, or + 48 h relative to the time of the initial infection. The vertical hashed lines represent the initial and second i.n. challenges of the hamsters with PTV (1.5 × 104 PFU) on day 0 and day 28 of the experiment. The effect of treatments on survival (n = 10 per treatment group; n = 5 in the sham-infected group) (A) and other disease parameters assessed on day 4 of infection (B) are shown. (A) For the initial infection, comparisons were made between DEF201-treated animals and corresponding EV-treated controls. For the second infection, groups with survivors were compared to the sham-infected animals, which were challenged with PTV on day 28. (B) Three of the five animals in the DEF201 +48 h group succumbed prior to time of sacrifice. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
The analysis of viral titers on day 4 of infection, for animals that were PTV infected and DEF201 treated in parallel, was representative of the survival data and supports the above assertion that viral replication is essential to eliciting protective immunity to rechallenge. Differences in ALT levels were not significant (Fig. 4B). As shown in Fig. 4C to E, viral replication in hamsters infected with PTV by the i.n. route was undetectable in the sera taken from the hamsters pretreated with DEF201, and only very low titers were present in liver and spleen samples of 20 to 40% of the animals. This inhibition of systemic viral replication correlated with the lack of protective immunity when the hamsters were rechallenged. On the other hand, the majority of the animals in the 24- and 48-h postexposure DEF201 treatment groups had measurable viral burden in tissues and serum (Fig. 4C to E). Thus, as observed with the PICV hamster infection model (7), the data suggest that an undefined level of viral replication appears to be necessary to elicit an adaptive immune response that confers protective immunity to reinfection.
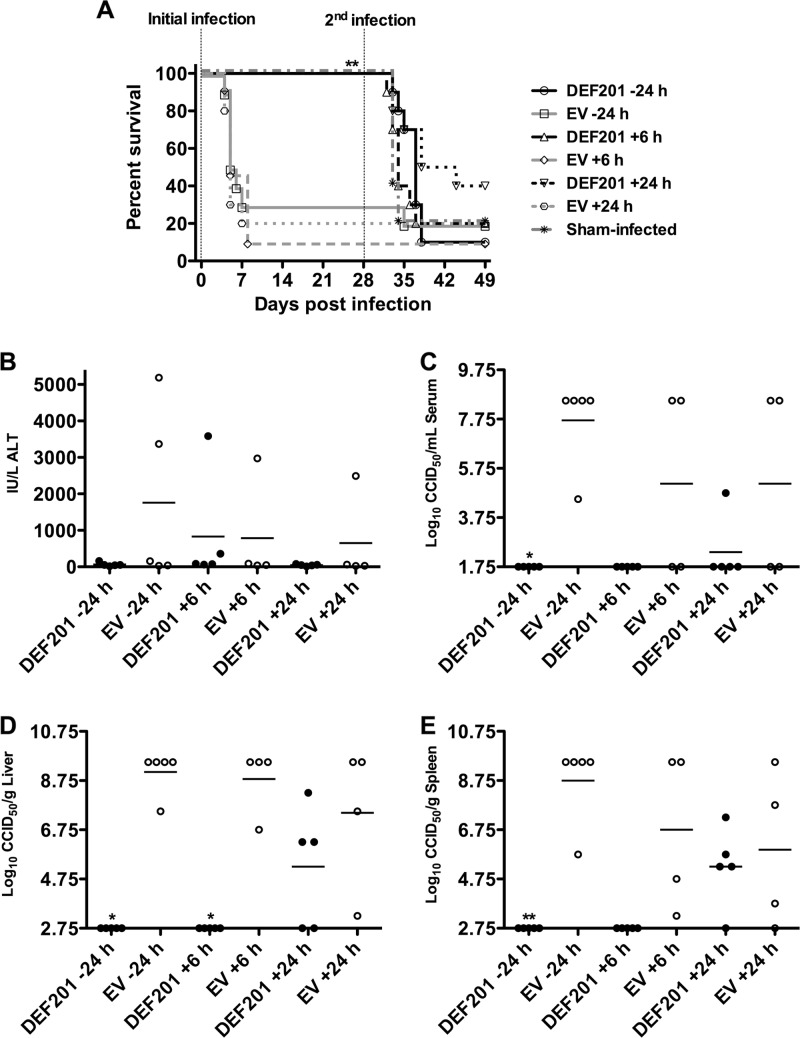
Because we expected s.c. challenge of hamsters with PTV (50 PFU) to be more aggressive compared to i.n. infection, we adjusted the timing of postexposure prophylaxis to 6 and 24 h postchallenge. In addition to the 24-h pretreatment, complete protection was seen at both the 6- and 24-h postchallenge treatment times (Fig. 5A). Unlike in the i.n. infection, the protective immunity to rechallenge elicited following postexposure treatment with DEF201 was limited in the s.c. challenge model. This may be explained in part by the fact that the s.c. challenge was not as aggressive as the i.n. challenge, with more of the EV-treated animals surviving the infection. Consequently, the DEF201 treatments largely controlled viral replication reflected in the virus titers in the serum and tissues, thereby preventing the development of protective immunity against rechallenge (Fig. 5). This effect is reflected in the survival results following the second s.c. challenge, wherein 5 of 6 (83%) of the surviving animals that received the EV control were protected, whereas only 7 of 30 (23%) hamsters treated with DEF201 survived the rechallenge. Interestingly, ALT levels in the EV-treated animals were not significantly elevated compared to those in the respective DEF201 groups (Fig. 4B and 5B). This may be due to low-level immunity stimulated by the EV control virus.
Fig 5.
Postexposure DEF201 treatment protects hamsters challenged with PTV by the s.c. route. Animals were treated once i.n. with a dose of 107 PFU of DEF201, the EV control virus, or PBS placebo −24 h, +6, or +24 h relative to the time of the initial infection. The vertical hashed lines represent the initial and second s.c. challenges of the hamsters with PTV (50 PFU) on day 0 and day 28 of the experiment. The effect of treatments on survival (n = 10 to 11 per treatment group; n = 5 in the sham-infected group) (A) and other disease parameters assessed on day 4 of infection (B) are shown. (A) For the initial infection, comparisons were made between DEF201-treated animals and corresponding EV-treated controls. For the second infection, groups with survivors were compared to the sham-infected animals, which were challenged with PTV on day 28. (B) One of the five animals in the EV +6 h and +24 h groups succumbed prior to time of sacrifice. *, P < 0.05; **, P < 0.01.
DISCUSSION
RVFV is a NIAID category A pathogen with dual select agent status (CDC and USDA) requiring effective interventions to prevent and treat RVF disease during natural outbreaks, laboratory exposure, or possible intentional release. As PTV infection in hamsters serves as a model for RVF (9), demonstration of preexposure prophylaxis in the i.n. challenge model would provide valuable insight into the prophylactic capacity of DEF201 as a possible countermeasure against acute phleboviral disease. The hamster PTV s.c. challenge model has been shown to respond well to cIFN-α protein administered through daily i.p. injections (10). Thus, it was hypothesized that in situ production of cIFN-α from the DEF201 construct would offer protection against PTV respiratory route challenge in hamsters. Compared to recombinant bacterially derived cIFN-α protein, DEF201 use offers distinct advantages that include (i) low manufacturing costs, (ii) enhanced stability, (iii) ease of administration, (iv) single dosing, and (v) native glycosylation, as seen for other proteins (1, 14).
Our data indicate that duration of cIFN-α expression following i.n. treatment of hamsters was longer than 8 days but less than 16 days and systemic levels varied in magnitude. These differences are believed to be associated with the inherent variability of the i.n. dosing process. Because cIFN-α was not detectable in the serum at 16 days in any of the DEF201-treated hamsters, the long-lasting anti-PTV prophylactic effect of DEF201 pretreatment is likely due to the establishment of an antiviral state characterized by the induction of IFN-stimulated genes (ISGs) and related pathways (4, 21). The detection of cIFN-α protein in the blood as early as 8 h post-DEF201 treatment, and the observed efficacy in protecting animals from lethal PTV infection when given within 24 h postchallenge, suggests that there may be great value in using DEF201 as a rapid-acting postexposure countermeasure. The steadier level of expression from DEF201-transduced cells would likely be preferable to the “bolus effect” that occurs with dosing of recombinant IFN protein drugs.
Weight loss in hamsters consistent with malaise generally correlated with the level of cIFN-α present in the serum. Thus, careful clinical evaluation is necessary to determine whether significant malaise may arise from DEF201 dosing in humans. Given the transient peak of high cIFN-α protein following DEF201 administration, no long-term effects due to the well-known toxicity profile of interferon would be expected. Preclinical safety/toxicity studies are under way to confirm this. Although we would expect hamsters to develop an immune response toward the adenovirus-vectored human cIFN-α protein, the occurrence of human antibodies directed at cIFN-α should be minimal, as the natively glycosylated protein is substantially similar to IFN-α produced endogenously.
Taken together, the data obtained are encouraging, particularly if they ultimately translate to the prevention of RVFV infections in humans and agriculturally important livestock. The present study builds upon previous findings in hamster models of viral diseases of biodefense interest demonstrating dramatic efficacy of DEF201 when dosed i.n. (7, 12). Importantly, the data support the idea that DEF201 may be effective in reducing RVF burden by administration of a single dose to at-risk individuals or livestock every 2 to 3 weeks during a natural outbreak, which may be predicted based on satellite measurements of global and regional sea surface temperatures, rainfall, and vegetation index data (2). Moreover, the respiratory route challenge simulates inhalation exposure, which could occur due to intentional release of the virus or through processing of infected livestock (17). Future studies exploring the effectiveness of DEF201 against RVFV challenge in rodents as well as vaccine enhancement/dose-sparing capabilities should be pursued.
ACKNOWLEDGMENTS
This work was supported by funding from the National Institutes of Health (contract HHSN272201000039I and grant AI-30063).
J.E. and J.D.T. are employed by Defyrus Inc., the manufacturer of DEF201.
Footnotes
Published ahead of print 21 May 2012
REFERENCES
- 1. Alkhatib G, Richardson C, Shen SH. 1990. Intracellular processing, glycosylation, and cell-surface expression of the measles virus fusion protein (F) encoded by a recombinant adenovirus. Virology 175:262–270 [DOI] [PubMed] [Google Scholar]
- 2. Anyamba A, et al. 2010. Prediction, assessment of the Rift Valley fever activity in East and Southern Africa 2006-2008 and possible vector control strategies. Am. J. Trop. Med. Hyg. 83:43–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bird BH, Ksiazek TG, Nichol ST, Maclachlan NJ. 2009. Rift Valley fever virus. J. Am. Vet. Med. Assoc. 234:883–893 [DOI] [PubMed] [Google Scholar]
- 4. Borden EC, Williams BR. 2011. Interferon-stimulated genes and their protein products: what and how? J. Interferon Cytokine Res. 31:1–4 [DOI] [PubMed] [Google Scholar]
- 5. Gerdes GH. 2002. Rift Valley fever. Vet. Clin. North Am. Food Anim. Pract. 18:549–555 [DOI] [PubMed] [Google Scholar]
- 6. Gowen BB, et al. 2005. Interferon alfacon-1 protects hamsters from lethal Pichinde virus infection. Antimicrob. Agents Chemother. 49:2378–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gowen BB, et al. 2011. Use of recombinant adenovirus vectored consensus IFN-α to avert severe arenavirus infection. PLoS One 6:e26072 doi:10.1371/journal.pone.0026072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gowen BB, et al. 2009. Prophylaxis with cationic liposome-DNA complexes protects hamsters from phleboviral disease: importance of liposomal delivery and CpG motifs. Antiviral Res. 81:37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gowen BB, Holbrook MR. 2008. Animal models of highly pathogenic RNA viral infections: hemorrhagic fever viruses. Antiviral Res. 78:79–90 [DOI] [PubMed] [Google Scholar]
- 10. Gowen BB, Wong MH, Jung KH, Blatt LM, Sidwell RW. 2008. Prophylactic and therapeutic intervention of Punta Toro virus (Phlebovirus, Bunyaviridae) infection in hamsters with interferon alfacon-1. Antiviral Res. 77:215–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gowen BB, et al. 2007. In vitro and in vivo activities of T-705 against arenavirus and bunyavirus infections. Antimicrob. Agents Chemother. 51:3168–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Julander JG, Ennis J, Turner J, Morrey JD. 2011. Treatment of yellow fever virus with an adenovirus-vectored interferon, DEF201, in a hamster model. Antimicrob. Agents Chemother. 55:2067–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kumaki Y, et al. 2011. Single-dose intranasal administration with mDEF201 (adenovirus vectored mouse interferon-alpha) confers protection from mortality in a lethal SARS-CoV BALB/c mouse model. Antiviral Res. 89:75–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marshall GS, Fenger DP, Stout GG, Knights ME, Hunt LA. 1996. Processing of human cytomegalovirus glycoprotein B in recombinant adenovirus-infected cells. J. Gen. Virol. 77:1549–1557 [DOI] [PubMed] [Google Scholar]
- 15. Morrill JC, Jennings GB, Cosgriff TM, Gibbs PH, Peters CJ. 1989. Prevention of Rift Valley fever in rhesus monkeys with interferon-alpha. Rev. Infect. Dis. 11(Suppl 4):S815–S825 [DOI] [PubMed] [Google Scholar]
- 16. Morrill JC, et al. 1990. Pathogenesis of Rift Valley fever in rhesus monkeys: role of interferon response. Arch. Virol. 110:195–212 [DOI] [PubMed] [Google Scholar]
- 17. NIAID 2002. Counter-bioterrorism research agenda of the National Institute of Allergy and Infectious Diseases (NIAID) for CDC category A agents. NIAID, Bethesda, MD [Google Scholar]
- 18. Peters CJ, Reynolds JA, Slone TW, Jones DE, Stephen EL. 1986. Prophylaxis of Rift Valley fever with antiviral drugs, immune serum, an interferon inducer, and a macrophage activator. Antiviral Res. 6:285–297 [DOI] [PubMed] [Google Scholar]
- 19. Reed LJ, Muench H. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. (Lond.) 27:493–497 [Google Scholar]
- 20. Samuel CE. 2001. Antiviral actions of interferons. Clin. Microbiol. Rev. 14:778–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sen GC. 2001. Viruses and interferons. Annu. Rev. Microbiol. 55:255–281 [DOI] [PubMed] [Google Scholar]
- 22. Sidwell RW, et al. 1994. Antiviral and immunomodulating inhibitors of experimentally induced Punta Toro virus infections. Antiviral Res. 25:105–122 [DOI] [PubMed] [Google Scholar]
- 23. Smee DF, Wong MH, Russell A, Ennis J, Turner JD. 2011. Therapy and long-term prophylaxis of vaccinia virus respiratory infections in mice with an adenovirus-vectored interferon alpha (mDEF201). PLoS One 6:e26330 doi:10.1371/journal.pone.0026330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weaver SC, Reisen WK. 2010. Present and future arboviral threats. Antiviral Res. 85:328–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wong G, et al. 2011. Evaluation of different strategies for post-exposure treatment of Ebola virus infection in rodents. J. Bioterr. Biodef. S1:007. doi:10.4172/2157–2526.S1-007 [DOI] [PMC free article] [PubMed] [Google Scholar]