Abstract
Cardiac toxicity may be associated with drugs used for malaria. Torsades de pointes (TdP) is a well-known adverse effect of quinidine when used for atrial fibrillation. Intravenous quinidine doses for resistant malaria are 2 to 3 times higher than those used for arrhythmias. Among 6 patients receiving quinidine for malaria or babesiosis, 4 developed QT interval prolongation and 2 experienced TdP. Clinicians should be aware that recommended doses of quinidine for malaria carry a high TdP risk.
TEXT
Cardiac toxicity may occur in association with drugs used for the treatment of malaria (5, 10). Quinidine-associated syncope was described nearly 100 years ago (15) and is due to QT interval prolongation and the life-threatening arrhythmia torsades de pointes (TdP) (2), which occurs in up to 8% of quinidine-treated patients (22). TdP is a polymorphic ventricular tachycardia associated with QT interval prolongation and appears on the electrocardiogram (ECG) as continuous “twisting of the pointes” of the QRS complex around the isoelectric baseline (24). TdP can degenerate into ventricular fibrillation, leading to sudden cardiac death.
In large part due to the risk of TdP, quinidine is no longer recommended for management of atrial fibrillation (AF) (6, 12–14, 25). However, intravenous (i.v.) quinidine gluconate is recommended by the Centers for Disease Control and Prevention (CDC) for treatment of resistant falciparum malaria (http://www.cdc.gov/malaria/diagnosis_treatment/clinicians3.html; accessed 9 April 2012) at doses that are 2 to 3 times higher than those that were used for AF (1), and it is also sometimes used for the management of resistant babesiosis (23). The CDC recommends a loading dose of 10 mg/kg of body weight quinidine gluconate over 1 to 2 h followed by a continuous infusion of 0.02 mg/kg/min or, alternatively, 24 mg/kg quinidine gluconate over 4 h followed by 12 mg/kg every 8 h (q8h). Even more conservative i.v. quinidine gluconate doses (10-mg/kg loading dose over 4 h, and then repeated every 8 h) prolong corrected QT (QTc) intervals by 24% ± 8% in patients with severe malaria (26). Few data exist regarding the proarrhythmic potential of i.v. quinidine using the aggressive CDC-recommended doses. In this case series, the risk of QT interval prolongation and TdP was assessed in patients receiving i.v. quinidine gluconate for babesiosis or resistant malaria using CDC-recommended dosing regimens.
The medical records of all patients receiving i.v. quinidine for treatment of severe malaria or babesiosis at a large urban multihospital system from January 2004 to December 2010 were reviewed. Data, including comorbid conditions, concurrent medications, diagnostic tests, and daily progress notes, were reviewed. All patients underwent 12-lead electrocardiograms (ECGs) within 48 h prior to i.v. quinidine and at least every 12 h during infusion. QT intervals were measured manually from the earliest QRS deflection to the end of the T wave in all leads by an investigator (H.W.) and were corrected for heart rate using Bazett's formula (3). Bazett's QT interval correction is associated with limitations, including overcorrection of QT intervals at more rapid heart rates (17). For this reason, other correction factors, such as the Fridericia correction (11), are often used in research studies (7). However, we report Bazett's-corrected QT intervals in this case series since the Bazett's correction is that which is uniformly used in clinical practice. QT and RR intervals were averaged over three consecutive complexes.
Six patients received i.v. quinidine (n = 5 for resistant malaria, and n = 1 for babesiosis). All patients underwent continuous telemetry or bedside ECG monitoring. Four (66.7%) patients developed QTc interval prolongation of ≥60 ms from baseline or ≥500 ms. Two patients (33.3%) experienced TdP. Characteristics of the patients are presented in Table 1. Comparisons of QTc intervals in the 6 patients are presented in Fig. 1. Plasma quinidine concentrations were not measured in any of these patients; monitoring of plasma quinidine concentrations is not recommended in the CDC guidelines for treatment of malaria. Monitoring of serum electrolyte concentrations and other laboratory values was performed as per routine clinical care, but the frequency of monitoring was not increased during quinidine therapy.
Table 1.
Summary of intravenous quinidine doses administered and risk factors for the development of torsades de pointesa
| Parameter | Result for patient no.: |
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Loading dose (mg/kg) | 6 | 20 | 10 | 10 | 0 | 10 |
| Infusion rate (mg/kg/h) | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| Total dose received (mg/kg) | 7.0 | 20.1 | 10.1 | 10.4 | 1.5 | 10.2 |
| Duration of therapy (h) | 48 | 7 | 11 | 22 | 75 | 9 |
| Baseline QTc (ms) | 465 | 433 | 602 | 498 | 428 | 451 |
| Maximum QTc (ms) | 531 | 543 | 704 | 550 | 450 | 473 |
| TdP | No | No | Yes | Yes | No | No |
| Advanced age (≥65 yr) | Yes (73 yr) | No | No | No | No | No |
| Sex (female) | Yes | No | Yes | Yes | Yes | No |
| Hypokalemia (K+ < 3.5 meq/liter) | No | Yes | Yes | No | Yes | No |
| Hypomagnesemia (Mg2+ < 1.8 mg/dl) | No | No | No | Yes | No | No |
| Hypocalcemia (Ca2+ < 7.6 mg/dl) | Yes | Yes | Yes | No | No | No |
| History of left ventricular dysfunction | No | No | No | Yes | No | No |
| Acute liver dysfunction | Yes | No | Yes | Yes | No | Yes |
| Concomitant administration of QTc-prolonging medication | No | No | No | Yes | No | No |
| Total no. of risk factors for drug-induced TdP | 3 | 2 | 4 | 5 | 2 | 0 |
Risk factors for torsades de pointes (TdP) included age of ≥65 years, female sex, hypokalemia at the time of quinidine administration, hypomagnesemia at the time of quinidine administration, hypocalcemia at the time of quinidine administration, documented history of left ventricular dysfunction, concomitant administration of QT interval-prolonging medications, and baseline QTc interval of ≥470 ms in males and ≥480 ms in females.
Fig 1.
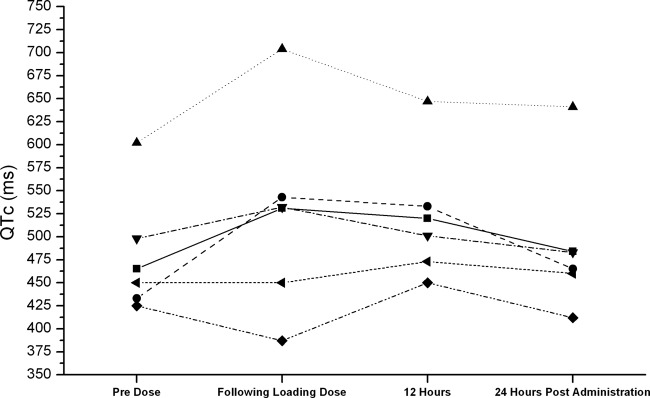
QTc intervals in 6 patients who received intravenous quinidine for management of resistant malaria or babesiosis. ■, patient 1; ●, patient 2; ▲, patient 3; ▼, patient 4; ♦, patient 5; ◀, patient 6. Note that patients 3 and 4 developed torsades de pointes.
Case 1 was a 73-year-old female with a history of orthotopic liver transplant who was transferred from another hospital with a 2-week history of chills, progressive jaundice, acute kidney injury, and possible urinary tract infection. Prior to initial hospital admission, she was taking 40 mg tacrolimus orally once daily and 80 mg propranolol orally once daily. The following medications had been initiated at the previous hospital and were continued: tacrolimus, 40 mg orally once daily; propranolol, 80 mg orally once daily; clindamycin, 900 mg i.v. every 8 h (q8h); esomeprazole, 40 mg orally once daily; trimethoprim-sulfamethoxazole, 160 mg/800 mg orally twice daily; and ursodiol, 250 mg orally q6h. New medications that were initiated were vancomycin at 750 mg i.v. q12h and piperacillin-tazobactam at 3.375 g i.v. q6h. A peripheral blood smear showed babesiosis, with 45 to 50% parasitemia. Upon admission, the heart rate was 62 beats per minute (bpm), the blood tacrolimus concentration was 10 ng/ml (therapeutic range, 5 to 15 ng/ml), and the QTc interval was 465 ms.
On day 1, 500 mg i.v. meropenem q8h was initiated, and the patient received two i.v. loading doses of quinidine gluconate, administered over 1 to 2 h and separated by 4 h, for a total loading dose of 800 mg (6 mg/kg). Following the second quinidine loading dose, ECG revealed a prolonged QTc interval of 531 ms. A continuous quinidine gluconate infusion (0.02 mg/kg/min) was initiated. She then became hypotensive, showed bradycardia following an exchange transfusion, and became unresponsive. Following intubation and treatment with vasopressors and continuous renal replacement therapy, her condition further deteriorated, and she developed pulseless electrical activity with bradycardia. Advanced cardiac life support was initiated, and she continued to have multiple episodes of pulseless electrical activity over the next 24 h which ultimately did not respond to resuscitation. After 68 h in the hospital, she expired from severe sepsis with multiorgan failure due to babesiosis. This patient was receiving tacrolimus on admission, which may have contributed to her admitting QTc interval of 465 ms (16); however, the only new QTc interval-prolonging medication that was initiated following admission was quinidine.
Case 2 was a 16-year-old male who was diagnosed with severe falciparum malaria after international travel. He was treated with 650 mg acetaminophen orally as needed (received four doses), 100 mg i.v. doxycycline q12h, and with an i.v. loading dose of 2,400 mg (20 mg/kg) quinidine gluconate, followed by a continuous quinidine gluconate infusion (0.02 mg/kg/min). The baseline QTc interval was 433 ms, and his heart rate was 104 bpm. Ten hours after initiation of quinidine, his heart rate was 84 bpm and his QTc interval was prolonged to 543 ms, at which time he was also hypocalcemic. Quinidine was discontinued, and he received oral quinine and i.v. doxycycline for 5 days. The QTc interval returned to baseline by hospital day 3. He was discharged to home after 7 days.
Case 3 was a 39-year-old female who was transferred from another hospital for severe malaria, which had been treated with oral doxycycline and quinine, which she tolerated poorly. Total serum bilirubin and transaminase concentrations were elevated. Upon transfer, doxycycline and quinine were discontinued, and she received 800 mg (10 mg/kg) quinidine gluconate i.v. followed by a continuous infusion (0.02 mg/kg/min). Her admission QTc interval was 602 ms, and her heart rate was 120 bpm. Twelve hours after initiation of quinidine, she developed TdP, which was treated with 4 g i.v. magnesium sulfate, restoring sinus rhythm. Prior to development of TdP, her QTc interval was 704 ms and heart rate was 98 bpm, and she was hypocalcemic (6.0 mg/dl) and hypokalemic (3.2 meq/liter). Quinidine was discontinued, and she was treated with 524 mg oral quinine three times daily for 4 days and 100 mg i.v. doxycycline q12h for 7 days. Daily ECGs revealed gradual normalization of QTc interval to 465 ms, and she developed no further arrhythmias. She was discharged to home on hospital day 11. It is possible that quinine may have also contributed to the admitting QTc interval of 602 ms and subsequent TdP in this case. Although rare, quinine has been associated with QT interval prolongation (8). There are only 3 reported cases of quinine-associated TdP in the literature (4, 19, 21), one of which occurred in the setting of a quinine overdose (4), and one of which occurred in the setting of digitalis toxicity (21). One of the published cases of quinine toxicity occurred in a patient receiving concomitant therapy with another QT interval-prolonging drug (astemizole) (19). However, in this patient, discontinuation of quinidine and reinitiation of therapy with quinine resulted in normalization of the QTc interval. Therefore, while contributing effects of quinine cannot be absolutely ruled out, quinidine was likely the predominant factor in this patient's TdP.
Case 4 was a 61-year-old white female with a history of end-stage kidney disease, gastrointestinal bleed secondary to angiodysplasia, thrombocytopenia, heart failure (left ventricular ejection fraction of approximately 30%), and diabetes mellitus who began experiencing nausea the day prior to presentation while undergoing hemodialysis. She was diagnosed with falciparum malaria based on a peripheral blood smear and was treated with i.v. fluids and vasopressors, and the following medications were initiated: ceftazidime, 1 g i.v. q12h; vancomycin, 1 g i.v. q12h; clindamycin, 900 mg i.v. q8h; enoxaparin, 40 mg subcutaneously (s.c.) daily; esomeprazole, 40 mg i.v. daily; sevelamer, 800 mg orally q8h; and simvastatin, 40 mg orally once daily. She received an i.v. loading dose of quinidine gluconate at 650 mg (10 mg/kg) followed by a continuous infusion (0.02 mg/kg/min). Her baseline QTc interval was 498 ms. She also presented with hyperbilirubinemia and hypomagnesemia, which was treated with i.v. magnesium sulfate. Her QTc interval was further prolonged to 550 ms within 12 h of initiation of quinidine. The quinidine gluconate infusion was discontinued after 48 h, and she was transitioned to oral quinine without further issues. Approximately 24 h after discontinuation of i.v. quinidine, the patient developed TdP, which converted to sinus rhythm after treatment with 4 g i.v. magnesium sulfate. Over the next 48 h, her severe sepsis due to falciparum malaria progressed, and she expired due to multiorgan failure. It is possible that quinine may have contributed to TdP in this case, although, as discussed above, quinine-associated QTc interval prolongation and TdP appear to be uncommon.
Cases 5 and 6 did not develop QTc interval prolongation. The first was a 58-year-old female with severe malaria who received a continuous infusion of quinidine gluconate (0.02 mg/kg/min) over 5 days (total of 10.76 mg). Her maximum QTc interval during infusion was 450 ms (38 ms increase from baseline). As her condition improved, she was transitioned to oral quinine for an additional 6 days. Additional medications included ampicillin, doxycycline, famotidine, vancomycin, phenytoin, levetiracetam, and levothyroxine. The second patient was a 40-year-old male with severe malaria who received an i.v. quinidine gluconate loading dose of 850 mg (10 mg/kg) followed by an infusion (0.02 mg/kg/min) for 13 h. Quinidine was discontinued as the patient stabilized, and he was transitioned to oral quinine. His maximum QTc interval was 473 ms (22-ms increase from baseline). Additional medications included doxycycline, ranitidine, ceftriaxone, and fentanyl. Both patients were discharged to home with no additional complications.
Primarily due to its propensity to provoke TdP (2, 15), quinidine is no longer recommended for management of AF (6, 12–14, 25). However, high doses of i.v. quinidine gluconate are recommended by the CDC for management of resistant falciparum malaria and have been used for the treatment of resistant babesiosis infections (23). Of six patients who received high doses of i.v. quinidine gluconate, four developed QTc interval prolongation and two developed TdP. All patients experienced the maximum QTc interval within the first 24 h of quinidine therapy. One of the patients who did not develop significant QTc interval prolongation did not receive a high loading dose of quinidine prior to the initiation of the continuous infusion.
Previous data regarding the risk of TdP associated with CDC-recommended doses of i.v. quinidine for resistant malaria are lacking. However, in one study, i.v. quinidine gluconate for the treatment of severe malaria, at doses lower than those recommended by the CDC (10-mg/kg loading dose over 4 h and then repeated every 8 h) increased the QTc interval by 24% ± 8% from baseline, which was associated with higher plasma quinidine concentrations (26).
In 2010, the CDC received 1,691 reported cases of malaria, which represented an increase of 14% in comparison to the number of reported cases in 2009 (18). Of these 1,691 cases, 176 were classified as severe, and 73 patients received i.v. quinidine (18). While this number may seem relatively small, ⅔ of the patients in our case series developed QT interval prolongation associated with quinidine, and ⅓ developed TdP. These data suggest that quinidine use for severe malaria could result in approximately 25 preventable cases of TdP annually in the United States. Furthermore, there were approximately 216 million cases of malaria reported worldwide in 2010, and i.v. quinidine is used for the management of severe malaria in countries other than the United States (18), indicating that the potential for quinidine-associated TdP during malaria treatment extends beyond the United States.
When used for AF, oral quinidine was well known to cause QT interval prolongation and TdP, which occurred most frequently in patients with at least one risk factor associated with TdP or who were receiving concomitant treatment with other proarrhythmic medications. All patients who developed QTc interval prolongation or TdP in this case series had multiple risk factors for TdP (24), including electrolyte imbalances and comorbid conditions, including heart failure. In addition, two patients had hepatic dysfunction, which may inhibit the metabolism and reduce the clearance of quinidine (20). Patients who did not develop QT interval prolongation also had multiple risk factors but in some cases were not administered aggressive loading doses. One patient (case 3) had a prequinidine QTc interval of 602 ms, which markedly increased her risk for quinidine-induced TdP (24). CDC guidelines recommend that quinidine gluconate infusions be slowed or discontinued if the QTc interval becomes prolonged to >600 ms. In this case, the treating physicians continued the quinidine gluconate despite a QTC interval of >600 ms because the patient had severe, refractory, life-threatening malaria and the risks associated with quinidine were felt to be acceptable in view of the severity of the disease.
CDC guidelines recommend continuous QT interval monitoring during quinidine therapy. In addition to QTc of >600 ms, the CDC recommends that infusions of quinidine gluconate be slowed or discontinued if the QRS duration increases by >50%, if the QTc interval becomes prolonged >25% from the pretreatment value, or if hypotension occurs that is not responsive to fluid challenge. CDC guidelines also recommend the following: “Consultation with a cardiologist and a physician with experience treating malaria is advised when treating malaria patients with quinidine.” Quinidine is primarily hepatically metabolized, but about 20% of a dose is eliminated unchanged by the kidneys (20). Few data exist to guide dose adjustment in patients with hepatic cirrhosis; monitoring of plasma quinidine concentrations in this population seems prudent. However, limited evidence suggests that quinidine doses should be reduced by 25% in patients with severe liver disease (9). The CDC does not recommend adjustment of initial doses (including loading doses) of i.v. quinidine in patients with kidney disease, but suggests that if kidney failure persists or if clinical improvement is not observed, the maintenance quinidine dose should be reduced by ⅓ to ½ on the third day of treatment.
The antimalarial drug artesunate is recommended by the World Health Organization as preferable to i.v. quinidine for management of severe malaria (27). Artesunate is not approved by the FDA for widespread use in the United States. However, i.v. artesunate is approved by the FDA under an investigational new drug designation on an emergency basis for patients with severe malaria, high levels of malarial parasites in the blood, inability to take oral medications, lack of timely access to i.v. quinidine, quinidine intolerance or contraindications, or failure of quinidine therapy (http://www.cdc.gov/malaria/diagnosis_treatment/artesunate.html; accessed 3 April 2012). Therefore, i.v. artesunate may be preferable to i.v. quinidine for patients with preexisting QT interval prolongation and/or other risk factors for drug-induced TdP.
In conclusion, clinicians using quinidine gluconate for management of resistant malaria at CDC-recommended doses should be aware of the potential for QT interval prolongation and TdP. When administering quinidine for this indication, clinicians must consider the cardiac risks and take steps to ensure safe administration. For patients receiving i.v. quinidine for management of resistant malaria, CDC guidelines recommend obtaining a cardiology consultation, initiating continuous telemetry monitoring, and reducing the infusion rate or discontinuing quinidine infusions if the QTc interval increases to >600 ms. We also recommend maintaining serum potassium concentrations of >4.0 meq/liter and serum magnesium concentrations of >2.0 mg/dl during i.v. quinidine therapy in patients with malaria. In addition, when possible, clinicians should consider reducing the rate and/or dose of infusion and consider omitting loading doses in patients with multiple risk factors for TdP.
ACKNOWLEDGMENT
R. J. Kovacs has served as an advisor to Eli Lilly & Co., Essentialis, Xenoport, Inc., and Synosia Therapeutics regarding issues related to the QT interval in drug development. There are no disclosures for the other authors.
Footnotes
Published ahead of print 21 May 2012
REFERENCES
- 1. Allen LaPointe NM, Li P. 2000. Continuous intravenous quinidine infusion for the treatment of atrial fibrillation or flutter: a case series. Am. Heart J. 139:114–121 [DOI] [PubMed] [Google Scholar]
- 2. Bauman JL, et al. 1984. Torsade de pointes due to quinidine: observations in 31 patients. Am. Heart J. 107:425–430 [DOI] [PubMed] [Google Scholar]
- 3. Bazett HC. 1920. An analysis of time relationships of the electrocardiogram. Heart 7:353–370 [Google Scholar]
- 4. Bodenhamer JE, Smilkstein MJ. 1993. Delayed cardiotoxicity following quinine overdose: a case report. J. Emerg. Med. 11:279–285 [DOI] [PubMed] [Google Scholar]
- 5. Bouchaud O, Bruneel F, Schiemann R, Peytavin G, Coulaud JP. 2002. Severe cardiac toxicity due to halofantrine: importance of underlying heart disease. J. Travel Med. 9:214–215 [DOI] [PubMed] [Google Scholar]
- 6. Camm AJ, et al. 2010. Guidelines for the management of atrial fibrillation. The Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace 12:1360–1420 [DOI] [PubMed] [Google Scholar]
- 7. Chiladakis J, Kalogeropoulos Arvanitis AP, Koutsogiannis N, Zagli F, Alexopoulos D. 2010. Preferred QT correction formula for the assessment of drug-induced QT interval prolongation. J. Cardiovasc. Electrophysiol. 21:905–913 [DOI] [PubMed] [Google Scholar]
- 8. Claessen FAP, et al. 1998. Quinine pharmacokinetics: ototoxic and cardiotoxic effects in healthy Caucasian subjects and in patients with falciparum malaria. Trop. Med. Int. Health 3:482–489 [DOI] [PubMed] [Google Scholar]
- 9. Fattinger K, Vozeh S, Ha HR, Borner M, Follath F. 1991. Population pharmacokinetics of quinidine. Br. J. Clin. Pharmacol. 31:279–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fragasso G, et al. 2009. Cardiotoxicity after low-dose chloroquine therapy. Heart Vessels 24:385–387 [DOI] [PubMed] [Google Scholar]
- 11. Fridericia LS. 1920. Die systolendauer im elektrokardiogramm bei normalen menchen und bei herzkranken. Acta Med. Scand. 53:469–486 [Google Scholar]
- 12. Fuster V, et al. 2001. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients with Atrial Fibrillation). Circulation 104:2118–2150 [PubMed] [Google Scholar]
- 13. Fuster V, et al. 2006. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients with Atrial Fibrillation). Circulation 114:700–752 [DOI] [PubMed] [Google Scholar]
- 14. Gillis AM, Verma A, Talajic M, Nattel S, Dorian P. 2010. CCS Atrial Fibrillation Guidelines Committee. Canadian Cardiovascular Society atrial fibrillation guidelines 2010: rate and rhythm management. Can. J. Cardiol. 27:47–59 [DOI] [PubMed] [Google Scholar]
- 15. Heist EK, Ruskin JN. 2010. Drug-induced arrhythmia. Circulation 122:1426–1435 [DOI] [PubMed] [Google Scholar]
- 16. Hodak SP, et al. 1998. QT prolongation and near fatal cardiac arrhythmia after intravenous tacrolimus administration: a case report. Transplantation 66:535–537 [DOI] [PubMed] [Google Scholar]
- 17. Hodges M. 1997. Rate correction of the QT interval. Cardiac Electrophysiol. Rev. 1:360–363 [Google Scholar]
- 18. Mali S, Kachur SP, Arguin PM. 2012. Malaria surveillance—United States, 2010. MMWR Surveill. Summ. 61:1–17 [PubMed] [Google Scholar]
- 19. Martin ES, Rogalski K, Black JN. 1997. Quinine may trigger torsades de pointes during astemizole therapy. Pacing Clin. Electrophysiol. 20:2024–2025 [DOI] [PubMed] [Google Scholar]
- 20. Ochs HR, Greenblatt DJ, Woo E. 1980. Clinical pharmacokinetics of quinidine. Clin. Pharmacokinet. 5:150–169 [DOI] [PubMed] [Google Scholar]
- 21. Ramírez A, Galván JM. 2007. Torsades-de-pointes-type ventricular tachycardia in a patient with digitalis intoxication under chronic treatment with quinine sulfate. Med. Intensiva 31:106–107 [DOI] [PubMed] [Google Scholar]
- 22. Roden DM, Thompson KA, Hoffman BF, Woosley RL. 1986. Clinical features and basic mechanisms of quinidine-induced arrhythmias. J. Am. Coll. Cardiol. 8(Suppl A):73A–78A [DOI] [PubMed] [Google Scholar]
- 23. Setty S, Khalil Z, Schori P, Azar M, Ferrieri P. 2003. Babesiosis. Two atypical cases from Minnesota and a review. Am. J. Clin. Pathol. 120:554–559 [DOI] [PubMed] [Google Scholar]
- 24. Tisdale JE. 2010. Ventricular arrhythmias, p 485–515 In Tisdale JE, Miller DA. (ed), Drug-induced diseases. Prevention, detection and management, 2nd ed American Society of Health-Systems Pharmacists, Bethesda, MD [Google Scholar]
- 25. Wann LS, et al. 2011. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (updating the 2006 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 123:104–123 [DOI] [PubMed] [Google Scholar]
- 26. White N, Looareesuwan S, Warrell D. 1983. Quinine and quinidine: a comparison of EKG effects during the treatment of malaria. J. Cardiovasc. Pharmacol. 5:173–175 [DOI] [PubMed] [Google Scholar]
- 27. World Health Organization 2010. Guidelines for the treatment of malaria, 2nd ed World Health Organization, Geneva, Switzerland [Google Scholar]


