Abstract
Candida albicans is a common cause of catheter-related bloodstream infections (CR-BSI). Ethanol (EtOH) lock therapy has been attempted despite limited data on optimal dose and duration. Concentrations of 35% EtOH or higher for a minimum of 4 h demonstrated a >99% reduction in mature C. albicans biofilm metabolic activity and prevented regrowth. Concentrations of 10% EtOH or higher reduced C. albicans biofilm formation by >99%. Further investigation of EtOH lock therapy for treatment and prevention of C. albicans CR-BSI is warranted.
TEXT
Biofilms are a major component of catheter-related bloodstream infections (CR-BSI) due to Candida species. Clinical trials have demonstrated worse outcomes in candidemic patients when indwelling venous catheters are retained, particularly in nonneutropenic populations; therefore, Infectious Diseases Society of America guidelines for treatment of CR-BSI due to Candida species recommend line removal in nonneutropenic patients and, if possible, in neutropenic patients (7). However, catheter removal may be high risk in a significant proportion of patients due to thrombocytopenia, coagulopathies, lack of alternative venous access, or critical illness. Antifungal lock therapy (AfLT) is a potential alternative strategy for catheter salvage. A limited number of in vitro studies, animal models, and clinical reports have used ethanol (EtOH) as an antiseptic agent as part of an AfLT strategy (1–3, 6, 9, 12, 13). These strategies have used differing concentrations of EtOH (12.5 to 70%) with various dwell times (2 to 24 h). However, there are few data that systematically examine the effects of EtOH concentration and dwell time in an AfLT model.
To determine the optimal concentration and time of EtOH needed to (i) inhibit mature Candida albicans biofilms and (ii) prevent biofilm formation, we used static microplate, silicone disk, and colony regrowth models for systematic in vitro analyses. Wild-type C. albicans SC5314 was used as a reference strain for detailed studies. C. albicans reference strains ATCC 10231, ATCC 14053, and ATCC 24433 and strains 42379 and 53264, two clinically derived C. albicans fks1 mutant isolates characterized by echinocandin resistance, were also studied (5, 11). Biofilm formation in 96-well microtiter plates was performed as described previously (10). A total of 100 μl of 200-proof EtOH diluted in buffered RPMI 1640 to concentrations of 5 to 50% (vol/vol) was added to biofilm-containing wells. The plates were incubated at 37°C for selected time points and analyzed using the XTT reduction assay (10). Each experiment was performed in triplicate or quadruplicate. Antifungal activity was expressed as a percentage relative to the metabolic activity of untreated biofilms.
Sessile MICs (sMIC) were defined as the concentrations of EtOH needed to reduce metabolic activity of the biofilms by 50% (sMIC50) and 80% (sMIC80). Metabolic activities of the treatment groups were compared to those of controls by using one- or two-way analyses of variance (ANOVAs) and Dunnett's multiple-comparison posttest. Differences were considered significant at a P value of <0.05. GraphPad Prism 5.0 (GraphPad Software, San Diego, CA) was used for statistical analyses and graphing.
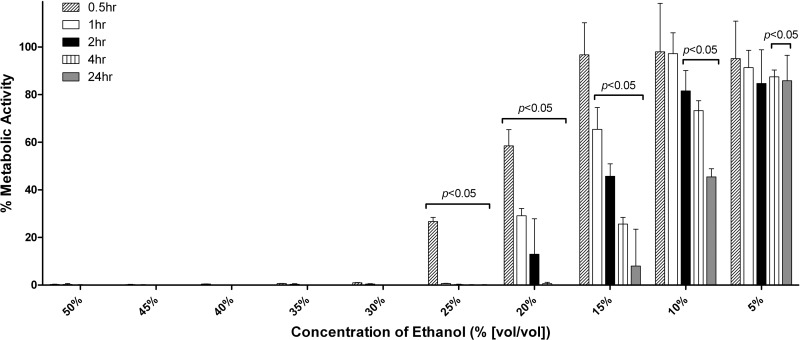
The antifungal effect of 5 to 50% EtOH against mature C. albicans SC5314 biofilms was tested after 0.5, 1, 2, 4, and 24-hour treatments (Fig. 1). EtOH concentrations of 20% or higher had statistically significant antifungal activity at all time points. The sMIC50 ranged from 10 to 25% EtOH depending on the treatment duration. The sMIC80 ranged from 15 to 30%. EtOH concentrations of 30% or higher inhibited biofilms by >99% for all time points tested. Concentrations of 35% EtOH inhibited biofilms by 99.85%, 99.86%, and 99.97% at 1, 2, and 4 h, respectively.
Fig 1.
Effect of various EtOH concentrations and incubation times on C. albicans SC5314 mature biofilms. The in vitro effect of ethanol (EtOH) against C. albicans SC5314 biofilms was assayed in a static microplate model using the XTT assay. EtOH concentrations of 5 to 50% were tested for incubation periods of 0.5, 1, 2, 4, or 24 h. The graph shows the averages of three to four replicates per treatment compared to the untreated control.
To test C. albicans SC5314 biofilm formation in the presence of EtOH, cells in RPMI were coincubated with 5 to 50% EtOH at 37°C for 24 h. Biofilm formation was reduced by >99% at concentrations of 10% or greater. Metabolic activity of biofilms incubated with 5% EtOH decreased 66.2%.
The effect of 5 to 50% EtOH on mature biofilms of three C. albicans reference isolates (ATCC 10231, ATCC 14053, and ATCC 24433) and two echinocandin-resistant strains (42379 and 53264) was tested at 2 and 24 h. The effect of EtOH on the biofilms varied significantly among strains for both incubation times (P < 0.0001). For the 2-h EtOH incubation, the sMIC50 ranged from 15 to 20% and the sMIC80 ranged from 20 to 25%. Concentrations of 25% and higher inhibited biofilms formed by all strains by >99%. For the 24-hour incubation, the sMIC50 was 10% and the sMIC80 ranged from 15 to 20%. Concentrations of 20% and higher fully inhibited the biofilms of all strains.
We next used a silicone disk model in 96-well plates to study the effect of EtOH on inhibition and formation of C. albicans SC5314 biofilms formed on silicone surfaces (4). Mature biofilms on silicone disks were treated with EtOH for 2 h. The sMIC50 of EtOH was 10%, and the sMIC80 was 20%. EtOH concentrations of 25% and higher inhibited the biofilms by >99%. EtOH significantly decreased biofilm formation at all concentrations and reduced biofilm formation by >99% at concentrations of 10% or greater.
Regrowth after EtOH treatment was tested as described by Raad et al., with slight modifications (8). C. albicans SC5314 biofilms formed on silicone disks were treated with 5 to 50% EtOH for 2 or 4 h (Table 1). Then, silicone disks were removed aseptically, scrape sonicated for 3 min, and incubated for 24 and 48 h at 30°C in Sabouraud's dextrose broth. Optical densities were read, and 100-μl dilutions were plated onto Sabouraud's dextrose agar and incubated for 48 h at 30°C for colony counting. For both 2- and 4-h treatments, cell regrowth was observed after treatment with 5 to 25% EtOH. EtOH concentrations of 35% or higher were sufficient to prevent regrowth after a 4-h treatment; a concentration of 50% was required to prevent regrowth after a 2-h treatment at both 24- and 48-h incubation periods.
Table 1.
Regrowth of Candida biofilm cells after 2- and 4-h EtOH treatments and 24 h of incubation of silicone disks
| EtOH concn (%) | Values for each EtOH treatmenta |
|||
|---|---|---|---|---|
| 2 h |
4 h |
|||
| OD600 | Mean CFU/ml | OD600 | Mean CFU/ml | |
| 0 | 0.439 | 3.0 × 104 | 0.492 | 2.6 × 105 |
| 5 | 0.437 | 3.0 × 104 | 0.480 | 2.6 × 105 |
| 25 | 0.247 | 3.0 × 104 | 0.120 | 5.2 × 104 |
| 35 | 0.022 | 1.6 × 103 | 0.026 | 0 |
| 50 | 0.019 | 0 | 0.026 | 0 |
| Negative control | 0.017 | 0 | 0.018 | 0 |
OD600, optical density at 600 nm.
Limited data currently preclude recommendations on the use of AfLT in the management of CR-BSI due to Candida species. Nonetheless, EtOH lock therapy offers a promising means of catheter salvage when removal is not viable. Blackwood et al. (2) described the successful use of 70% EtOH lock solutions instilled for at least 2 h daily for 14 days to treat three pediatric patients with candidemia. Venkatesh et al. (13) reported that 12.5% EtOH reduced C. albicans biofilm mass by 50% after 24 h of incubation in a 96-well plate model; however, biofilms were not eradicated. Raad et al. (9) investigated the effects of a 25% EtOH solution on Candida parapsilosis biofilms; although biofilm growth was initially suppressed after 15 min (modified Robbins device) or 1 h (silicone disk model) of EtOH exposure, biofilm regrowth occurred after 24 h of incubation.
This study suggests that at 35% EtOH, incubation times should be at least 4 h to ensure adequate sterilization. Higher EtOH concentrations are effective at eradicating biofilms in vitro, but potential issues include catheter occlusion from precipitation of 100% EtOH (6) and alterations in mechanical integrity/elasticity of polyurethane catheters (3). Catheter type must therefore be taken into account when considering EtOH lock therapy. Limitations of this study include a focus on C. albicans and not non-albicans Candida species. Furthermore, this study utilized a series of in vitro biofilm models, which are highly simplified representations of central venous catheter (CVC)-related infection. Additional studies in a biofilm flow and/or animal model would assist development of a clinically useful EtOH lock protocol. Further studies of EtOH for prevention of CR-BSI due to Candida species are also warranted.
ACKNOWLEDGMENTS
This work was supported by grants from the Department of Veterans' Affairs (Merit Award to S.A.L.) and from the Biomedical Research Institute of New Mexico (to S.A.L.).
We thank William Fonzi (Georgetown University) for providing strain SC5314.
Footnotes
Published ahead of print 21 May 2012
REFERENCES
- 1. Balestrino D, et al. 2009. Eradication of microorganisms embedded in biofilm by an ethanol-based catheter lock solution. Nephrol. Dial. Transplant. 24:3204–3209 [DOI] [PubMed] [Google Scholar]
- 2. Blackwood RA, et al. 2011. Ethanol locks therapy for resolution of fungal catheter infections. Pediatr. Infect. Dis. J. 30:1105–1107 [DOI] [PubMed] [Google Scholar]
- 3. Crnich CJ, Halfmann JA, Crone WC, Maki DG. 2005. The effects of prolonged ethanol exposure on the mechanical properties of polyurethane and silicone catheters used for intravascular access. Infect. Control Hosp. Epidemiol. 26:708–714 [DOI] [PubMed] [Google Scholar]
- 4. Ku TSN, Bernardo SM, Lee SA. 2011. In vitro assessment of the antifungal and paradoxical activity of different echinocandins against Candida tropicalis biofilms. J. Med. Microbiol. 60:1708–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. LaFleur MD, Kumamoto CA, Lewis K. 2006. Candida albicans biofilms produce antifungal-tolerant persister cells. Antimicrob. Agents Chemother. 50:3839–3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Laird J, Soutar R, Butcher I. 2005. Complications of the ethanol-lock technique in the treatment of central venous catheter sepsis. J. Infect. 51:338. [DOI] [PubMed] [Google Scholar]
- 7. Mermel LA, et al. 2009. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 49:1–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Raad I, et al. 2008. Role of ethylene diamine tetra-acetic acid (EDTA) in catheter lock solutions: EDTA enhances the antifungal activity of amphotericin B lipid complex against Candida embedded in biofilm. Int. J. Antimicrob. Agents. 32:515–518 [DOI] [PubMed] [Google Scholar]
- 9. Raad I, Hanna H, Dvorak T, Chaiban G, Hachem R. 2007. Optimal antimicrobial catheter lock solution, using different combinations of minocycline, EDTA, and 25-percent ethanol, rapidly eradicates organisms embedded in biofilm. Antimicrob. Agents Chemother. 51:78–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ramage G, Lòpez-Ribot JL. 2005. Techniques for antifungal susceptibility testing of Candida albicans biofilms. Methods Mol. Med. 118:71–79 [DOI] [PubMed] [Google Scholar]
- 11. Ramage G, Mowat E, Jones B, Williams C, Lòpez-Ribot JL. 2009. Our current understanding of fungal biofilms. Crit. Rev. Microbiol. 35:340–355 [DOI] [PubMed] [Google Scholar]
- 12. Slobbe L, et al. 2010. Prevention of catheter-related bacteremia with a daily ethanol lock in patients with tunneled catheters: a randomized placebo-controlled trial. PLoS One 5:e10840 doi:10.1371/journal.pone.0010840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Venkatesh M, Rong L, Raad I, Versalovic J. 2009. Novel synergistic antibiofilm combinations for salvage of infected catheters. J. Med. Microbiol. 58:936–944 [DOI] [PubMed] [Google Scholar]