Abstract
Hepatitis E virus (HEV), an enterically transmitted pathogen, is one of the major causes of acute hepatitis in humans worldwide, being responsible for outbreaks and epidemics in regions with suboptimal sanitary conditions, in many of which it is endemic. In industrialized countries, hepatitis E is rarely reported, but recent studies have revealed quite high human seroprevalence rates and the possibility of porcine zoonotic transmission. There is currently no specific therapy or licensed vaccine against HEV infection, and little is known about its intracellular growth cycle, as until very recently no efficient cell culture system has been available. In the present study, vaccinia viruses have been used to express recombinant HEV ORF2 proteins, allowing the study of their glycosylation patterns and subcellular localization. Furthermore, the expressed proteins have been shown to be good antigens for diagnostic purposes and to elicit high and long-lasting specific anti-HEV titers of antibodies in mice that are passively transferred to the offspring by both transplacental and lactation routes.
INTRODUCTION
Hepatitis E virus (HEV) is a spherical, nonenveloped virus around 27 to 34 nm in diameter, whose genome is a single-stranded RNA molecule of positive polarity and approximately 7.2 kb in length, containing 3 open reading frames (ORFs) and a 3′ poly(A) tail (39, 47). ORF2 is synthesized as a large glycoprotein precursor that is cleaved into the mature viral capsid protein, which harbors immunoreactive epitopes; therefore, it has largely been used in diagnostic and vaccine development. ORF2 contains three potential N-glycosylation sites and a putative signal peptide at its 3′ end (17, 50), and it has been implicated in viral replication, as it binds to the 5′ end of the viral genome (44). Until very recently (42, 43, 48, 49), no efficient HEV-susceptible cell culture was available; thus, different heterologous expression systems have been used to study ORF2 glycosylation and intracellular traffic, with disparate results (12, 17, 50, 51).
HEV is classified into four genotypes (gt) that infect humans, although only gt3 and gt4 have been isolated in other animal species, mainly pigs (2, 6, 29, 36) and, recently, rats (21, 23, 37). HEV is enterically transmitted and causes epidemic outbreaks in areas with inadequate hygienic conditions and/or water supplies, and thus, it has become an important public health concern. Lately, an increase in the number of sporadic autochthonous cases has been reported in industrialized countries, and its zoonotic potential has been documented (7, 35).
The course of hepatitis E disease is variable, including icteric and anicteric hepatitis, and severe hepatitis with development of fulminate hepatic failure (FHF), but it can also be asymptomatic (25) or become chronic in immunosuppressed and organ transplant patients (6, 22). Although still controversial (30), HEV may cause substantial morbidity and mortality in pregnant women, with mortality rates of up to 25% due to FHF, which may lead to spontaneous abortion and stillbirths (33).
Since viremia is usually limited to the acute phase of the infection, the diagnosis of the disease is mainly dependent on serology. Currently, commercially available kits are designed to detect anti-HEV in humans and include short fragments of ORF2 and ORF3 of gt1 and gt2, but not of gt3 or gt4, the most prevalent in industrialized countries in swine and humans (31). However, various reports indicate that commercial assays sometimes failed to detect specific antibodies in sera from patients with proven HEV gt3 infections, and thus, the number of autochthonous HEV infections in industrialized regions may have been underestimated (2, 3, 8, 14, 20, 28).
No specific treatment of HEV infection or approved vaccine is yet available, although due to the lack of an efficient cell culture system, several recombinant proteins and peptides have been assayed (1), with two already in clinical trials (41, 52, 53).
In the present study, the glycosylation and intracellular transport of vaccinia virus-expressed HEV ORF2 proteins have been analyzed in mammalian cells, and their antigenic and immunogenic capabilities have been further evaluated.
MATERIALS AND METHODS
Generation of vaccinia viruses expressing recombinant ORF2 proteins of HEV gt3.
Amplification and cloning of the complete HEV gt3 ORF2 (GenBank accession number JQ522948) and a truncated Δ(1-111)-ORF2 fragment of it into TOPO-TA plasmid (Invitrogen, Carlsbad, CA) using specific primers (sequence available upon request) were performed as previously described (19). Then, genes were transferred to pRB21-Myc-His, which includes a Myc epitope followed by a tail of 6 His residues at the C termini of the proteins, to yield plasmids pRB21-ORF2-Myc-His and pRB21-Δ(1-111)-ORF2-Myc-His, respectively. These plasmids were used to generate the corresponding recombinant vaccinia viruses, rVV-ORF2 and rVV-Δ(1-111)-ORF2, which were further purified through a sucrose cushion (4). The Western Reserve (WR) strain of vaccinia virus, grown and purified under the same conditions, was used as the control virus.
Protein expression and characterization.
Monolayers of BHK-21 or BSC-1 cells were infected with recombinant vaccinia viruses or WR (multiplicity of infection [MOI] = 1 PFU/cell). When cytopathic effect was complete, cells were collected and centrifuged at 210 × g for 5 min. The pellet was resuspended in lysis buffer (1% Nonidet P-40, 50 mM Tris-HCl [pH 7.6], 150 mM NaCl, 2 mM EDTA) supplemented with complete protease inhibitor cocktail (Roche, Mannheim, Germany) for 30 min at 4°C, vortexed for 5 min, sonicated 3 times for 10 s each time, and centrifuged at 15,680 × g for 2 min. The supernatant was recovered and kept at −20°C until use. Protein expression was analyzed by Western blotting (WB) (19, 20).
Glycosylation of the recombinant HEV proteins was analyzed upon infection (MOI = 2) of BHK-21 cells. One microgram/microliter of tunicamycin (Sigma, St. Louis, MO), an inhibitor of N-glycosylation (9, 46), was added after viral adsorption (0 h postinfection [h p.i.]) or 4 h p.i. As a positive control, N-glycosylation of the later-expressed B5 vaccinia virus protein (10) was analyzed in WR-infected cells. Brefeldin A (BFA) (Sigma), an inhibitor of the transport between the Golgi and the endoplasmic reticulum (ER) (24), was also included in the assays at a final concentration of 5 μg/ml in dimethyl sulfoxide (DMSO). As a control, cells were similarly treated with equal amounts of the drug solvents.
Protein extracts were tested as enzyme-linked immunosorbent assay (ELISA) antigens. Microplates (Polysorp; Nunc, Roskilde, Denmark) were coated with 50 μl/well of serial dilutions of proteins extracts in 50 mM carbonate-bicarbonate buffer (pH 9.6), and the ELISA was performed as described previously (19, 20).
Antibodies, staining, and reagents.
Recombinant proteins were detected using an anti-C-Myc mouse monoclonal antibody (Roche), an antihistidine mouse monoclonal antibody (Clontech Laboratories, Inc., Mountain View, CA), or 4G11, an anti-rΔ(1-111)-ORF2 mouse monoclonal antibody (available upon request), conjugated to Alexa Fluor 488 (AF-488). Wheat germ agglutinin (WGA; Invitrogen), a lectin that labels specifically the Golgi complex (34), conjugated with AF-594 was used to reveal the position of the Golgi complex. Likewise, as specific markers of the ER, an anticalnexin rabbit polyclonal antibody (Stressgen, MI) or an anticalreticulin rabbit polyclonal antibody (Abcam, Cambridge, United Kingdom) was used. To analyze the localization of vaccinia virus proteins to the cell surface, a rat monoclonal antibody that recognizes the B5 vaccinia virus protein expressed in the extracellular surface of vaccinia virus-infected cells (40) was used. Secondary antibodies against mouse, rabbit, or rat anti-IgG coupled to AF-488 or AF-594 were purchased from Invitrogen.
Immunofluorescence and confocal microscopy.
Cell monolayers were grown on glass coverslips (Menzel-Glaser, Braunschweig, Germany), infected with the tested recombinant vaccinia viruses at an MOI of 1 PFU/cell, and processed, 7 or 17 h p.i. as described previously (27). Depending on the experiment, cells were fixed in 4% paraformaldehyde (PFA) or in cold absolute methanol. In some instances, PFA-fixed cells were permeabilized before processing (27). All cells were further incubated with phosphate-buffered saline (PBS)-0.1 M glycine and primary and fluorescently labeled secondary antibodies and then mounted with Fluoromount-G (Southern Biotech, Birmingham, AL). Preparations were observed with a Leica TCS SPE confocal laser scanning microscope using an HCX PL APO 63×/1.4 oil immersion objective. Images were acquired using Leica advanced fluorescence software (LAS AF). Optical slice thickness for all confocal images displayed was 1 Airy unit.
Animals and vaccination schedule.
Groups (6 to 9) of 8-week-old Swiss outbred mice were inoculated intraperitoneally (i.p.), three times at a 2-week intervals, with sucrose cushion-purified recombinant vaccinia viruses or WR. For each recombinant virus, mice were inoculated with an initial dose of either 105 or 106 PFU/mouse, followed by two additional doses of 104 or 105 PFU/mouse. Blood samples were collected from the jaw vein (maxillary sinus) previously to the first inoculation and 21, 41, and 119 days later. Throughout the experiments, animals were given food and water ad libitum and monitored daily. Experiments were approved and performed according to the guidelines for animal experimentation of the animal safety committee of our institution.
To analyze vertical transmission of acquired immunity from vaccinated mothers to their offspring, two additional groups of 4 female mice were inoculated under the same schedule with the highest doses of virus as described previously (18) and crossed after the third inoculation. In parallel, 8 unvaccinated female black-furred B6D2 mice were also crossed. At delivery, half of the pups born to vaccinated mothers were interchanged with half of the pups born to their assigned control unvaccinated B6D2 mothers. In this way, vaccinated and nonvaccinated mothers nursed some of their own pups and some fostered ones. To avoid the interference of colostrum, some pups born to nonvaccinated mothers were transferred to vaccinated mothers 9 days postpartum. Pups (4 to 10) from the different groups were anesthetized, bled, and sacrificed at different times (3, 9, 18, and 30 days postdelivery). To analyze intrauterine transmission, cesarean sections were performed in two additional vaccinated mice. Antibodies raised in mice were tested by an ELISA based on rΔ(1-111)-ORF2 HEV protein partially purified from insect larvae as previously described (20).
RESULTS
Expression of HEV gt3 rORF2 proteins in mammalian cells and glycosylation analysis.
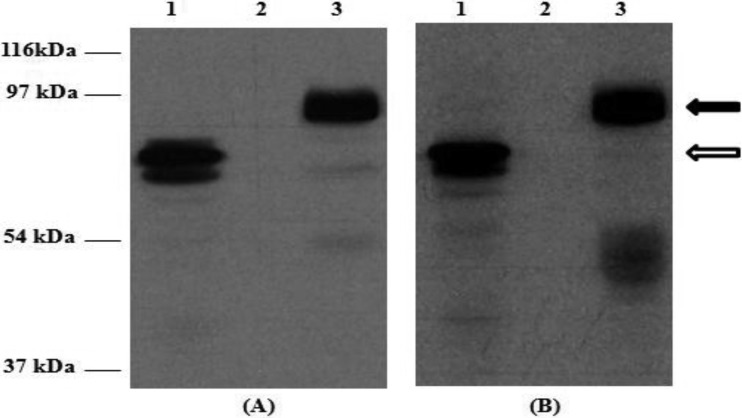
Western blot analyses of extracts from mammalian cells infected with the recombinant vaccinia viruses rVV-ORF2 and rVV-Δ(1-111)-ORF2 showed proteins with apparent masses of around 80 to 90 kDa and a doublet of around 60 to 62 kDa, respectively (Fig. 1), although some minor species could also be detected. The specificity of the bands was confirmed, as none of them were detected in WR-infected cellular extracts (Fig. 1) or when anti-HEV IgG-negative swine sera were used (data not shown).
Fig 1.
Western blot analysis of recombinant rVV-ORF2 and rVV-Δ(1-111)-ORF2 proteins in BHK-21 mammalian cells. Western blots were stained with a monoclonal anti-His antibody (A) or an HEV-positive porcine serum (B), as described previously (19, 20). Lane 1, rVV-Δ(1-111)-ORF2-infected cells; lane 2, WR vaccinia virus-infected cells; lane 3, rVV-ORF2-infected cells. Molecular mass markers are shown on the left. Black and white arrows mark the positions of the rORF2-vv and rΔ(1-111)-ORF2-vv proteins, respectively.
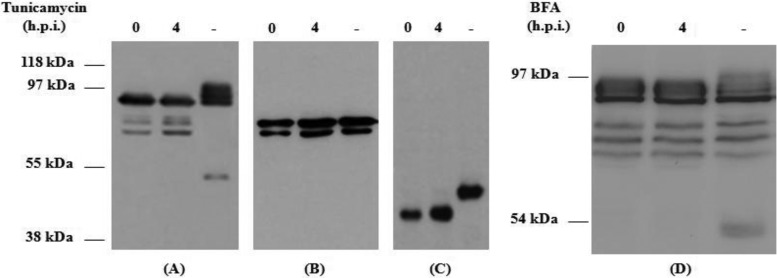
Glycosylation analyses showed that in the presence of tunicamycin, an N-glycosylation inhibitor, only the complete rORF2-vv presented a different mobility pattern with several isoforms, while rΔ(1-111)-ORF2-vv mobility pattern was not altered (Fig. 2A and B). Control of the assay confirmed the inhibitory action of the tunicamycin, as no glycosylated form of vaccinia virus B5 protein was detected in its presence (Fig. 2C). Western blot analysis of rORF2-vv expression after treatment with BFA, a drug that inhibits the transport between the Golgi and the ER, showed that although some specific isoforms could be detected only in the absence of the drug, most of them were not altered by the treatment (Fig. 2D).
Fig 2.
Western blot analysis of the glycosylation patterns of HEV recombinant rORF2-vv (A and D) and rΔ(1-111)-ORF2-vv (B) proteins and of B5 WR vaccinia virus protein (C) in infected BHK-21 cells. Western blots were stained with a monoclonal anti-His antibody (A, B, and D) or with an anti-B5 rat monoclonal antibody (C). Infected-cell extracts treated with the drugs at 0 or 4 h p.i. or not treated (−) were harvested 7 h p.i. and processed as described in Materials and Methods. Molecular mass markers are shown on the left.
Subcellular location of recombinant proteins in mammalian cells.
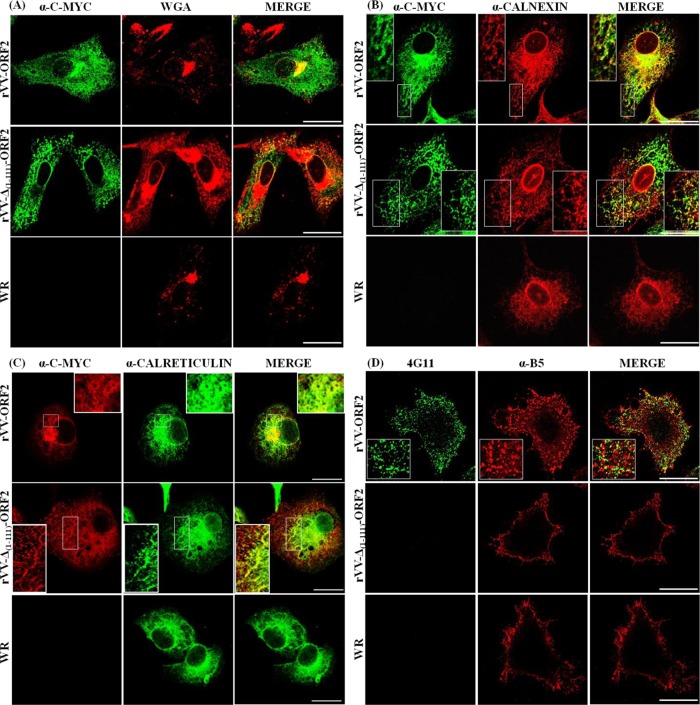
Immunofluorescence and confocal microscopy analyses of BHK-21 or BSC-1 cells infected with recombinant vaccinia virus showed a reticular pattern for both proteins, with a juxtanuclear accumulation of rORF2-vv. As displayed in Fig. 3A, the juxtanuclear ORF2 signal in rVV-ORF2-infected cells was coincident with the Golgi complex, and this signal was absent in rVV-Δ(1-111)-ORF2-infected cells. Double-labeling experiments with ER-specific markers anticalnexin and anticalreticulin showed that both recombinant proteins localized to the ER (Fig. 3B and C). By using a specific anti-ORF2 monoclonal antibody (4G11) conjugated with Alexa Fluor 488, it was observed that rORF2-vv, but not rΔ(1-111)-ORF2, was located in the cell surface. The rORF2-vv labeling appeared as a punctate pattern that was not coincident with mature vaccinia viruses, as revealed by B5 staining (Fig. 3D).
Fig 3.
Cells infected with HEV recombinant rVV-ORF2, rVV-Δ(1-111)-ORF2, and WR vaccinia virus (MOI = 1 PFU/cell) were fixed and processed for immunofluorescence and confocal microscopy as described in Materials and Methods. Recombinant HEV proteins were detected using anti-C-Myc or anti-rΔ(1-111)-ORF2 (4G11) mouse monoclonal antibodies. Vaccinia viruses on the cell surface were visualized by staining nonpermeabilized cells with an anti-B5 rat monoclonal antibody (α-B5). Cellular structures were labeled by using specific antibodies: wheat germ agglutinin (WGA) for Golgi and anticalnexin and anticalreticulin rabbit polyclonal antibodies for ER. Suitable secondary antibodies coupled to AF-488 were used. Scale bar, 25 μm.
ELISA based on the expressed proteins.
The antigenicity of the expressed HEV recombinant proteins was assessed by ELISA after establishing the optimal antigen (1:500 and 1:2,500 for rORF2-vv and rΔ(1-111)-ORF2-vv, respectively) and serum (1:100) dilutions by using a battery of previously well-characterized positive and negative swine sera (20). As a negative control, protein extracts from WR-infected cells were used. The cutoff the assays was established as 2-fold the absorbance of the negative control (<0.15; range, 0.05 to 0.14). Comparison of the results obtained with both recombinant proteins showed a 94.5% (52/55) concordance. Further comparison with two previously validated ELISAs based on rORF2 proteins expressed in insect larvae (20) showed 89.5% (51/57) and 92.8% (65/70) concordances. These proteins were obtained upon inoculation of Trichoplusia ni larvae with recombinant baculoviruses and partially purified in their native forms by means of their His tails (19, 20). Likewise, a concordance of 76.8% was found between the ELISA based on the vaccinia virus-expressed truncated rΔ(1-111)-ORF2-vv and a widely used commercial kit (MP diagnostic kit; Genelabs) modified for use with swine sera as described previously (20).
To further confirm the accuracy of the assays, all sera with discordant ELISA results, that is, those that were positive by one of the assays and negative with the others, and 10 randomly selected concordant sera were tested by WB as a reference assay (14, 20). The sensitivity and specificity of the in-house assay for detecting anti-HEV antibodies were 91.3% and 100%, while those of the commercial assay were 74% and 87%, respectively.
Inmunogenicity of the expressed proteins.
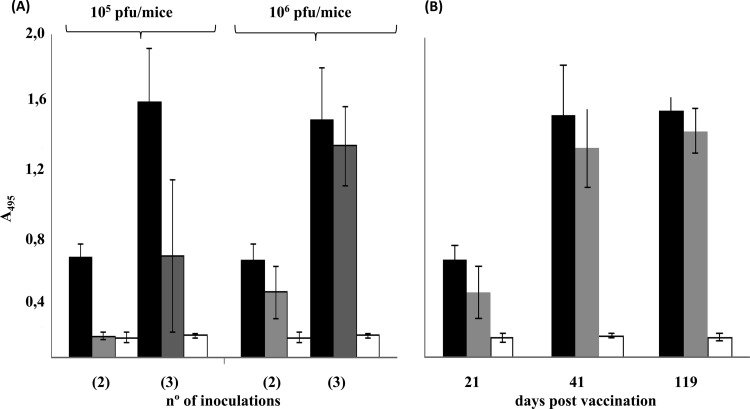
All mice vaccinated with rVV-ORF2 presented specific antibodies in sera after two inoculations (22 days postinoculation [d.p.i.]), regardless of the viral dose inoculated. In contrast, 3 infections with the highest dose (106 PFU) were needed to elicit an antibody response in all rVV-Δ(1-111)-ORF2-vaccinated animals (Table 1). As expected, antibody levels increased with the number of inoculations (Fig. 4A) and reached high titers through the end of the experiment, 119 d.p.i. (Fig. 4B). Antibody titers were consistently higher in mice inoculated with rVV-ORF2, in particular at a 105-PFU dose.
Table 1.
Number of mice with specific anti-HEV antibodies at 21 and 41 days after vaccination with different doses of the vaccinia viruses
| Inoculated virus | Dose (PFU/mouse) | No. of mice with specific anti-HEV antibodies/total no. of mice (%) |
|
|---|---|---|---|
| 22 d.p.v.a | 41 d.p.v. | ||
| rVV-ORF2 | 105 | 6/6 (100) | 6/6 (100) |
| 106 | 9/9 (100) | 9/9 (100) | |
| rVV-Δ(1-111)-ORF2 | 105 | 0/6 (0) | 4/6 (66.6) |
| 106 | 4/9 (44.4) | 9/9 (100) | |
| WR | 106 | 0/6 (0) | 0/6 (0) |
d.p.v., days postvaccination.
Fig 4.
Anti-HEV IgG levels elicited in mice vaccinated with vaccinia viruses. (A) Anti-HEV IgG antibodies levels raised in mice after two and three inoculations with either 105 or 106 PFU/mouse of rVV-ORF2 (black bars), rVV-Δ(1-111)-ORF2 (gray bars), or WR (white bars). (B) Levels of anti-HEV IgG antibodies raised over time by mice vaccinated with 106 PFU/mouse of rVV-ORF2 (black bars), rVV-Δ(1-111)-ORF2 (gray bars), or WR (white bars). ELISAs were conducted as described previously (20). Results are expressed as the average absorbance (A495) values. Standard deviations are shown by error bars.
Vertical transfer of acquired immunity.
As shown in Table 2, all pups (n = 73) nursed by mothers vaccinated with any of the two recombinant vaccinia viruses (rVV-ORF2 or rVV-Δ(1-111)-ORF2), either their own pups (n = 39) or those fostered from nonvaccinated mothers (n = 34), presented specific antibodies in sera that were maintained until the end of the experiment (30 days postpartum [d.p.p.]). Similar results were obtained in those cases in which colostrum contribution to vertical transmission of the acquired immunity was avoided (data not shown). On the other hand, around 80% (n = 26) of the pups born to vaccinated mothers and nursed by nonvaccinated ones presented specific antibodies in sera at 9 d.p.p., but by day 30 p.p. no specific antibodies were detected in any of the pups tested (Table 2). All pups (n = 18) born by cesarean section of 2 vaccinated mothers also presented specific antibodies (data not shown). As expected, none of the pups (n = 31) born to nonvaccinated mothers and nursed by them presented specific antibodies in sera.
Table 2.
Number of newborn mice with anti-HEV antibodies and route of transmissiona
| Mothers | Origin of pups (no.) | No. of anti-HEV-positive newborns/total tested (%) |
||||
|---|---|---|---|---|---|---|
| 0 d.p.d. | 3 d.p.d. | 9 d.p.d. | 18 d.p.d. | 30 d.p.d. | ||
| Vaccinated | Own, i.u + L (39) | 12/12 (100) | 9/9 (100) | 5/5 (100) | 6/6 (100) | 7/7 (100) |
| Fostered, L (34) | 7/7 (100) | 7/7 (100) | 9/9 (100) | 11/11 (100) | ||
| Unvaccinated | Own, none (31) | 0/4 (0) | 0/9 (0) | 0/5 (0) | 0/9 (0) | 0/4 (0) |
| Fostered, i.u. (26) | 7/8 (87.5) | 4/5 (80) | 2/8 (25) | 0/5 (0) | ||
Mice were tested at different days postdelivery (d.p.d.). i.u, intrauterine; L, lactation.
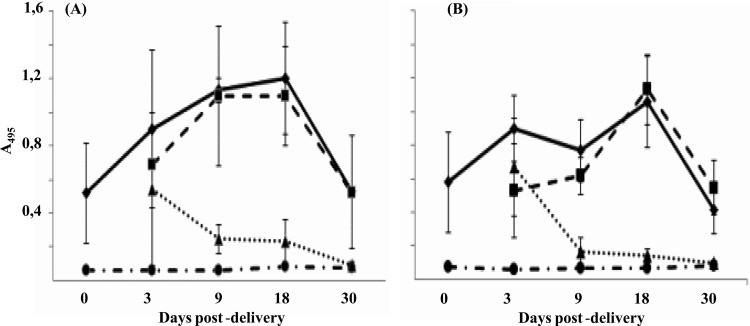
Levels of antibodies transmitted by rVV-ORF2-vaccinated mothers, transplacentally and/or by nursing, to their offspring were overall higher than those transmitted by rVV-Δ(1-111)-ORF2-vaccinated mothers (Fig. 5).
Fig 5.
Levels of anti-HEV IgG antibodies over time in the offspring of mice vaccinated with rVV-ORF2 (A) or rVV-Δ(1-111)-ORF2 (B). Anti-HEV IgG antibody levels were measured in pups born to vaccinated mothers and raised by them (diamonds) or by unvaccinated foster mothers (triangles) and in pups born to unvaccinated mothers and nursed by them (circles) or by vaccinated ones (squares). ELISAs were conducted as described previously (20). Results are expressed as the average absorbance (A495) values. Standard deviations are shown by error bars.
DISCUSSION
Only very recently has HEV-susceptible cell culture been described (42, 43, 48, 49), and this fact has hampered the study of the viral growth cycle, including the possible role of ORF2 glycosylation. Glycosylation frequently affects protein folding and intracellular transport along the exocytic pathway (13), and although nonenveloped viruses were not considered to have glycosylated protein, there are well-known exceptions, such as that of rotaviruses (11).
HEV ORF2 contains three putative N-glycosylation sites and a putative signal peptide at its 3′ end (17, 51), but reports describing its glycosylation status using different expression systems are contradictory. Glycosylation of ORF2 at the ER, due to the presence of a putative signal peptide (51) or of 3 sequons (12, 50), has been described. This glycosylation status may affect ORF2 intracellular trafficking, allowing its translocation from the cytosol to the ER (45). Even more, the presence of mannose-rich residues on ORF2 has been related with a circulation of ORF2 throughout the Golgi (17). However, a cytoplasmic localization that excluded the ER has also been reported (12).
In the present study, the two recombinant ORF2 proteins were expressed at similar levels in mammalian cells with the expected mass, and while, in accordance with most of the aforementioned studies, ORF2 was glycosylated, the truncated form was not. This may be a consequence of the absence of the signal peptide region on rΔ(1-111)-ORF2-vv, which would hamper its translocation to the ER and the consequent glycosylation. Immunofluorescence and confocal microscopy analyses of both recombinant proteins showed that they localize at the ER, but only rORF2-vv circulates throughout the Golgi complex. These results are supported by treatment with BFA, a drug that inhibits the transport between the ER and Golgi (24), where it was shown that although some glycosylation may occur at the Golgi, it more likely takes place at other cellular locations. Even more, confirming previous studies (17, 51), our data with permeabilized and nonpermeabilized cells revealed that rORF2-vv could also be observed at the cell surface, while rΔ(1-111)-ORF2-vv could not. This could be again due either to an incorrect glycosylation pattern or to the absence of the hydrophobic N-terminal motif on rΔ(1-111)-ORF2-vv, which would impair its association to the membranes. Our data show that rΔ(1-111)-ORF2-vv presents a uniform localization characteristic of ER even in the absence of a putative signal peptide. This observation suggests that the association of ORF2 with the ER may be mediated by the hydrophobic region near its C terminus or by sequences not yet identified. Although our results cannot completely rule out that both proteins interact with cellular or vaccinia virus proteins, which may facilitate their transport to the ER, they are indirectly supported by reports indicating that both proteins react with the 5′ end of HEV RNA (44), and by the ER localization of the viral replicase (38). Thus, our analysis showed that the complete ORF2 was localized to the ER, to the Golgi, and at the cell surface, confirming the expected trafficking of the protein, while the truncated form was mainly observed at the ER. These different intracellular localization patterns are unlikely due to experimental artifacts as a consequence of protein overexpression, as previously suggested (12, 50), since the two proteins were expressed at similar levels.
An early report describing the expression of ORF2 and ORF3 proteins from HEV genotype 1 suggested their putative utility to analyze the immune response to HEV (5); therefore, once the glycosylation and subcellular patterns of the expressed proteins were partially characterized, their antigenicity and immunogenicity were evaluated. Even though commercial assays for detection of anti-HEV antibodies in humans, which are based on proteins of gt1 and/or gt2, are available, there are still concerns about their utility for infections caused by other genotypes (2, 3, 14, 20, 28). In addition, they are not designed to be used with samples from swine, a likely natural reservoir of HEV (29). The utility of the vaccinia virus recombinant-expressed proteins as ELISA antigens was confirmed after comparison with a previously validated in-house ELISA (20) and with a widely used commercial kit (Genelabs Diagnostics Inc., CA), to which they present a very good concordance and an even better sensitivity and specificity. The in-house test developed in this study allows 7,500 individual ELISA determinations from the infection of 107 mammalian cells, making it a relatively cheap and easy-to-apply tool for use in regions where other alternatives are less available.
As mentioned above, until the recent description of HEV-susceptible cell culture (42, 43, 48, 49), the lack of an efficient, productive cell culture for HEV has made unfeasible the use of live or attenuated virus vaccines; thus, several recombinant proteins and DNA candidates have been evaluated (1), some of which are in clinical trials (41, 52, 53). However, no approved vaccine is commercially available, and several aspects about its potential use remain to be elucidated, such as the duration of the response, its usefulness against a fulminant course of the disease, and its cost-effectiveness. Furthermore, an important aspect to address in an HEV vaccine is its safety for use in pregnant women, because, although still debated (30), HEV may account for higher rates of mortality, spontaneous abortion, and neonatal death (33).
Since vaccinia virus has been widely used as a vaccine vector (16, 32) and its potential for expressing HEV recombinant proteins had been described in an early report (5), we analyzed the potential of recombinant vaccinia viruses expressing HEV proteins as immunogens in mice. Significantly, both recombinant vaccinia viruses obtained (rVV-ORF2 and rVV-Δ(1-111)-ORF2) elicited high and long-lasting (up to 4 months) anti-HEV specific antibody levels in mice, although those vaccinated with rVV-ORF2 presented higher and earlier (after just two inoculations) levels. These results are in concordance with those previously reported for humans (52, 53) and mice (18). Differences between the two immunogens could be due to their different intracellular trafficking and glycosylation statuses, which may influence their immunogenic capabilities.
As previously found using purified baculovirus-expressed recombinant proteins (18), the immunity acquired after infection of pregnant mice with recombinant vaccine viruses was transmitted to their offspring by both intrauterine and lactation routes, the latter route being more efficient, as antibody titers raised by newborns nursed by vaccinated mothers were higher and longer-lasting than those raised by pups born to vaccinated mothers but nursed by nonvaccinated ones. However, antibody titers were lower and less lasting than those previously observed after vaccination with baculovirus-expressed proteins (18). Although HEV has recently been detected in rats with shedding of virus (21, 23, 37), and HEV experimental infection has been reported with nude mice (15), HEV does not seem to infect immunocompetent mice (26); therefore, the protective capacity of the immunological status acquired by these animals after vaccination with the recombinant vaccinia viruses was not evaluated.
In summary, our results show that vaccinia virus is a good system for expression of HEV recombinant proteins that allows the study of their intracellular localization and glycosylation patterns. The vaccinia virus-expressed proteins are also valuable diagnostic reagents and vaccine candidates that deserve further study.
ACKNOWLEDGMENTS
We thank M. Calvo for technical assistance.
This work was supported by grant CSD2006-0007 and BIO2008-03713 from the Spanish Ministerio de Ciencia e Innovación (MICINN) and S2009/TIC-1476 from the Comunidad Autónoma de Madrid.
Footnotes
Published ahead of print 16 May 2012
REFERENCES
- 1. Aggarwal R, Jameel S. 2008. Hepatitis E vaccine. Hepatol. Int. 2:308–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baechlein C, et al. 2010. Prevalence of hepatitis E virus-specific antibodies in sera of German domestic pigs estimated by using different assays. Vet. Microbiol. 144:187–191 [DOI] [PubMed] [Google Scholar]
- 3. Bendall R, Ellis V, Ijaz S, Ali R, Dalton H. 2010. A comparison of two commercially available anti-HEV IgG kits and a re-evaluation of anti-HEV IgG seroprevalence data in developed countries. J. Med. Virol. 82:799–805 [DOI] [PubMed] [Google Scholar]
- 4. Blasco R, Moss B. 1995. Selection of recombinant vaccinia viruses on the basis of plaque formation. Gene 158:157–162 [DOI] [PubMed] [Google Scholar]
- 5. Carl M, et al. 1994. Expression of hepatitis E virus putative structural proteins in recombinant vaccinia viruses. Clin. Diagn. Lab. Immunol. 1:253–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chandra V, Taneja S, Kalia M, Jameel S. 2008. Molecular biology and pathogenesis of hepatitis E virus. J. Biosci. 33:451–464 [DOI] [PubMed] [Google Scholar]
- 7. Dalton HR, et al. 2008. Autochthonous hepatitis E in Southwest England: natural history, complications and seasonal variation, and hepatitis E virus IgG seroprevalence in blood donors, the elderly and patients with chronic liver disease. Eur. J. Gastroenterol. Hepatol. 20:784–790 [DOI] [PubMed] [Google Scholar]
- 8. Daniel HD, Warier A, Abraham P, Sridharan G. 2004. Age-wise exposure rates to hepatitis E virus in a Southern Indian patient population without liver disease. Am. J. Trop. Med. Hyg. 71:675–678 [PubMed] [Google Scholar]
- 9. Elbein AD. 1987. Inhibitors of the biosynthesis and processing of N-linked oligosaccharide chains. Annu. Rev. Biochem. 56:497–534 [DOI] [PubMed] [Google Scholar]
- 10. Engelstad M, Howard ST, Smith GL. 1992. A constitutively expressed vaccinia gene encodes a 42-kDa glycoprotein related to complement control factors that form a part of the extracellular virus envelope. Virology 188:801–810 [DOI] [PubMed] [Google Scholar]
- 11. Estes MK. 2007. Rotavirus, p 1747–1786 In Knipe DM, et al. (ed), Fields virology. 5th ed, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 12. Graff J, et al. 2008. Mutations within potential glycosylation sites in the capsid protein of hepatitis E virus prevent the formation of infectious virus particles. J. Virol. 82:1185–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Helenius A. 1994. How N-linked oligosaccharides affect glycoprotein folding in the endoplasmic reticulum. Mol. Biol. Cell 5:253–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herremans M, Bakker J, Duizer E, Vennema H, Koopmans MP. 2007. Use of serological assays for diagnosis of hepatitis E virus genotype 1 and 3 infections in a setting of low endemicity. Clin. Vaccine Immunol. 14:562–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang F, et al. 2009. Experimental infection of Balb/c nude mice with hepatitis E virus. BMC Infect. Dis. 9:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jacobs BL, et al. 2009. Vaccinia virus vaccines: past, present and future. Antiviral Res. 84:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jameel S, Zafrullah M, Ozdener MH, Panda SK. 1996. Expression in animal cells and characterization of the hepatitis E virus structural proteins. J. Virol. 70:207–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jiménez de Oya N, et al. 2011. Maternal transfer of antibodies to the offspring after mice immunization with insect larvae-derived recombinant hepatitis E virus ORF-2 proteins. Virus Res. 158:28–32 [DOI] [PubMed] [Google Scholar]
- 19. Jiménez de Oya N, et al. 2009. Expression and immunoreactivities of hepatitis E virus genotype 3 open reading frame 2 (ORF-2) recombinant proteins expressed in insect cells. Food Environ. Virol. 1:77–84 [Google Scholar]
- 20. Jiménez de Oya N, et al. 2009. Serological immunoassay for detection of hepatitis E virus on the basis of genotype 3 open reading frame 2 recombinant proteins produced in Trichoplusia ni larvae. J. Clin. Microbiol. 47:3276–3282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johne R, et al. 2010. Detection of hepatitis E virus in faeces of wild rats using a nested broad spectrum RT-PCR. J. Gen. Virol. 91:750–758 [DOI] [PubMed] [Google Scholar]
- 22. Kamar N, et al. 2008. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N. Engl. J. Med. 358:811–817 [DOI] [PubMed] [Google Scholar]
- 23. Kanai Y, et al. 2012. Hepatitis E virus in Norway rats (Rattus norvegicus) captured around a pig farm. BME Res. Notes 5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klausner RD, Donaldson JG, Lippincott-Schwartz J. 1992. Brefeldin A: insights into the control of membrane traffic and organelle structure. J. Cell Biol. 116:1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krawczynski K, Aggarwal R, Kamil S. 2000. Hepatitis E. Infect. Dis. Clin. North Am. 14:669–687 [DOI] [PubMed] [Google Scholar]
- 26. Li TC, et al. 2008. Mice are not susceptible to hepatitis E virus infection. J. Vet. Med. Sci. 70:1359–1362 [DOI] [PubMed] [Google Scholar]
- 27. Martín-Acebes MA, Blázquez AB, Jiménez de Oya N, Escribano-Romero E, Saiz JC. 2011. West Nile virus replication requires fatty acid synthesis but is independent of phosphatidylinositol-4-phosphate lipids. PLoS One 6:e24970 doi:10.1371/journal.pone.0024970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mast EE, Alter MJ, Holland PV, Purcell RH. 1998. Evaluation of assays for antibody to hepatitis E virus by a serum panel. Hepatitis E Antibody Serum Panel Evaluation Group. Hepatology 27:857–861 [DOI] [PubMed] [Google Scholar]
- 29. Meng XJ. 2010. Hepatitis E virus: animal reservoirs and zoonotic risk. Vet. Microbiol. 140:256–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Meng XJ. 2010. Recent advances in hepatitis E virus. J. Viral Hepat. 17:153–161 [DOI] [PubMed] [Google Scholar]
- 31. Meng XJ, et al. 2002. Prevalence of antibodies to hepatitis E virus in veterinarians working with swine and normal blood donors in the United States and other countries. J. Clin. Microbiol. 40:117–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moss B. 2011. Smallpox vaccines: targets of protective immunity. Immunol. Rev. 239:8–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Navaneethan U, Al Mohajer M, Shata MT. 2008. Hepatitis E and pregnancy: understanding the pathogenesis. Liver Int. 28:1190–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Parkkinen JJ, et al. 1997. Polyamine-dependent alterations in the structure of microfilaments, Golgi apparatus, endoplasmic reticulum, and proteoglycan synthesis in BHK cells. J. Cell. Biochem. 66:165–174 [PubMed] [Google Scholar]
- 35. Pavio N, Meng XJ, Renou C. 2010. Zoonotic hepatitis E: animal reservoirs and emerging risks. Vet. Res. 41(6):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Purcell RH, Emerson SU. 2010. Hidden danger: the raw facts about hepatitis E virus. J. Infect. Dis. 202:819–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Purcell RH, et al. 2011. Hepatitis E virus in rats. Los Angeles, California, U. S. A. Emerg. Infect. Dis. 17:2216–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rehman S, Kapur N, Durgapal H, Panda KS. 2008. Subcellular localization of hepatitis E virus (HEV) replicase. Virology 370:77–92 [DOI] [PubMed] [Google Scholar]
- 39. Reyes GR, et al. 1990. Isolation of a cDNA from the virus responsible for enterically transmitted non-A, non-B hepatitis. Science 247:1335–1339 [DOI] [PubMed] [Google Scholar]
- 40. Schmelz M, et al. 1994. Assembly of vaccinia virus: the second wrapping cisterna is derived from the trans Golgi network. J. Virol. 68:130–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shrestha MP, et al. 2007. Safety and efficacy of a recombinant hepatitis E vaccine. N. Engl. J. Med. 356:895–903 [DOI] [PubMed] [Google Scholar]
- 42. Shukla P, et al. 2012. Adaptation of genotype 3 hepatitis E virus to efficient growth in cell culture depends on an inserted human gene segment acquired by recombination. J. Virol. 86:5697–5707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shukla P, et al. 2011. Cross-species infections of cultured cells by hepatitis E virus and discovery of an infectious virus-host recombinant. Proc. Natl. Acad. Sci. U. S. A. 108:2438–2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Surjit M, Jameel S, Lai SK. 2004. The ORF2 protein of hepatitis E virus binds to the 5′ region of viral RNA. J. Virol. 78:320–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Surjit M, Jameel S, Lai SK. 2007. Cytoplasmic localization of the ORF2 protein of hepatitis E virus is dependent on its ability to undergo retrotranslocation from the endoplasmic reticulum. J. Virol. 81:3339–3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Takatsuki A, Arima K, Tamura G. 1971. Tunicamycin, a new antibiotic. I: isolation and characterization of tunicamycin. J. Antibiot. (Tokyo) 24:215–223 [DOI] [PubMed] [Google Scholar]
- 47. Tam AW, et al. 1991. Hepatitis E virus (HEV): molecular cloning and sequencing of the full-length viral genome. Virology 185:120–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tanaka T, Takahashi M, Kusano E, Okamoto H. 2007. Development and evaluation of an efficient cell-culture system for hepatitis E virus. J. Gen. Virol. 88:903–911 [DOI] [PubMed] [Google Scholar]
- 49. Tanaka T, et al. 2009. Development and characterization of a genotype 4 hepatitis E virus cell culture system using HE-JF5/15F strain recovered from a fulminant hepatitis patient. J. Clin. Microbiol. 47:1906–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Torresi J, Li F, Locarnini SA, Anderson DA. 1999. Only the non-glycosylated fraction of hepatitis E virus capsid (open reading frame 2) protein is stable in mammalian cells. J. Gen. Virol. 80:1185–1188 [DOI] [PubMed] [Google Scholar]
- 51. Zafrullah M, Ozdener MH, Kumar R, Panda SK, Jameel S. 1999. Mutational analysis of glycosylation, membrane translocation, and cell surface expression of the hepatitis E virus ORF2 protein. J. Virol. 73:4074–4082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhang J, et al. 2009. Randomized-controlled phase II clinical trial of a bacterially expressed recombinant hepatitis E vaccine. Vaccine 27:1869–1874 [DOI] [PubMed] [Google Scholar]
- 53. Zhu FC, et al. 2010. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomized, double-blind placebo-controlled, phase 3 trial. Lancet 376:895–902 [DOI] [PubMed] [Google Scholar]