Abstract
Adeno-associated virus (AAV) vectors have the potential to promote long-term gene expression. Unfortunately, humoral immunity restricts patient treatment and in addition provides an obstacle to the potential option of vector readministration. In this study, we describe a comprehensive characterization of the neutralizing antibody (NAb) response to AAV type 1 (AAV1) through AAV5 both in vitro and in vivo. These results demonstrated that NAbs generated from one AAV type are unable to neutralize the transduction of other types. We extended this observation by demonstrating that a rationally engineered, muscle-tropic AAV2 mutant containing 5 amino acid substitutions from AAV1 displayed a NAb profile different from those of parental AAV2 and AAV1. Here we found that a single insertion of Thr from AAV1 into AAV2 capsid at residue 265 preserved high muscle transduction, while also changing the immune profile. To better understand the role of Thr insertion at position 265, we replaced all 20 amino acids and evaluated both muscle transduction and the NAb response. Of these variants, 8 mutants induced higher muscle transduction than AAV2. Additionally, three classes of capsid NAb immune profile were defined based on the ability to inhibit transduction from AAV2 or mutants. While no relationship was found between transduction, amino acid properties, and NAb titer or its cross-reactivity, these studies map a critical capsid motif involved in all steps of AAV infectivity. Our results suggest that AAV types can be utilized not only as templates to generate mutants with enhanced transduction efficiency but also as substrates for repeat administration.
INTRODUCTION
Adeno-associated virus (AAV) vectors have been safely and successfully used in phase I clinical trials (39, 40, 54). In particular, therapeutic effects have been achieved in patients with Leber's congenital amaurosis and hemophilia B. Among 30 patients with Leber's congenital amaurosis, 28 demonstrated remarkably improved eyesight after AAV type 2 (AAV2) delivery of the rpe65 gene to the retina. Regarding the recent hemophilia B trial, all six patients had therapeutic factor IX (FIX) expression within a beneficial 1% to 8% of normal levels as long as 18 months after intravenous delivery of an AAV vector encoding an optimized FIX cassette (54).
AAV is a nonenveloped, single-stranded DNA virus that requires a helper virus, such as adenovirus (Ad) or herpes simplex virus, for efficient replication. Despite the lack of inflammation or other AAV-associated complications following administration of AAV2 vectors in several organs, neutralizing antibody (NAb) titers against the AAV2 capsid were found to be significantly increased following vector administration, particularly in the lung, muscle, and liver (7, 8, 43–45, 49, 54, 63). NAbs in circulation are able to block AAV transduction after systemic administration. Recently, Manno et al. (44) performed a phase I study of AAV-mediated FIX transgene delivery in patients with hemophilia B, in which one patient with a higher preexisting NAb titer demonstrated lower levels of FIX in the serum than another patient with lower preexisting NAbs against AAV2. These results suggest that preexisting NAbs in the human population can attenuate vector transduction efficiency and inhibit transgene expression upon systemic application (44).
In the general human population, over 95% of individuals have been infected by AAV2, with 50% of them having NAbs (3, 4, 6, 10, 18, 21, 24, 27, 35, 48, 62, 65). The prevalence of NAb in children is lower, ranging from 13 to 25% (9, 35). Although 11 additional types of AAV have been isolated for gene therapy purposes, little to no cross-reactivity of NAbs is demonstrated among these types in animals (27, 30, 38, 60, 65). In humans, recent studies have shown that different degrees of NAb cross-reactivity exist between AAV2 and other types (6, 10, 27, 45). There is a lower prevalence of NAbs against AAV1, -5, -6, -7, and -8 than against AAV2 (6, 10). These findings present a concern to the gene therapy community as to how we can avoid antagonistic NAb activity during systemic delivery of AAV vectors. To overcome this concern, several approaches have been exploited in our lab and by other groups, including (i) the utilization of polymers to cover the AAV capsid and block NAb recognition (11, 32, 33), (ii) the development of NAb escape mutants by error-prone PCR in vitro (31, 41, 56), (iii) the application of other types of AAV vectors (27, 30, 38, 60, 65), and (iv) the generation of chimeric types via AAV shuffling (2, 36, 37). In this study, we have systematically explored the possibility of using different types and AAV mutants as alternative vectors for intramuscular gene delivery in mice preimmunized against different AAV types.
MATERIALS AND METHODS
Plasmids and viruses.
We first generated a series of AAV type vectors (AAV1 to -6) as described previously (57). The plasmid pRep6cap6 was a kind gift from David Russell (University of Washington) (61). All constructs were generated in the pXR2 backbone (57). Site-directed mutagenesis (Stratagene QuikChange site-directed mutagenesis kit) was used to insert nucleotides or substitute point mutations. The pXR2.5 plasmid was constructed by replacement of amino acids at positions 263, 265, 709, 712, and 720 of AAV2 by the corresponding amino acids in AAV1. Primer 5′-CCAGCCAATCANNKGGAGCCTCGAACG-3′ was used to generate clones with insertion of an amino acid(s) at residue 265 of the AAV2 capsid. AAV was produced in 293 cells using a triple-transfection protocol (68). Virus was concentrated by double cesium chloride gradient centrifugation. The titer was quantitated by Southern dot blotting, and the contamination of preparations with empty particle was less than 5%.
Determination of immunoglobulin titers for different AAV types.
For comparison of the humoral immune response to AAV types 1 to 6, 1 × 1010 particles of AAV/green fluorescent protein (GFP) (100 μl) were intraperitoneally injected into 6- to 10-week-old mice (BALB/c; Jackson Laboratory, ME) at day 0, and mice were boosted with the same virus as for the primary immunization at day 14. Blood sera were collected via the retro-orbital plexus at the indicated time points for NAb assays. Serially diluted serum samples from immunized mice were analyzed for AAV-specific immunoglobulins (total Ig, IgG, IgM, IgG1, IgG2a, IgG2b, and IgG3) by enzyme-linked immunosorbent assay (ELISA). High-binding, 96-well flat-bottom plates (Costar, Cambridge, MA) were coated with 100 μl coating buffer (boric acid, pH 9.4) containing 1 × 109 AAV particles/ml at 4°C overnight. The wells were then washed twice with wash buffer (phosphate-buffered saline [PBS] with 0.05% Tween 20) and blocked in PBS with 3% BSA for 1 h at 37°C. Serially diluted samples were then added to antigen-coated plates and incubated at 37°C for 2 h. Plates were then washed four times and incubated with 100 μl peroxidase-conjugated goat anti-mouse IgG, IgM, IgG1, IgG2a, IgG2b, and IgG3 (ICN Biomedicals, Inc., Costa Mesa, CA) for 2 h at 37°C. Plates were then washed four times and optical densities (OD) were determined using tetramethylbenzidine (TMB) substrate (Pierce Chemical Company, Rockford, IL) at 452 nm on an MRX microplate reader. The highest dilution was defined as positive when the OD value was 20% higher than that for the control group without AAV immunization.
NAb-mediated inhibition of AAV transduction in vitro.
NAb-mediated inhibition of AAV transduction in vitro was studied using a method described before (34). Briefly, 293 cells (for AAV1 to -3 and AAV5) and Cos-7 cells (for AAV4) were seeded in a 48-well plate at a density of 1 × 105 cells/well in 200 μl Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS). The cells were cultured for 3 to 4 h at 37°C and treated with a mixture containing AAV/GFP (1 × 108 particles) preincubated with serially diluted mouse serum (total volume, 25 μl) for 2 h at 4°C. Then, 4 × 106 particles of Ad dl309 were added and incubated for 48 h at 37°C. The number of GFP-expressing cells was then determined using a Nikon fluorescence microscope. The NAb titer was defined as the highest dilution required for decreasing the percentage of GFP-expressing cells by 50% in comparison to the control (no serum added).
Muscle transduction with AAV2 mutants.
AAV2 variants carrying the CBA-luciferase transgene cassette were injected into mice at various viral genome amounts. Female or male BALB/c mice were intramuscularly injected with AAV2 and AAV2 mutants at 1 × 1010 vector genome particles unless indicated otherwise. After 4 weeks postinjection, mice were injected with d-luciferin substrate (150 mg/kg; Invitrogen, CA) and 5 min later were imaged in the Xenogen IVIS system. Images were quantified using the Igor Pro 3.0 software.
NAb-mediated inhibition of AAV Transduction in vivo.
For readministration of AAV vectors in vivo, mice preimmunized with AAV/GFP vectors through intramuscular injection were challenged with AAV/FIX or AAV/AAT vectors after 2 months. Transgene expression was then measured 6 weeks after administration of AAV vectors. Canine FIX levels and human α1-antitrypsin levels in mouse sera were detected by ELISA as previously described (14, 57).
Western dot blotting.
Production of empty and full capsids was determined by loading 2 μl of the virus fractions onto a nitrocellulose membrane in a dot blot apparatus. Membranes were blocked in 10% milk in PBS for 30 min at room temperature (RT) and incubated with the A20 primary antibody (dilution, 1:20) in 2% milk for 1 h at RT. Membranes were washed 5 times with 1× PBS and incubated with goat anti-mouse horseradish peroxidase-conjugated secondary antibody (Pierce; dilution, 1:5,000) for 30 min. The membranes were washed as described above, and capsid production was visualized using the SuperSignal West Femto Maximum Sensitivity Substrate chemiluminescence kit from Pierce.
Statistical analyses.
Student's two-tailed t test was used to analyze the significance of differences between experimental groups and controls.
RESULTS
Characterization of NAbs against AAV capsids.
To study the humoral immune response to AAV1 to -5 vectors, AAV1 to -5 (1 × 1010 particles) packaging the GFP transgene were injected intraperitoneally, and the same number of viral particles was used as a booster dose 2 weeks later. At the indicated time points, sera were collected from mice and screened for the production of specific antibodies. All mice immunized with the 5 types of recombinant AAV (rAAV)/GFP virus generated very high titers of specific antibody that persisted for an extended time (data not shown). A high cross-reactivity between specific antibodies was demonstrated between AAV2 and AAV3b (data not shown). All mice produced IgM and IgG, with IgG2a being the predominant IgG subclass after immunization (Table 1). IgG1 was detected only in mice immunized with AAV2, -4, and -5, with AAV5 eliciting the highest titers of IgG1 (Table 1). Interestingly, IgG3 was detected only in mice immunized with AAV5, albeit at a low titer (Table 1), which suggests that AAV5 may use a different mechanism to elicit a humoral immune response.
Table 1.
Ig subclasses in mice immunized with different types of rAAV
| Virus | Ig in seruma |
|||||
|---|---|---|---|---|---|---|
| IgM | IgG subclass |
|||||
| IgG | IgG1 | IgG2a | IgG2b | IgG3 | ||
| AAV1 | +/++ | ++/+++ | − | ++/+++ | − | − |
| AAV2 | ++ | ++++ | + | +++/++++ | − | − |
| AAV3 | ++ | ++++ | − | ++++ | − | − |
| AAV4 | + | ++++ | + | ++++ | − | − |
| AAV5 | ++ | ++++ | +++ | +++/++++ | − | + |
Mice were immunized with different types of AAV. Two weeks later, after boost, sera were collected and used for Ig subclass analysis. −, 102-fold dilution; +, 103-fold dilution; ++, 104-fold dilution; +++, 105-fold dilution; ++++, 106-fold dilution.
Consistent with observations related to the specific antibody titers, high-titer NAbs were also generated in mice immunized with different AAV types, albeit at 1 to 2 log units lower (data not shown). A more or less identical kinetic profile for type-specific NAb generation was observed (data not shown). This can possibly be explained by the production of total specific antibodies, where neutralizing activity was induced mainly by IgM in the early stage of immunization (within 2 weeks) and then by IgG, primarily the IgG2a subclass. For AAV5, it is likely that IgG1 may play some role in neutralizing activity shortly after the second antigen challenge. Similar to cross-reactivity of specific antibodies, there was no cross-reactivity of NAb among AAV types 1 to 5 except for partial cross-neutralizing activity between sera from AAV2 and AAV3 immunizations (Table 2).
Table 2.
NAb production in mice immunized with different types of rAAV
| Virus | Serum NAb productiona |
|||||
|---|---|---|---|---|---|---|
| AAV1 | AAV2 | AAV3 | AAV4 | AAV5 | AAV6 | |
| AAV1 | ++/+++ | − | − | − | − | ++/+++ |
| AAV2 | − | ++++ | +/++ | − | − | ND |
| AAV3 | − | +/++ | +++/++++ | − | − | ND |
| AAV4 | − | − | − | ++++ | − | ND |
| AAV5 | − | − | − | − | ++++ | ND |
| AAV6 | ++/+++ | ND | ND | ND | ND | ++/+++ |
Mice were immunized with different types of AAV. Two weeks later, after boost, sera were collected and used for NAb analysis. −, 2-fold dilution; +, 102-fold dilution; ++, 103-fold dilution; +++, 104-fold dilution; ++++, 105-fold dilution; ND, not determined.
AAV1 and -6 display identical NAb profiles.
AAV1 and -6 share 99% amino acid identity in the capsid, with only 6 amino acid differences (61, 65). In order to compare the neutralizing activities of antibodies produced against AAV1 and AAV6, mice were immunized with AAV1 or AAV6 at day 0 and boosted with the same virus or the other type 2 weeks later. Mouse sera were collected at week 4 after primary immunization. Although higher total Ig against AAV1 than against AAV6 was observed (data not shown), the NAb titers for AAV1 and AAV6 were similar regardless of the immunization schedule (Table 2 and data not shown).
Alternative types allow repeat administration in AAV2-preimmunized mice.
In vitro data demonstrated that there was an absence of NAb cross-reactivity between AAV2 and other types, except for AAV3b (Table 2). When translated to an in vivo setting, mice first treated with AAV2/GFP vectors were challenged after 2 months with AAV1 to 5 expressing the canine FIX reporter gene (AAV/cFIX) by intramuscular injection. At 1 week postadministration, serum cFIX levels were measured by ELISA. As shown in Fig. 1A, except for AAV2 and AAV3b, similar cFIX levels were detected in mice following administration of the other types (1, 4, and 5) regardless of preexisting immunity to AAV2. As expected, the cFIX levels significantly decreased in the case of AAV2 and AAV3b vectors administered in mice preimmunized against AAV2 (Fig. 1A). Reciprocal experiments were then designed to further demonstrate that there is no NAb cross-reactivity between AAV2 and types 1, 4, and 5. Mice were treated with different types (1 to 5) of AAV/cFIX vectors and challenged 2 months later with AAV2 vectors expressing the human alpha1 antitrypsin transgene (AAV2/hAAT). At 6 weeks postadministration, serum hAAT levels were undetectable in mice preimmunized against AAV2 or AAV3b vector but were largely unaffected compared to control values (∼100 ng/ml) in mice preimmunized with AAV1, -4, or -5 vector (Fig. 1B). These in vivo results corroborate in vitro findings that NAbs against AAV2 are cross-reactive with those against AAV3b but not AAV1, -4, or -5.
Fig 1.
In vivo evaluation of NAb cross-reactivity among types 1 to 5. (A) AAV2 NAb against other types. Type AAV1 to -5/FIX vectors were injected into AAV2/GFP-pretreated mice. At 1 week postinjection, the FIX level in the serum was measured. The data represents the average ± standard deviation (SD) from 4 mice. (B) Other AAV types induce NAb against AAV2 vectors. The mice were initially injected with AAV/F9 vectors of types 1 to 5, and 2 months later AAV2/AAT vectors were administered. AAT concentrations were determined at week 6 after injection of AAV2/AAT vectors. The data represent the average from 4 mice. On the x axis, that the first number represents which type of AAV vector was used for primary immunization, while the second number represents the AAV type used for the following injection (e.g., AAV2/1 means that mice were injected with AAV2 vector followed by AAV1). Zero means no injection.
A small number of amino acid changes alter the AAV2 NAb profile.
AAV1 and AAV6 are known to transduce muscle at a very high efficiency compared to other AAV types (5, 12, 13, 22). Recent studies in our lab have resulted in the rational design of an AAV2 mutant that can transduce skeletal muscle with high efficiency (7). This mutant was generated by substitution of five amino acid residues in the 2-fold dimple region of the AAV capsid with the corresponding amino acid residues from AAV1 (designated AAV2.5) (7). We have demonstrated that the NAb titer against AAV2.5 in sera from AAV2-immunized mice was 5-fold lower than that against AAV2 (7). Serum NAb titers obtained from AAV2.5-immunized mice were 4-fold higher than those cross-reacting with the AAV2 capsid. A similar phenomenon was demonstrated with the NAb profiles of AAV1 and AAV2.5 capsids (7). These observations suggested that the engineered AAV2.5 mutant has a unique NAb profile and might therefore facilitate repeat administration (7).
To further dissect which amino acid change influences AAV2.5 muscle transduction and the induced immunological profile, we made different clones with mutations. The mutant with amino acid substitutions at residues 709, 712, and 720 did not change virion muscle transduction efficacy compared to AAV2 (data not shown). The substitution of AAV2 residue 263 from AAV1 capsid did not influence AAV2 muscle tropism either (Fig. 2). However, after injection of AAV2-263/265T (with a mutation at residue 263 and a Thr insertion at residue 265) and AAV2/265T (with a Thr insertion at residue 265) into muscles, transduction similar to that for AAV2.5 was observed (Fig. 2). This result indicated that the insertion of a threonine at the 265 location (AAV2/265T) played a major role in enhanced muscle transduction of AAV2.5 (Fig. 2). Also, similar patterns of NAbs were shown for AAV2.5 and AAV2/265T (Table 3). It was noted that the NAb titers generated from AAV2.5 and AAV2/265T were lower than those from AAV1 and AAV2 (Table 3).
Fig 2.
Transgene expression from AAV2 mutants following muscular injection. Mice were injected intramuscularly with1 × 1010 particles of AAV/luciferase. Four weeks later, images were taken and the total photons (luciferase activity) were calculated as described in Materials and Methods. The data represents the average from 4 mice.
Table 3.
Neutralizing antibody to AAV and mutants
| Vector | Serum NAb titera |
|||
|---|---|---|---|---|
| AAV1 | AAV2 | AAV2.5 | AAV2 265T | |
| AAV1 | 800 | ND | 100 | 8 |
| AAV2 | ND | 20,000 | 100 | 40 |
| AAV2.5 | ND | ND | 200 | 80 |
| AAV2 265T | 4 | 200 | 200 | 80 |
Mice were administered AAV vector via muscular injection. Four weeks later, sera from mice were collected and used for NAb assay. ND, not determined.
In vitro characterization of AAV2 265 insertion mutants.
To determine whether the efficient muscle transduction of AAV2/265T (compared to AAV2) and its immune profile change were related to the specific amino acid inserted at residue 265 (threonine) or to a general structure change due to the insertion within the 2-fold loop, we generated AAV variants that have insertions with all 20 amino acids at position 265 of the AAV2 capsid. Viral titers from all mutant capsids were similar to that for wild-type AAV2 (Table 4). Generally, the infectivity in 293 cells was lower for the residue 265 insertion mutants than for AAV2. However, similar transduction efficiencies were observed between mutants tested and AAV2 in differentiated C2C12 myotubes (Table 4).
Table 4.
Phenotypic comparison of AAV2 265 insertion mutants
| Insertion mutant | Phenotypea |
||||
|---|---|---|---|---|---|
| Western blotb | A20 recognitionc | Titer | 293 infectivity | C2C12 infectivity | |
| AAV2 | ++++ | ++++ | ++++ | ++++ | ++++ |
| AAV2 A | ++++ | − | ++++ | + | ND |
| AAV2 C | ++++ | − | ++++ | + | ND |
| AAV2 D | ++++ | − | ++++ | +++ | ++++ |
| AAV2 E | ++++ | − | ++++ | ++ | ++++ |
| AAV2 F | ++++ | − | ++++ | ++ | ND |
| AAV2 G | ++++ | ++ | ++++ | +++ | ND |
| AAV2 H | ++++ | − | ++++ | ++ | ND |
| AAV2 K | ++++ | − | ++++ | ++ | +++ |
| AAV2 L | ++++ | − | ++++ | ++ | ++++ |
| AAV2 M | ++++ | − | ++++ | + | ND |
| AAV2 N | ++++ | − | ++++ | ++ | ND |
| AAV2 P | ++++ | − | ++++ | ++ | ++++ |
| AAV2 Q | ++++ | − | ++++ | ++ | ND |
| AAV2 R | ++++ | − | ++++ | ++ | ND |
| AAV2 S | ++++ | − | ++++ | +++ | ND |
| AAV2 T | ++++ | − | ++++ | +++ | ++++ |
| AAV2 V | ++++ | − | ++++ | + | ++++ |
| AAV2 W | ++++ | − | ++++ | ++ | ND |
| AAV2 Y | ++++ | − | ++++ | ++ | ND |
++++, similar to wild-type AAV2 capsids; +++, within 10-fold similarity; ++, 10-fold less similarity; +, more than 10-fold-lower similarity; ND, not determined.
Western blot with B1 primary antibody (1:20).
Western dot blot with A20 primary antibody (1:20). −, lack of A20 recognition.
In vivo muscle transduction with AAV2 residue 265 insertion mutants.
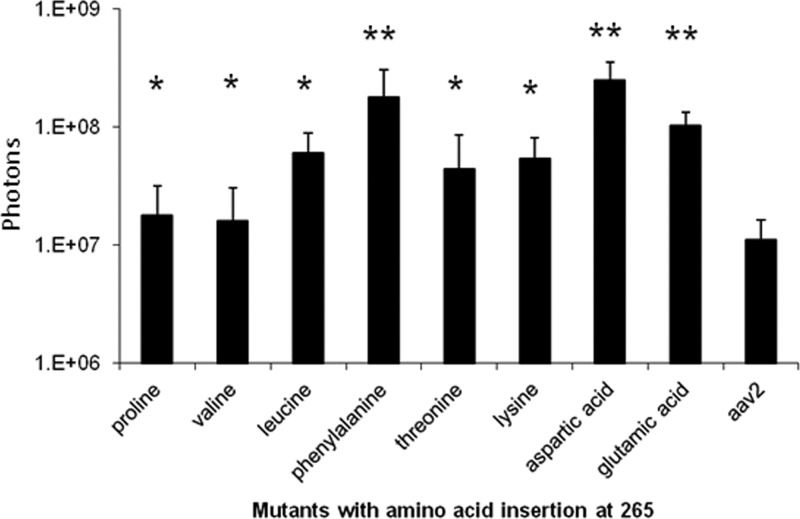
To examine the in vivo muscular tropism of AAV2 residue 265 insertion mutants, we injected all mutants carrying the firefly luciferase transgene into the right and left gastrocnemius muscles at a vector genome copy number of 1 × 1010. Compared to AAV2, higher muscular transduction (over 2-fold the value of AAV2 transduction) was observed in 8 mutants, i.e., those with insertion of either Asp, Glu, Phe, Lys, Leu, Pro, Thr, or Val at position 265 (Fig. 3). Three mutants, i.e., those with insertion of Cys, Ser, or Gln, induced lower muscle transduction than AAV2, while similar muscle transduction was achieved in the other 9 mutants. It was interesting to note that insertions of Phe, Glu, and Asp at residue 265 of the AAV2 capsid demonstrated dramatically enhanced muscle transduction compared to that with the threonine insertion (Fig. 3).
Fig 3.
Transgene expression with AAV2 265 insertion mutants following muscular injection. AAV2/luciferase mutants with the indicated single amino acid insertion at residue 265 were injected into mouse muscle at 1 × 1010 particles. The imaging was taken at 4 weeks postinjection, and luciferase activity was quantitated as described in Materials and Methods.
Analysis of NAb profiles in sera from AAV2 mutant immunizations.
Our previous study demonstrated that AAV2.5 diminished A20 antibody binding affinity (7); therefore, we evaluated whether insertions at residue 265 also ablated the A20 binding site. Analysis of A20 binding affinity by Western dot blotting showed that no mutants were recognized by A20, similar to the result for AAV2.5 (Table 4). An exception was noted for AAV2 265G, with only a decreased binding affinity, highlighting the importance of this site in the antibody-capsid recognition footprint.
To study the neutralizing antibody profiles of AAV2 mutants with 20 different single amino acid insertions at residue 265, we injected AAV2 and mutants into the muscles of C57BL/6 mice at 1 × 1010 particles. One month later, sera from mice were collected for NAb analyses. Three classes of NAb immune profile were defined (Fig. 4). Class I included mutants with insertion of alanine, arginine, glycine, glutamic acid, lysine, phenylalanine, tryptophan, or tyrosine. In this class, the sera generated from AAV2 immunization equally neutralized the transduction from both wild-type AAV2 and mutants; however, the sera from mutant immunizations inhibited transduction of mutants much more efficiently than that of AAV2 immunization. Class II had mutants of insertion of asparagine, aspartic acid, cysteine, glutamine, methionine, or serine. In class II, similar to class I, sera from AAV2 immunization suppressed AAV2 and mutant transductions with similar efficiencies. However, the sera from mutant immunization demonstrated similar efficiency in inhibition transduction of both AAV2 and mutants. Class III was composed of mutants with insertion of histidine, isoleucine, proline, threonine, or valine. In class III, similar inhibition of transduction from AAV2 and mutants was achieved with sera from mutant immunizations; however, higher NAb titers to AAV2 than to mutants was observed with sera from AAV2 immunization. It should be noted that the NAb titer induced from some mutants was lower than that from AAV2 based on NAb activity to its cognates, including arginine, cystine, glutamine, histidine, isoleucine, lysine, methionine, proline, threonine, and valine. No obvious relationship was found between muscle transduction from mutants and inserted amino acid properties and NAb titer or its cross-reactivity.
Fig 4.
NAb analysis of immunization of AAV2 265 insertion mutants. Mice were administered 1 × 1010 particles of AAV2 265 insertion mutants via muscular injection. One month later, the pooled sera were collected from three mice and NAb assay was performed. (A) Sera from AAV2-immunized mice. (B) Sera from mice immunized with the indicated AAV2 265 insertion mutants.
DISCUSSION
The current study was focused on characterization of NAbs against AAV types and capsid mutants in mice. Among the 5 types tested, except for partial cross-reactivity between NAbs against AAV2 and AAV3b, there was no cross-reactivity between any other two types. After immunization of AAV vectors in mice, IgM was found during the early phase of the humoral immune response, followed by class switching to IgG. Insertion of 20 different amino acids at residue 265 of the AAV2 capsid diminished the AAV2 binding affinity to A20 antibody and changed the immune profile, with enhanced muscle transduction in some mutants.
The generation of AAV NAbs is a T cell-dependent B cell response.
Antigens can be classified as either T lymphocyte dependent (TD) or T lymphocyte independent (TI) with regard to their capacity for antibody induction (46). In the immune response to a TD antigen, a variety of cells are involved in the development of antibody responses. Antigen-presenting cells (APCs) take up and process the antigen and present fragments of the antigen (T cell epitopes) to T cells. The presentation of the T cell epitopes in the context of major histocompatibility complex (MHC) class II molecules, together with the costimulatory signals provided by APCs, results in the activation of T cells. Activated T cells then regulate B cell proliferation, production of immunoglobulins (Ig), Ig class switching, rescue of B lymphocytes from apoptotic death, germinal center formation, and generation of B lymphocyte memory (25). In contrast to TD antigens, TI antigens induce antibody production without the help of T lymphocytes. TI antigens can be further divided into TI type 1 antigens, which are polyclonal B lymphocyte activators (e.g., lipopolysaccharides), and TI type 2 antigens with properties of repetitive biochemical structures (e.g., bacterial capsular polysaccharides) (58). Although it is unknown whether the AAV virion surface contains highly repetitive sequences or carbohydrates, a TI B cell response can be induced from AAV infection only via intraspleen injection in mice (66). However, studies in animals and humans have suggested that the AAV capsid mounts a very strong TD immune response. The evidence includes the following: (i) no NAb was detected after muscular injection, and AAV vector transgene expression was observed following readministration of AAV2 vector via muscular injection in MHC class II-deficient mice (42); (ii) transient immunosuppression with CD4 antibody treatment at the time of primary infection elicited no NAbs and allowed transgene expression in wild-type mice following readministration of AAV vectors (19, 42, 66); (iii) our prior work has demonstrated that a high NAb titer was achieved after immunization with AAV-pulsed dendritic cells in mice (34); (iv) IgG subclasses were produced in mice and primates with AAV administration (19, 52, 53, 69); and (v) IgG subclasses were observed in humans and in patients following AAV vector treatment (6, 51, 54). In this study, isotype assays suggested that IgG2a is the predominant IgG subclass in all mice immunized with AAV1 to -5, which is consistent with other viruses that also elicit markedly increased IgG2a production in mice (20). This is in contrast to human-derived data, in which IgG1 is predominant (6, 51, 54). IgG1 was transiently generated in mice after boosting with AAV2 and AAV4. Surprisingly, in mice immunized with AAV5, IgG1 reached its peak (5 log units) at 1 week after the booster dose and persisted at 4 log units higher than levels achieved with AAV2 and AAV4. The kinetics of IgG and IgM generation and IgG subclass switching further supports that AAV types mount a T cell-dependent B cell response (46). It is tempting to speculate that blocking T-B cell interactions might represent an effective approach to overcome AAV NAb activity and allow repeat administration (1, 19, 29, 42).
Is there a need for repeat administration of AAV2 vectors?
AAV2 vectors induce long-term transgene expression in mice, dogs, and primates (1, 17, 59, 67). Persistent stable transgene expression has been observed in rhesus monkeys over 6 years and in dogs over 3 years (1, 59). Such animal studies suggest that a single application of AAV vector may induce stable lifelong therapeutic gene expression that might exclude the need for repeat administration. However, natural infection of wild-type AAV2 is prevalent in the human population. Different studies suggest that specific antibodies against AAV2 can be found in 30 to 96% of individuals, with 30 to 70% containing NAbs against AAV2 (3, 4, 18, 21, 24, 27, 48, 65). Accordingly, several studies have shown that the presence of NAbs may prevent repeat administration of the same vector (28, 29, 42, 44, 48, 55, 67). It is therefore imperative to develop effective approaches to overcome existing NAbs and allow readministration of AAV vectors to achieve therapeutically significant transgene levels.
Alternative types of AAV allow repeat administration.
To overcome AAV NAb inhibition of AAV vector transduction, several approaches have been exploited in the lab. One of them is to use a polymer to cover the AAV surface and block NAb recognition (11, 32, 33). The second approach is to use error-prone PCR to generate a library of AAV variants and select for NAb escape mutants in the presence of NAb in vitro (31, 41, 56). The third approach is to randomly modify AAV capsids and to escape NAbs (2, 36, 37) and yield novel capsids with preferred tropism. The fourth approach is to use other types of AAV which have shown no or low NAb cross-reactivity in mice (27, 30, 38, 60, 65). To date, 12 types and several variants of AAV have been isolated and sequenced (15, 16, 22, 23, 47, 50, 61, 65). Although a high prevalence of NAbs against AAV2 is seen in humans, the incidence of NAbs to alternative types appears to be nonexistent or at lower titers (23, 27). In vitro NAb assays, including those performed in this study, have demonstrated a lack of cross-reactivity between different types (22, 23, 26). The utilization of other types of AAV vectors to overcome AAV2 NAb activity has been performed in several animal models (27, 55, 64). Consistent with these studies, AAV1, AAV4, and AAV5 were found to transduce skeletal muscle regardless of preimmunization with AAV2 (Fig. 1A). The corollary, where AAV2 vectors transduced skeletal muscle in mice preimmunized against AAV1, -4, and -5 was also established (Fig. 1B). These observations support the application of alternative AAV types to circumvent preexisting NAb activity against a given AAV type.
A rationally designed AAV mutant allows vector readministration.
The majority of the human population has a natural AAV2 infection, and about half of these individuals have NAbs in the blood, which can prevent AAV transduction after systemic application. A number of surveys have demonstrated that NAb cross-activity exists among types of AAV (6, 10, 27, 45). Most recent studies, including our own, have been focused on how to evade NAb activity, and our efforts have included modification of the AAV virion or blocking NAb activity with immunosuppressants. Maheshri et al. recently demonstrated the use of DNA shuffling to generate a mutant AAV2 capsid library and isolated several mutants that retained AAV2 vector transduction efficiency in the presence of AAV2 NAbs. These mutants can potentially be utilized for repeat administration in patients with preexisting immunity to AAV2 (41). Our lab recently generated an AAV2 mutant (AAV2.5) by replacing five amino acids at the 2-fold axis of symmetry of the AAV capsid with corresponding residues from AAV1. The resulting AAV2.5 mutant was found to transduce skeletal muscle with an efficiency higher than that of AAV2 but lower than that of AAV1. Interestingly, the AAV2.5 mutant displayed a unique NAb profile different from those of AAV1 and AAV2 and was capable of promoting higher transgene expression levels in mice preimmunized against AAV2 (7). In this study, we further elucidated that the insertion of single amino acid at residue 265 of AAV2 VP1 changed the humoral immune profiles as well as the efficiency of muscle transduction. As this result was not correlated to a particular amino acid (Fig. 3 and 4), it is suggested that the enhanced muscle transduction and immune profile change from mutants with insertions at residue 265 are related to a structural change of the mutant virion.
In summary, no NAb cross-reactivity was demonstrated between the tested AAV types, and insertion of different amino acids at residue 265 of the AAV2 capsid changed the humoral immune profile and transduction efficiency. These collective results provide a platform for rational manipulation of any AAV type to obtain novel mutants that can escape preexisting immunity to specific AAV types.
ACKNOWLEDGMENTS
We thank Nathan Allen and Jennifer Giles for their comments and for proofreading the manuscript.
This study was partly supported by NIH research grants R01 AI080726, DK084033, AI072176, and U54 AR056953 to R.J.S. and R01 GM082946 to M.A.-M.
Footnotes
Published ahead of print 16 May 2012
REFERENCES
- 1. Arruda VR, et al. 2005. Regional intravascular delivery of AAV-2-F.IX to skeletal muscle achieves long-term correction of hemophilia B in a large animal model. Blood 105:3458–3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Asokan A, et al. 2010. Reengineering a receptor footprint of adeno-associated virus enables selective and systemic gene transfer to muscle. Nat. Biotechnol. 28:79–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blacklow NR, Hoggan MD, Kapikian AZ, Austin JB, Rowe WP. 1968. Epidemiology of adenovirus-associated virus infection in a nursery population. Am. J. Epidemiol. 88:368–378 [DOI] [PubMed] [Google Scholar]
- 4. Blacklow NR, Hoggan MD, Rowe WP. 1968. Serologic evidence for human infection with adenovirus-associated viruses. J. Natl. Cancer Inst. 40:319–327 [PubMed] [Google Scholar]
- 5. Blankinship MJ, et al. 2004. Efficient transduction of skeletal muscle using vectors based on adeno-associated virus serotype 6. Mol. Ther. 10:671–678 [DOI] [PubMed] [Google Scholar]
- 6. Boutin S, et al. 2010. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum. Gene Ther. 21:704–712 [DOI] [PubMed] [Google Scholar]
- 7. Bowles DE, et al. 2012. Phase 1 gene therapy for Duchenne muscular dystrophy using a translational optimized AAV vector. Mol. Ther. 20:443–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brantly ML, et al. 2009. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc. Natl. Acad. Sci. U. S. A. 106:16363–16368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Calcedo R, et al. 2011. Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin. Vaccine Immunol. 18:1586–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Calcedo R, Vandenberghe LH, Gao G, Lin J, Wilson JM. 2009. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J. Infect. Dis. 199:381–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carlisle RC, et al. 2008. Coating of adeno-associated virus with reactive polymers can ablate virus tropism, enable retargeting and provide resistance to neutralising antisera. J. Gene Med. 10:400–411 [DOI] [PubMed] [Google Scholar]
- 12. Chao H, et al. 2000. Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol. Ther. 2:619–623 [DOI] [PubMed] [Google Scholar]
- 13. Chao H, Monahan PE, Liu Y, Samulski RJ, Walsh CE. 2001. Sustained and complete phenotype correction of hemophilia B mice following intramuscular injection of AAV1 serotype vectors. Mol. Ther. 4:217–222 [DOI] [PubMed] [Google Scholar]
- 14. Chao H, et al. 1999. Persistent expression of canine factor IX in hemophilia B canines. Gene Ther. 6:1695–1704 [DOI] [PubMed] [Google Scholar]
- 15. Chiorini JA, Kim F, Yang L, Kotin RM. 1999. Cloning and characterization of adeno-associated virus type 5. J. Virol. 73:1309–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chiorini JA, Yang L, Liu Y, Safer B, Kotin RM. 1997. Cloning of adeno-associated virus type 4 (AAV4) and generation of recombinant AAV4 particles. J. Virol. 71:6823–6833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chiorini JA, Yang L, Safer B, Kotin RM. 1995. Determination of adeno-associated virus Rep68 and Rep78 binding sites by random sequence oligonucleotide selection. J. Virol. 69:7334–7338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chirmule N, et al. 1999. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 6:1574–1583 [DOI] [PubMed] [Google Scholar]
- 19. Chirmule N, et al. 2000. Humoral immunity to adeno-associated virus type 2 vectors following administration to murine and nonhuman primate muscle. J. Virol. 74:2420–2425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Coutelier JP, van der Logt JT, Heessen FW, Vink A, van Snick J. 1988. Virally induced modulation of murine IgG antibody subclasses. J. Exp. Med. 168:2373–2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Erles K, Sebokova P, Schlehofer JR. 1999. Update on the prevalence of serum antibodies (IgG and IgM) to adeno-associated virus (AAV). J. Med. Virol. 59:406–411 [DOI] [PubMed] [Google Scholar]
- 22. Gao G, et al. 2004. Clades of adeno-associated viruses are widely disseminated in human tissues. J. Virol. 78:6381–6388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gao GP, et al. 2002. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc. Natl. Acad. Sci. U. S. A. 99:11854–11859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Georg-Fries B, Biederlack S, Wolf J, zur Hausen H. 1984. Analysis of proteins, helper dependence, and seroepidemiology of a new human parvovirus. Virology 134:64–71 [DOI] [PubMed] [Google Scholar]
- 25. Grewal IS, Flavell RA. 1998. CD40 and CD154 in cell-mediated immunity. Annu. Rev. Immunol. 16:111–135 [DOI] [PubMed] [Google Scholar]
- 26. Grimm D, et al. 2003. Preclinical in vivo evaluation of pseudotyped adeno-associated virus vectors for liver gene therapy. Blood 102:2412–2419 [DOI] [PubMed] [Google Scholar]
- 27. Halbert CL, Rutledge EA, Allen JM, Russell DW, Miller AD. 2000. Repeat transduction in the mouse lung by using adeno-associated virus vectors with different serotypes. J. Virol. 74:1524–1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Halbert CL, et al. 1997. Transduction by adeno-associated virus vectors in the rabbit airway: efficiency, persistence, and readministration. J. Virol. 71:5932–5941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Halbert CL, Standaert TA, Wilson CB, Miller AD. 1998. Successful readministration of adeno-associated virus vectors to the mouse lung requires transient immunosuppression during the initial exposure. J. Virol. 72:9795–9805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hildinger M, et al. 2001. Hybrid vectors based on adeno-associated virus serotypes 2 and 5 for muscle-directed gene transfer. J. Virol. 75:6199–6203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huttner NA, et al. 2003. Genetic modifications of the adeno-associated virus type 2 capsid reduce the affinity and the neutralizing effects of human serum antibodies. Gene Ther. 10:2139–2147 [DOI] [PubMed] [Google Scholar]
- 32. Le HT, Yu QC, Wilson JM, Croyle MA. 2005. Utility of PEGylated recombinant adeno-associated viruses for gene transfer. J. Control Release 108:161–177 [DOI] [PubMed] [Google Scholar]
- 33. Lee GK, Maheshri N, Kaspar B, Schaffer DV. 2005. PEG conjugation moderately protects adeno-associated viral vectors against antibody neutralization. Biotechnol. Bioeng. 92:24–34 [DOI] [PubMed] [Google Scholar]
- 34. Li C, et al. 2007. Adeno-associated virus type 2 (AAV2) capsid-specific cytotoxic T lymphocytes eliminate only vector-transduced cells coexpressing the AAV2 capsid in vivo. J. Virol. 81:7540–7547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li C, et al. 2012. Neutralizing antibodies against adeno-associated virus examined prospectively in pediatric patients with hemophilia. Gene Ther. 19:288–294 [DOI] [PubMed] [Google Scholar]
- 36. Li W, et al. 2008. Engineering and selection of shuffled AAV genomes: a new strategy for producing targeted biological nanoparticles. Mol. Ther. 16:1252–1260 [DOI] [PubMed] [Google Scholar]
- 37. Lochrie MA, et al. 2006. Mutations on the external surfaces of adeno-associated virus type 2 capsids that affect transduction and neutralization. J. Virol. 80:821–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Maersch S, Huber A, Buning H, Hallek M, Perabo L. 2010. Optimization of stealth adeno-associated virus vectors by randomization of immunogenic epitopes. Virology 397:167–175 [DOI] [PubMed] [Google Scholar]
- 39. Maguire AM, et al. 2009. Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial. Lancet 374:1597–1605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maguire AM, et al. 2008. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N. Engl. J. Med. 358:2240–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Maheshri N, Koerber JT, Kaspar BK, Schaffer DV. 2006. Directed evolution of adeno-associated virus yields enhanced gene delivery vectors. Nat. Biotechnol. 24:198–204 [DOI] [PubMed] [Google Scholar]
- 42. Manning WC, Zhou S, Bland MP, Escobedo JA, Dwarki V. 1998. Transient immunosuppression allows transgene expression following readministration of adeno-associated viral vectors. Hum. Gene Ther. 9:477–485 [DOI] [PubMed] [Google Scholar]
- 43. Manno CS, et al. 2003. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood 101:2963–2972 [DOI] [PubMed] [Google Scholar]
- 44. Manno CS, et al. 2006. Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat. Med. 12:342–347 [DOI] [PubMed] [Google Scholar]
- 45. Mendell JR, et al. 2010. Dystrophin immunity in Duchenne's muscular dystrophy. N. Engl. J. Med. 363:1429–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mond JJ, Vos Q, Lees A, Snapper CM. 1995. T cell independent antigens. Curr. Opin. Immunol. 7:349–354 [DOI] [PubMed] [Google Scholar]
- 47. Mori S, Wang L, Takeuchi T, Kanda T. 2004. Two novel adeno-associated viruses from cynomolgus monkey: pseudotyping characterization of capsid protein. Virology 330:375–383 [DOI] [PubMed] [Google Scholar]
- 48. Moskalenko M, et al. 2000. Epitope mapping of human anti-adeno-associated virus type 2 neutralizing antibodies: implications for gene therapy and virus structure. J. Virol. 74:1761–1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moss RB, et al. 2004. Repeated adeno-associated virus serotype 2 aerosol-mediated cystic fibrosis transmembrane regulator gene transfer to the lungs of patients with cystic fibrosis: a multicenter, double-blind, placebo-controlled trial. Chest 125:509–521 [DOI] [PubMed] [Google Scholar]
- 50. Muramatsu S, Mizukami H, Young NS, Brown KE. 1996. Nucleotide sequencing and generation of an infectious clone of adeno-associated virus 3. Virology 221:208–217 [DOI] [PubMed] [Google Scholar]
- 51. Murphy SL, et al. 2009. Diverse IgG subclass responses to adeno-associated virus infection and vector administration. J. Med. Virol. 81:65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nathwani AC, et al. 2007. Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates. Blood 109:1414–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nathwani AC, et al. 2006. Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood 107:2653–2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nathwani AC, et al. 2011. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N. Engl. J. Med. 365:2357–2365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Peden CS, Burger C, Muzyczka N, Mandel RJ. 2004. Circulating anti-wild-type adeno-associated virus type 2 (AAV2) antibodies inhibit recombinant AAV2 (rAAV2)-mediated, but not rAAV5-mediated, gene transfer in the brain. J. Virol. 78:6344–6359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Perabo L, et al. 2006. Combinatorial engineering of a gene therapy vector: directed evolution of adeno-associated virus. J. Gene Med. 8:155–162 [DOI] [PubMed] [Google Scholar]
- 57. Rabinowitz JE, et al. 2002. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J. Virol. 76:791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rijkers GT, Mosier DE. 1985. Pneumococcal polysaccharides induce antibody formation by human B lymphocytes in vitro. J. Immunol. 135:1–4 [PubMed] [Google Scholar]
- 59. Rivera VM, et al. 2005. Long-term pharmacologically regulated expression of erythropoietin in primates following AAV-mediated gene transfer. Blood 105:1424–1430 [DOI] [PubMed] [Google Scholar]
- 60. Riviere C, Danos O, Douar AM. 2006. Long-term expression and repeated administration of AAV type 1, 2 and 5 vectors in skeletal muscle of immunocompetent adult mice. Gene Ther. 13:1300–1308 [DOI] [PubMed] [Google Scholar]
- 61. Rutledge EA, Halbert CL, Russell DW. 1998. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J. Virol. 72:309–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Scallan CD, et al. 2006. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood 107:1810–1817 [DOI] [PubMed] [Google Scholar]
- 63. Wagner JA, et al. 2002. A phase II, double-blind, randomized, placebo-controlled clinical trial of tgAAVCF using maxillary sinus delivery in patients with cystic fibrosis with antrostomies. Hum. Gene Ther. 13:1349–1359 [DOI] [PubMed] [Google Scholar]
- 64. Wang L, et al. 2005. Sustained correction of disease in naive and AAV2-pretreated hemophilia B dogs: AAV2/8-mediated, liver-directed gene therapy. Blood 105:3079–3086 [DOI] [PubMed] [Google Scholar]
- 65. Xiao W, et al. 1999. Gene therapy vectors based on adeno-associated virus type 1. J. Virol. 73:3994–4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xiao W, et al. 2000. Route of administration determines induction of T-cell-independent humoral responses to adeno-associated virus vectors. Mol. Ther. 1:323–329 [DOI] [PubMed] [Google Scholar]
- 67. Xiao X, Li J, Samulski RJ. 1996. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J. Virol. 70:8098–8108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Xiao X, Li J, Samulski RJ. 1998. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J. Virol. 72:2224–2232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang HG, et al. 2005. Genetic analysis of the antibody response to AAV2 and factor IX. Mol. Ther. 11:866–874 [DOI] [PubMed] [Google Scholar]