Abstract
Aberrant expression of regulatory receptors programmed death-1 (PD-1) and B- and T-lymphocyte attenuator (BTLA) is linked with dysregulation and exhaustion of T lymphocytes during chronic human immunodeficiency virus type 1 (HIV-1) infection; however, less is known about whether a similar process impacts B-lymphocyte function during HIV-1 infection. We reasoned that disruption of the peripheral B cell compartment might be associated with decreased neutralizing antibody activity. Expression of markers that indicate dysregulation (BTLA and PD-1), immune activation (CD95), and proliferation (Ki-67) was evaluated in B cells from HIV-1-infected viremic and aviremic subjects and healthy subjects, in conjunction with immunoglobulin production and CD4 T cell count. Viral load and cross-clade neutralizing activity in plasma from viremic subjects were also assessed. Dysregulation of B lymphocytes was indicated by a marked disruption of peripheral B cell subsets, increased levels of PD-1 expression, and decreased levels of BTLA expression in viremic subjects compared to aviremic subjects and healthy controls. PD-1 and BTLA were correlated in a divergent fashion with immune activation, CD4 T cell count, and the total plasma IgG level, a functional correlate of B cell dysfunction. Within viremic subjects, the total IgG level correlated directly with cross-clade neutralizing activity in plasma. The findings demonstrate that even in chronically infected subjects in which B lymphocytes display multiple indications of dysfunction, antibodies that mediate cross-clade neutralization breadth continue to circulate in plasma.
INTRODUCTION
Infection with human immunodeficiency virus type 1 (HIV-1) leads to widespread dysfunction of the immune system, including B lymphocytes. One sign of B cell dysfunction in HIV-1 infection is an increase in the production of IgG, or hypergammaglobulinemia (8, 21, 29). B lymphocytes of HIV-1-infected persons also exhibit signs of polyclonal activation and autoreactivity (46) and impaired responses to both T-dependent and -independent antigenic stimuli or immunization (19, 20, 36, 39). These dysfunctions have been attributed, in part, to an imbalance of four major subsets within the B cell compartment (31, 32). Combination antiretroviral therapy (cART) only partially restores the balance, even after 12 months of treatment (31).
Since first introduced by Ascher and Sheppard in the late 1980s, the concept of immune activation as a causative mechanism of HIV-1 pathogenesis/AIDS has garnered immense consideration and experimental evaluation (1). The degree of immune activation has been implicated in disease progression pace (15). Normally, a delicate interplay among several regulatory receptors tightly governs activation of the immune system. Recently, the importance of programmed death-1 (PD-1, CD279) has been emphasized in the development of hyperimmune activation and exhaustion within T lymphocytes during chronic viral infections, including HIV-1 (2, 6, 7, 17, 18, 52). Less is known about the role of PD-1 in the maintenance of B cell function, but a recent study demonstrated that PD-1 expression on activated memory B cells in simian immunodeficiency virus (SIV) infection was associated with rapid disease progression (49). Similar to PD-1, B- and T-lymphocyte attenuator (BTLA, CD272) is another member of the B7/CD28 superfamily (51). This regulatory receptor is decreased on CD4 and CD8 T cells during chronic HIV-1 infection, and its expression is inversely correlated with disease progression (53). Thus, aberrant expression of PD-1 and BTLA on T cells in HIV-1 infection has been associated with disease progression.
Antibodies that can mediate neutralization of heterologous HIV-1 viruses are desirable from a vaccine perspective, but it is unclear how they arise or if they provide any benefit to the patient. Furthermore, these types of neutralizing antibodies (nAbs) are detected only after several years of infection and in only a subset of infected individuals (3, 5, 10, 13, 14, 35, 42, 47, 50). Factors that have been suggested to promote the development of neutralization breadth include prolonged exposure to antigen, higher envelope diversity, and plasma viral load (9, 12, 14, 34, 37, 42). Nevertheless, neutralization breadth does not delay disease progression (13, 14, 37). Others have demonstrated that peripheral B cell decline and other perturbations do not necessarily impede nAb activity as measured in vitro (4, 35), but to date no one has measured neutralization breadth in a cohort of HIV-1-infected subjects for which multiple aspects of B cell dysfunction have been evaluated in parallel.
Here we evaluated the state of the peripheral B cell compartment in chronically HIV-1-infected individuals, infected but aviremic subjects treated with cART, and healthy controls by evaluating levels of PD-1 and BTLA expression on total B cells and within peripheral B cell subsets. Aberrant expression of these receptors was observed in viremic individuals and was correlated with increased levels of immune activation, proliferation, IgG production, and CD4 T cell decline. We also investigated whether individuals experiencing these signs of B cell dysfunction possessed antibody-mediated neutralization capacity against pseudotyped heterologous HIV-1 envelope (Env) glycoproteins. Strong cross-clade neutralizing antibody activity was detected in the plasma of a subset of these infected individuals, even though the B cell compartment was perturbed.
MATERIALS AND METHODS
Study subjects.
In compliance with procedures approved by the Emory University Institutional Review Board (IRB), 41 individuals were enrolled with informed consent for this study. Participants were categorized into three groups: healthy controls (HC, n = 12) included persons without HIV-1 infection or any clinical symptoms at the time of enrollment; viremic subjects (VI, n = 16) had clinical records of HIV-1 infection but were cART naïve and had plasma viral loads greater than 1,000 copies/ml; and aviremic subjects (AV, n = 13) were HIV-1 infected and currently on cART with a plasma viral load of fewer than 100 copies/ml. Median age, CD4 T cell count, and viral load, as well as the gender and ethnicity of the study participants, are listed in Table 1.
Table 1.
Characteristics of the study participants
| Characteristic | HC | AVa,b | VIb |
|---|---|---|---|
| No. of subjects | 12 | 13 | 16 |
| Gender | |||
| Male | 8 | 9 | 14 |
| Female | 4 | 4 | 2 |
| Ethnicity | |||
| Caucasian | 5 | 0 | 2 |
| African American | 3 | 13 | 14 |
| Asian | 2 | 0 | 0 |
| Mixed | 2 | 0 | 0 |
| Median age, yr (range) | 32 (20–56) | 46 (33–65) | 37 (22–50) |
| Median CD4 count,c cells/μl (range) | 632 (375–1,094) | 329 (31–988) | 104 (4–465) |
| Median viral load, copies/ml (range) | NAd | <100 | 129,092 (4,189–676,811) |
The duration of the cART regimen was greater than 6 months for all aviremic subjects.
All HIV-1-infected subjects (aviremic and viremic) were classified as CDC stage C3.
P < 0.05 for HC versus VI and AV versus VI.
NA, not applicable.
PBMC isolation.
Approximately 50 ml blood was collected from each participant in acid-citrate-dextrose (ACD)-containing BD-Vacutainer blood collection tubes, with informed consent from the donor. Peripheral blood mononuclear cells (PBMCs) were isolated from fresh blood by standard Ficoll-Paque density gradient centrifugation (Ficoll-Paque Plus, GE Healthcare). PBMCs were then aliquoted and cryopreserved in liquid nitrogen (−160°C) until needed for flow cytometry.
Flow-cytometric analysis of peripheral B cells.
PBMCs were thawed and washed twice with phosphate-buffered saline (PBS) and then resuspended in fluorescence-activated cell sorter (FACS) buffer (PBS with 1% bovine serum albumin [BSA] and 0.1% sodium azide). Two million cells were used for surface staining with the following antibodies: yellow fluorescent reactive dye (live/dead stain), anti-CD3 V500 (SP34-2), anti-CD14 V500 (M5E2), anti-PD-1 APC (EH12.2H7), anti-BTLA PE (J168-540), anti-CD19 Qdot655 (SJ25C1), anti-CD10 APC-Cy7 (HI10a), anti-CD21 PE-Cy5 (B-ly4), anti-CD27 PE-Cy7 (1A4CD27), and anti-CD95 FITC (DX2). Following live/dead cell staining, PBMCs were incubated with antibodies at 4°C for 30 min; cells were fixed, and any contamination of red blood cells (RBC) was removed by incubation in 1× lysing solution (BD Bioscience) for 10 min at room temperature. For intracellular staining, PBMCs were further washed twice with FACS buffer and permeabilized with 1× permeabilizing solution (BD Bioscience) for 30 min at room temperature. Anti-Ki-67 Alexa Fluor 700 (B56) antibody was used for the intracellular staining at room temperature for 30 min. After washing twice, cells were resuspended in 400 μl FACS buffer containing 1% paraformaldehyde. Fluorescence minus one (FMO) negative controls were included for staining. An LSR-II cell analyzer (BD Bioscience) was used to acquire data. Lymphocytes were gated based on forward versus side scatter profile, and B lymphocytes were gated as CD19+ cells after exclusion of dead, CD3+, and CD14+ cells. Data were analyzed using FlowJo software (version 9.3.1; TreeStar Inc., USA).
ELISA for plasma IgG.
Total IgG concentration in plasma was measured by using a human IgG enzyme-linked immunosorbent assay (ELISA) quantitation set (Bethyl Laboratories Inc.) according to the manufacturer's directions. Plasma was heat inactivated (56°C for 60 min) and then diluted to 1:100,000 for the experiments. Endpoint absorbance was measured at 450 nm with a BioTek Synergy multidetection microplate reader, and data were analyzed with KC4 v3.4 software. A human reference serum was used to normalize total IgG concentrations in plasma.
ELISA for binding to monomeric gp120.
Immulon microtiter 96-well plates were coated with 100 μl of HIV-1 BaL gp120 diluted to 5 μg/ml in coating buffer (Institute of Human Virology, μQuant Facility). Plates were washed 3 times and then blocked for 30 min at 37°C. Following washing, 100 μl of heat-inactivated plasma was added to each well and incubated for 1 h. Plates were washed 3 times, and 100 μl of horseradish peroxidase (HRP)-conjugated goat anti-human IgG was added to each well. After a 1-h incubation at 37°C, plates were washed, and 3,3′,5,5′-tetramethylbenzidine (TMB) substrate was added. After 10 min, reactions were stopped with 4 N H2SO4, endpoint absorbance was measured at 450 nm with a BioTek Synergy multidetection microplate reader, and data were analyzed with KC4 v3.4 software.
Neutralization assay.
The ability of plasma from 16 viremic individuals to neutralize a cross-clade panel of 13 HIV-1 envelope (Env) pseudotyped virions was measured using the Tzm-bl luciferase assay as described previously (22, 27, 40, 41). Each plasma-Env combination was analyzed independently at least two times with duplicate wells. The neutralization 50% inhibitory concentration (IC50) for each plasma-Env combination was calculated using linear regression analysis in GraphPad Prism version 5.0. IC50s that were less than the highest dilution of plasma tested (1:100) were assigned a score of 1:50. Neutralization breadth was calculated as the number of pseudoviruses neutralized with an IC50 of greater than 1:100, and potency was defined by (i) dividing the IC50 for each given plasma-Env combination by the median IC50 for that pseudovirus against all plasma samples and (ii) adding the scores for each plasma sample, as described in reference 26. Higher scores indicate greater breadth and potency. All Env clones were obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: 6535.3, SS1196.1, TRO.11, AC10.0.29, and PVO.4 are from the standard reference panel for subtype B HIV-1 Env clones (23); ZM197M.PB7, Du172.17, Du156.12, ZM109F.PB4, CAP45.2.00.G3, and ZM214M.PL15 are from the subtype C HIV-1 reference panel of Env clones (24); subtype A Env clones Q23ENV17 (38) and Q769ENVd22 (25) were contributed by Julie Overbaugh.
Blood CD4 T cell count and plasma HIV-1 viral load.
Blood CD4 T cell count was measured by the Emory University CFAR Immunology core, and plasma viral load was quantified by the Virology core. Briefly, the absolute number of peripheral blood lymphocytes was calculated from the total white blood cell (WBC) count determined with an automated hematology analyzer, and the percentage of CD4 T-lymphocyte population was determined by flow cytometry. The plasma HIV-1 RNA level was measured using the Cobas Amplicor HIV Monitor test (version 1.5; Roche) or the Abbott Real Time HIV-1 assay on an automated m2000 system, according to the manufacturer's directions.
Statistical analysis.
Nonparametric one-way analysis of variance (1-way ANOVA, Kruskal-Wallis with Dunn's posttest) and Spearman's rank correlation tests were performed with GraphPad Prism version 5.0 to evaluate the data. A P value of less than 0.05 (95% confidence level) was considered significant.
RESULTS
Regulatory receptors PD-1 and BTLA are aberrantly expressed on B lymphocytes during chronic HIV-1 infection.
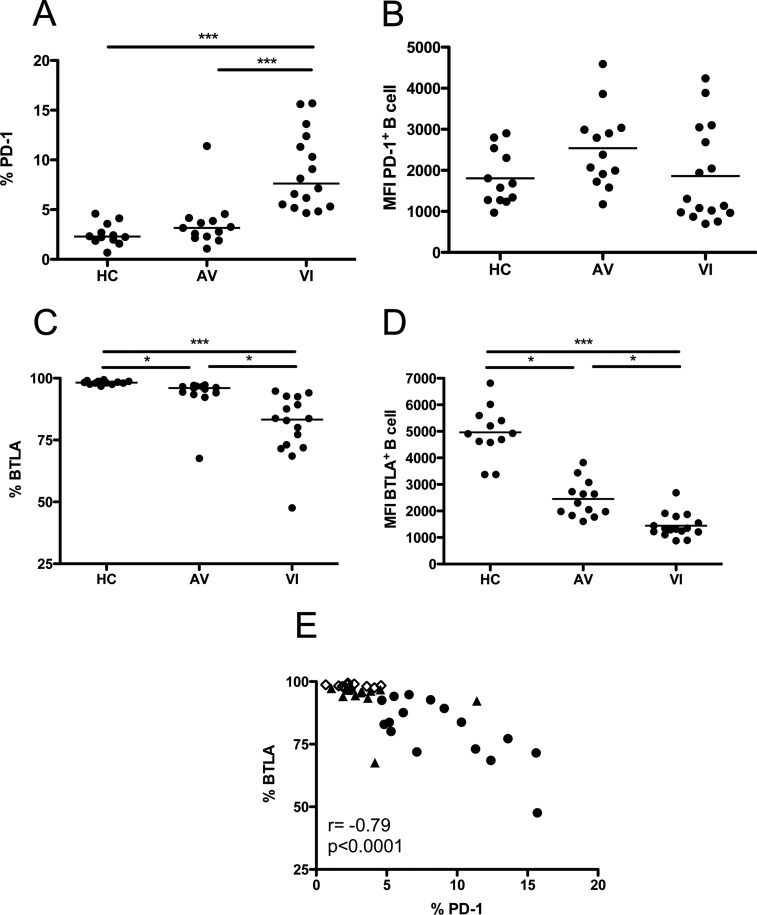
To investigate dysregulation within the B cell compartment during chronic HIV-1 infection, expression of the inhibitory receptors PD-1 and BTLA was assessed by flow cytometry and compared among healthy controls (HC), aviremic subjects (AV), and viremic subjects (VI) (Fig. 1). In HC, only a minor proportion of B cells expressed PD-1 (Fig. 1A), consistent with what is observed in T lymphocytes (7, 52). In VI, expression of PD-1 was significantly increased (Fig. 1A, P < 0.001). AV subjects had PD-1 expression levels that were significantly lower than those of VI (Fig. 1A, P < 0.001) but were not significantly different from HC. Thus, active viral replication in VI is associated with a significant increase in PD-1 expression on B cells, which is alleviated by cART. Despite a greater percentage of B cells expressing PD-1 in VI, the level of receptor expression per CD19+ PD-1+ cell was not different from that of HC or AV (Fig. 1B). Instead, comparable mean fluorescence intensity (MFI) values were observed across the three groups.
Fig 1.
Expression of PD-1 and BTLA by B lymphocytes. (A and C) Percentages of total B cells (CD19+) that express PD-1 (A) and BTLA (C) in HC, AV, and VI subjects. (B and D) Mean fluorescence intensity (MFI) for PD-1 (B) and BTLA (D) expression by individual PD-1+ or BTLA+ CD19+ B cells. Each point represents data from a single subject. Horizontal bars within the point plots indicate the median percentage for each group. Significance between groups determined by 1-way ANOVA is indicated above the groups: *, P < 0.05; **, P < 0.01; ***, P < 0.001. (E) Correlation between percentages of total B cells that express PD-1 and BTLA. Spearman's rank correlation coefficient (r) and level of significance (p) are indicated within the graph. Open diamonds, HC; closed triangles, AV; closed circles, VI.
The majority of B cells in HC expressed BTLA on their surface (Fig. 1C). However, VI showed a significant decline in BTLA expression compared to HC (Fig. 1C, P < 0.001). AV individuals had intermediate levels of BTLA expression that were significantly different from that of both HC and VI (Fig. 1C, P < 0.05), representing only partial restoration of normal BTLA levels. In addition to the decrease in percentage of BTLA-expressing B cells in VI and AV, the MFIs of individual CD19+ BTLA+ cells were significantly lower in VI and AV than in HC (Fig. 1D, P < 0.001 and P < 0.05, respectively). Thus, modulation of BTLA expression by HIV-1 infection occurred at both the population and single-cell level and remained depressed even when viral replication was suppressed by cART. HIV-1 infection exerts a differential effect on B cell expression of PD-1 and BTLA, as evidenced by the strong inverse correlation between the two receptors (Fig. 1E, P < 0.0001). The aberrant expression of these receptors in VI indicates that homeostasis within the B cell compartment is significantly disrupted.
Peripheral B cell subsets are dysregulated during chronic HIV-1 infection.
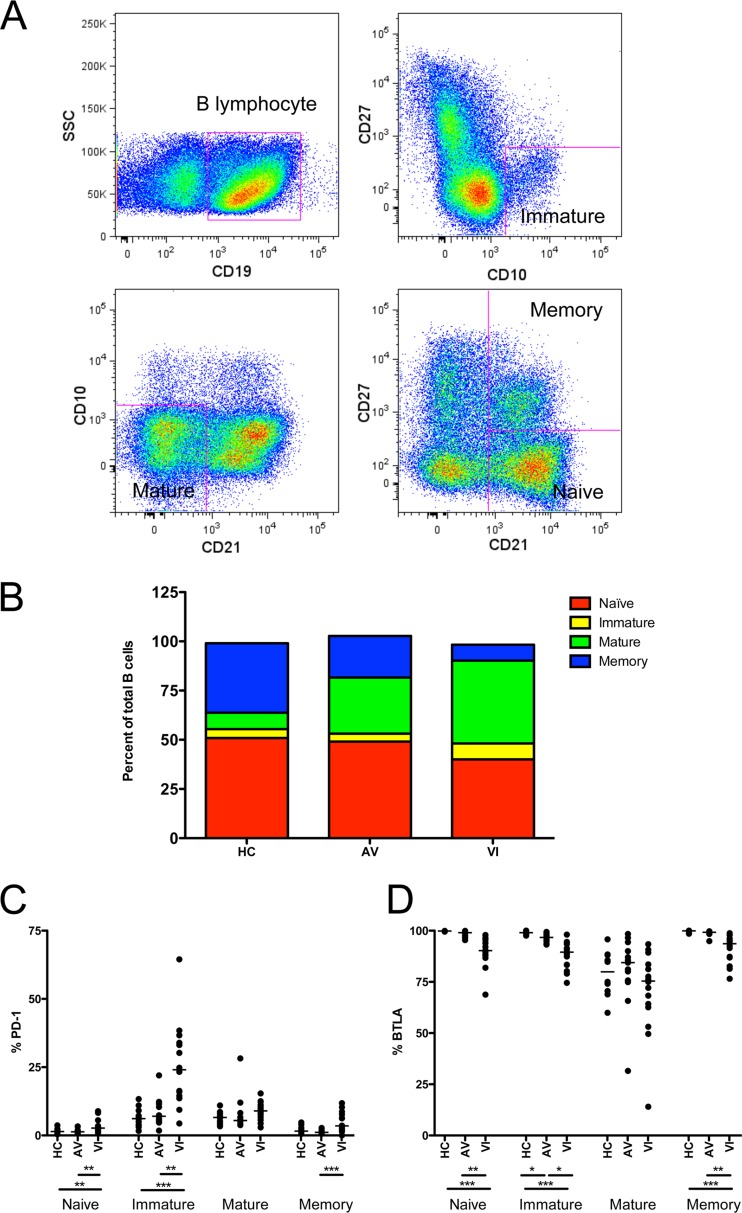
We next examined whether altered PD-1 and BTLA expression levels in the total B cells of VI were reflected in specific B-lymphocyte subsets. Figure 2A displays the strategy used for separating total B cells (CD19+) into 4 phenotypic subsets: immature (CD10+CD27−), mature (CD10−CD21lo), naïve (CD10−CD21hiCD27−), and classical memory (CD10−CD21hiCD27+), as described previously (31). Similar to a published study by Moir et al. (31), a decrease in the proportion of naïve and memory B cells and an increase in the immature and mature populations were observed in VI compared to HC (Fig. 2B). The change in the proportion of mature B cells was dramatic, increasing from 8% in HC to 43% in VI. Likewise, a substantial decline in the memory B cell subset, from 36% in HC to 8% in VI, was observed. Thus, mature B cells came to dominate the peripheral B cell compartment in VI. The balance within B cell subsets in AV was partially restored, falling somewhere between the proportions seen in HC and in VI.
Fig 2.
Distribution of B-lymphocyte subsets and expression of PD-1 and BTLA. (A) Flow cytometry gating strategy for separating B cell subsets. Within the B lymphocytes (CD19+), cells were further gated into four subsets, defined as immature (CD10+CD27−), mature (CD10−CD21lo), naive (CD10−CD21hiCD27−), and memory (CD10−CD21hiCD27+). (B) Mean proportions of naive, immature, mature, and memory subsets within the total B cell population in HC, AV, and VI subjects. (C and D) Percentages of naive, immature, mature, and memory B cells that express PD-1 (C) and BTLA (D) in HC, AV, and VI. Each point represents data from a single subject. Horizontal bars within the point plots indicate the median percentage for each group. Significance between groups determined by 1-way ANOVA is indicated below the groups: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
The B cell subsets differed in their respective PD-1 and BTLA expression patterns (Fig. 2C and D, respectively). In HC, low PD-1 expression was observed in all subsets, but particularly within naive and memory B cells (Fig. 2C). In VI, PD-1 expression was significantly increased in the naive and immature subpopulations of B cells compared to HC (Fig. 2C, P < 0.01 and P < 0.001, respectively). However, PD-1 expression on naive and immature B cells in AV was not different from HC, indicating some partial restoration (Fig. 2C). BTLA expression in VI was significantly decreased in the naive, immature, and memory B cell subsets compared to HC and AV (Fig. 2D, P < 0.001 and at least P < 0.05, respectively). BTLA expression in AV was not different from that of HC except in immature B cells, where it remained significantly lower than in HC (Fig. 2D, P < 0.05). In the mature B cell subset, significant differences in the expression of PD-1 and BTLA were not detected among HC, AV, and VI. However, because the mature B cell subset is expanded in VI (Fig. 2B), these B cells may contribute disproportionately to the overall increase in PD-1 and decrease in BTLA expression.
PD-1 and BTLA expression on B lymphocytes is correlated with markers of immune activation, proliferation, and disease progression.
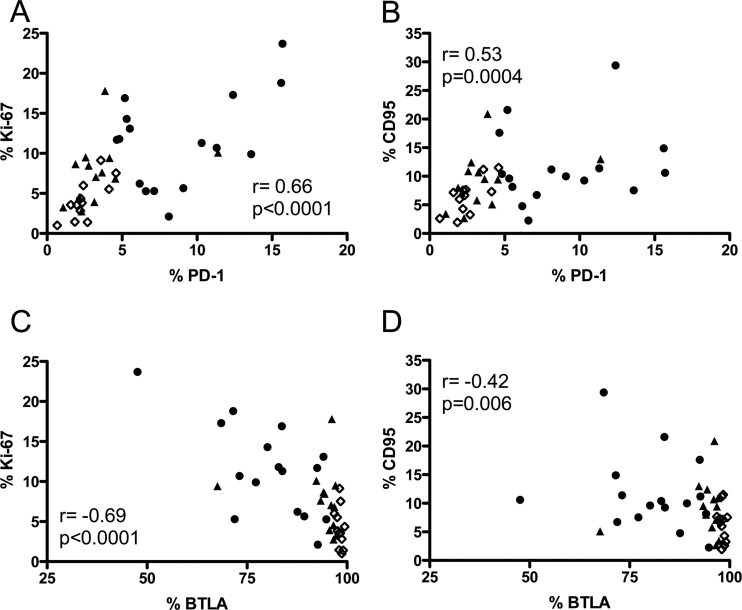
Because generalized immune activation is an important factor in determining the course of HIV-1 infection, we also investigated whether PD-1 and BTLA expression was associated with Ki-67 or CD95 on total B cells. A strong positive correlation was found between PD-1 expression and Ki-67 (P < 0.0001) and CD95 (P = 0.0004) (Fig. 3A and B, respectively), whereas BTLA exhibited an inverse correlation with these markers (Fig. 3C and D, P < 0.0001 and P = 0.006, respectively). These results suggest a direct link between dysregulation and immune activation in the B cell compartment.
Fig 3.
Correlation of PD-1 and BTLA expression on B lymphocytes with Ki-67 and CD95. (A and B) Correlations between percentages of total B cells (CD19+) that express PD-1 and percentages expressing Ki-67 (A) or CD95 (B). (C and D) Correlations between BTLA and Ki-67 (C) or CD95 (D) expression. Spearman's rank correlation coefficient (r) and level of significance (p) are indicated within each graph. Each point represents data from a single subject. Open diamonds, HC; closed triangles, AV; closed circles, VI.
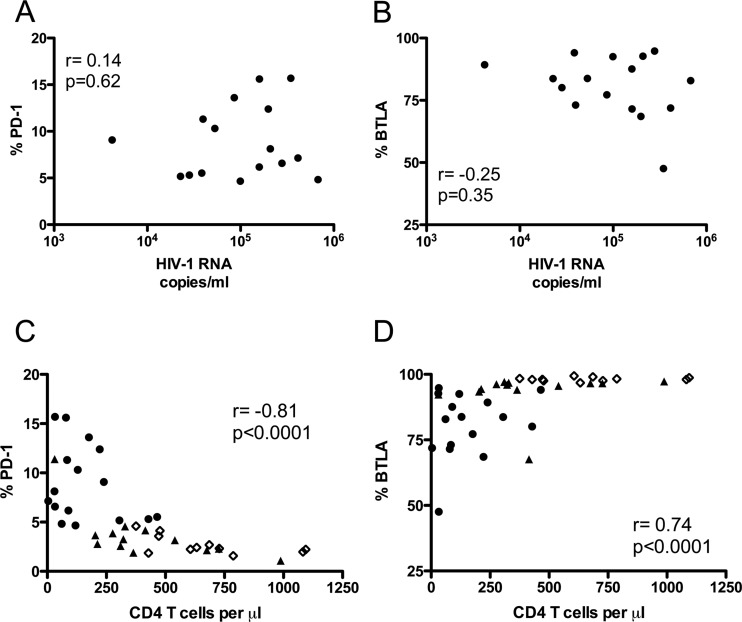
The relationship between PD-1 and BTLA expression on total B cells and two indicators of disease progression, plasma viral load and blood CD4 T cell count, was also assessed. PD-1 or BTLA expression on B lymphocytes was not significantly associated with plasma viral load in VI (Fig. 4A and B, respectively). However, a significant correlation was observed between CD4 T cell count and PD-1 or BTLA expression, including data from the three subject groups (Fig. 4C and D, respectively, P < 0.0001). PD-1 expression on total B cells was inversely correlated with CD4 T cell count, while the correlation for BTLA expression and CD4 T cell count was direct.
Fig 4.
Correlation of PD-1 and BTLA expression on B lymphocytes with markers of HIV-1 disease progression. (A and B) Correlations between plasma viral load (HIV-1 RNA copies/ml) and percentage of total B cells (CD19+) that express PD-1 (A) or BTLA (B). (C and D) Correlations between blood CD4 count (CD4 T cells/μl) and percentage of total B cells (CD19+) that express PD-1 (C) or BTLA (D). Spearman's rank correlation coefficient (r) and level of significance (p) are indicated in each graph. Each point represents data from a single subject. Open diamonds, HC; closed triangles, AV; closed circles, VI.
PD-1 and BTLA expression on B lymphocytes is correlated with plasma IgG level.
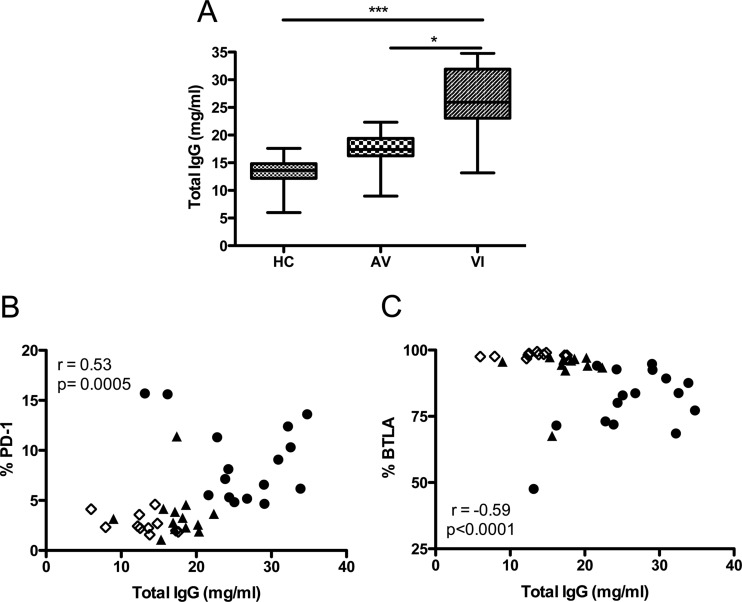
Hypergammaglobulinemia is a direct manifestation of B cell dysfunction during HIV-1 infection. We therefore examined the relationship between PD-1 and BTLA expression on B cells and plasma total IgG level for each group of subjects. Concurrent with previous reports, a significant increase in the plasma total IgG level was observed in VI compared to both AV and HC (Fig. 5A, P < 0.05 and P < 0.001, respectively). Viral suppression mediated by cART resulted in lower levels of total IgG production. In addition, highly significant direct and indirect correlations between total IgG level and PD-1 or BTLA expression on B cells were identified (Fig. 5B and C, P = 0.0005 and P < 0.0001, respectively). Thus, regulatory receptor expression is linked with this functional anomaly of the B cell compartment.
Fig 5.
Correlation of PD-1 and BTLA expression on B lymphocytes with total plasma IgG levels. (A) Concentrations of total IgG (mg/ml) in the plasma of HC, AV, and VI subjects. Horizontal lines within the boxes indicate the median value for each group. Boxes represent the 25th to 75th percentiles, and brackets represent the minimum to maximum values in each group. Significance between groups by 1-way ANOVA is indicated above the groups: *, P < 0.05; ***, P < 0.001. (B and C) Correlations between total IgG concentration (mg/ml) in plasma and percentage of total B cells (CD19+) that express PD-1 (B) or BTLA (C). Spearman's rank correlation coefficient (r) and level of significance (p) are indicated within each graph. Each point represents data from a single subject. Open diamonds, HC; closed triangles, AV; closed circles, VI.
Total IgG level in plasma but not immune dysregulation is associated with HIV-1 neutralization breadth in viremic individuals.
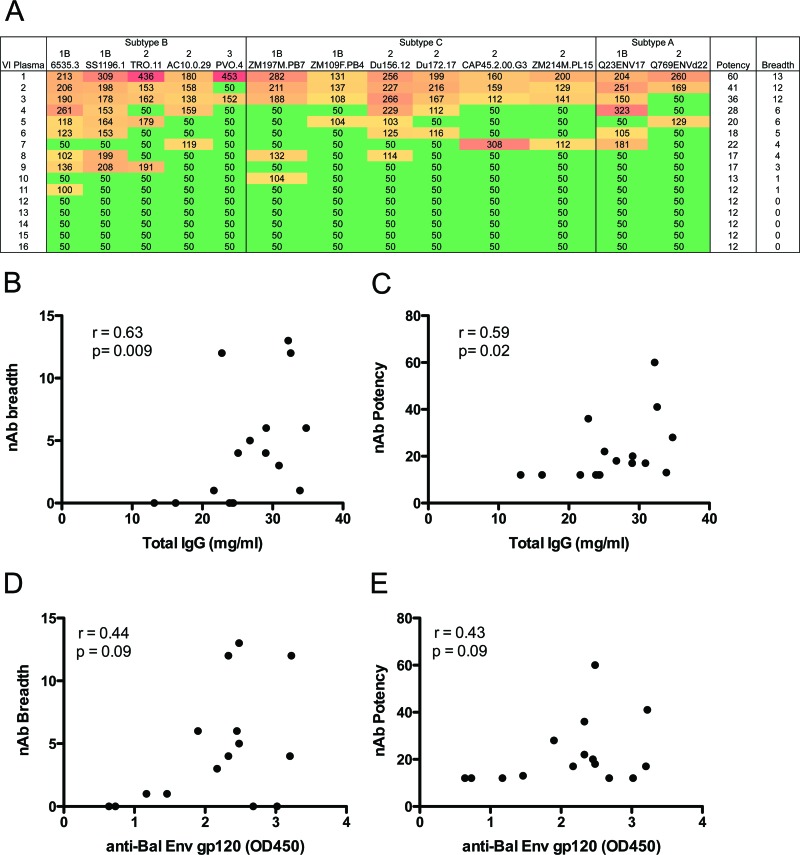
We next investigated if heterologous neutralizing activity was present in VI with established B cell dysfunction and if nAb breadth was dependent upon the level of B cell activation or dysfunction. Plasma samples from VI were tested for their ability to neutralize a panel of 13 HIV-1 envelope (Env) pseudotyped virions from clades A, B, and C, which included three tiers of sensitivity, as determined by Seaman et al. (45). While HIV-1 subtypes were not determined, our cohort of viremic subjects was most likely infected with subtype B, as this viral clade predominates in the southeastern United States. The neutralization IC50 was calculated for each plasma-Env combination, and these data were used to calculate a breadth (how many Envs were neutralized) and potency (the strength of neutralization) score for each plasma sample, as described in reference 37. Infectivity curves for each plasma sample are shown in Fig. S1 in the supplemental material. A range of neutralization breadth was observed in these 16 subjects: three plasma samples (19%) demonstrated widespread neutralizing activity against this panel of Envs, while five subjects (31%) exhibited a complete lack of detectable neutralization at the lowest dilution of plasma tested (1:100) (Fig. 6A). No correlation was observed between neutralization breadth or potency and parameters of B cell dysfunction (PD-1, BTLA), immune activation (Ki-67, CD95), or disease progression (CD4 T cell count, plasma viral load) (data not shown). However, the level of total IgG in each VI plasma sample was significantly correlated with both neutralization breadth and potency (Fig. 6B and C, P = 0.009 and P = 0.02, respectively). We next quantitated the level of antibodies that bind to the monomeric form of a subtype B Env gp120 (HIV-1 BaL) in each VI plasma sample and determined whether antibodies with this specificity were correlated with nAb breadth or potency. Like total IgG, anti-gp120 antibodies were positively correlated with nAb breadth and potency, but in this case the correlations only trended toward significance (Fig. 6D and E, respectively, P = 0.09 for both). Anti-gp120 antibodies did not correlate with parameters of B cell dysfunction, immune activation, disease progression, or total IgG level. These findings suggest that gp120 binding and other IgG antibody specificities contribute to nAb breadth, but neither is overtly influenced by perturbations in the B cell compartment during chronic HIV-1 infection.
Fig 6.
Neutralization breadth of plasma from VI subjects correlates with total IgG level. Sixteen plasma samples from VI were evaluated for their neutralization breadth and potency against a cross-clade panel of 13 HIV-1 Env pseudotypes. (A) The neutralization IC50 was calculated for each plasma-Env combination using linear regression. IC50s of less than 1:100 were assigned a value of 1:50. Color shading indicates the potency of neutralization: red > dark orange > light orange > green. HIV-1 Envs are listed along the top and are grouped by subtype. The tier designation for each Env (1B, 2, or 3) is shown and represents overall neutralization phenotype, as described in reference 45. Tier 1B viruses are “easy” to neutralize; tier 2 viruses are representative of most patient viruses; tier 3 viruses are “difficult” to neutralize. Higher breadth and potency scores indicate greater neutralization capacity. (B and C) Correlations between neutralization breadth (the number of Env pseudotypes neutralized) or potency (the sum of IC50s for each plasma-Env combination divided by the median IC50 for that virus against all plasma samples) and total IgG level for each plasma sample. (D and E) Correlations between nAb breadth and potency and the level of anti-gp120 binding antibodies in plasma, detected by ELISA (plotted as the optical density reading at 450 nm). Spearman's rank correlation coefficient (r) and level of significance (p) are indicated within each graph. Results given in panels D and E showed positive r values and trended toward significance but did not reach a level of P < 0.05.
DISCUSSION
An effective humoral immune response, in concert with cell-mediated immunity, may contribute to the control of HIV-1 replication. Several lines of evidence from SIV and simian-human immunodeficiency virus (SHIV) infection of nonhuman primates and from studies of HIV-1 infection support the importance of B lymphocytes. A suboptimal antibody response can influence disease progression and even lead to a fatal outcome during SIV/SHIV infections (11, 43, 44, 48, 49, 54). Furthermore, studies of HIV-1 infection have shown that B-lymphocyte dysfunction correlates with markers of disease progression (28, 30, 33). In one HIV-1-infected individual, monoclonal antibody-mediated depletion of B cells resulted in a decrease in neutralizing antibody titer and an increase in plasma viral load, which was reversed when the neutralizing antibody titer recovered to the pretreatment level (16). Thus, strategies to reverse or limit B cell dysfunction during HIV-1 infection could potentially limit disease progression.
Here we have demonstrated that PD-1 and BTLA, previously recognized mainly for their effects on T cells, are also aberrantly expressed on B lymphocytes during chronic HIV-1 infection. Our data demonstrate that expression of PD-1 was increased and that of BTLA decreased on B lymphocytes during persistent HIV-1 viremia and that alteration in PD-1 and BTLA expression on B cells is comparable to the patterns observed in T cells (7, 52, 53). Expanded analysis into the four major subsets of B lymphocytes revealed that PD-1 expression was notably higher in naive and immature B cells, and BTLA was lower in naive, immature, and memory B cells in VI. Interestingly, the mature B cell subset exhibited the least quantifiable differences in expression of these regulatory markers among VI, AV, and HC but was the most affected with respect to the peripheral B cell subset distribution.
Plasma viral load in VI was not significantly correlated with either PD-1 or BTLA expression on B cells. In contrast, other studies have reported correlations between PD-1 or BTLA expression on T cells and plasma viral load (7, 53). These studies also demonstrated that the CD4 T cell count was inversely correlated with PD-1 expression and directly correlated with BTLA expression on T cells (7, 53). Similarly in our study, peripheral blood CD4 T cell count was also indirectly and directly correlated with PD-1 and BTLA expression, respectively, on B lymphocytes. Thus, an imbalance in immune homeostasis, rather than simply the presence of persistent viral antigen, could be reflected in the aberrant expression of these regulatory receptors on B cells. A strong correlation was also observed between PD-1 and BTLA expression on B cells and markers of cell proliferation and activation. These findings suggest a possible role for aberrant PD-1 and BTLA expression in driving increased B cell activation. Finally, this report is among the first to link B cell dysregulation with the extent of hypergammaglobulinemia, a functional measure of B cell dysfunction in HIV-1 infection.
Having established multiple tiers of disruption within the B cell compartment in the VI cohort, we investigated whether plasma from these individuals contained nAbs with cross neutralizing capacity. Broad and potent neutralization was observed in 3 of the 16 subjects analyzed here. This frequency of 19% is consistent with that reported for individuals possessing greater nAb breadth in other cohorts. These three individuals did not systematically differ from the others exhibiting less nAb activity with regard to measures of immune activation, dysregulation, CD4 T cell count, or plasma viral load. Instead, in this cohort of typical progressor patients, nAb breadth and potency were associated directly with the level of hypergammaglobulinemia and gp120 binding antibodies, even though the latter did not reach statistical significance. A recent study from Oballah et al. demonstrated that the absolute B cell count in a subtype A HIV-1-infected cohort in Uganda was inversely correlated with neutralizing activity against heterologous Envs (35). In our study, we did not find a correlation between total B cell count and nAb breadth or potency (data not shown). However, consistent with their results, we did observe that relatively strong and broad nAbs are present in individuals that exhibit B cell dysregulation and hypergammaglobulinemia. Others have reported that the time since infection (14, 42) and plasma viral load or CD4 T cell count were associated with nAb breadth (9, 13, 37, 42). It is likely that these associations did not emerge in our study because of the smaller cohort size, which was targeted toward facilitating an extensive flow-cytometric analysis of B cells in addition to measuring nAb breadth.
In summary, this paper is among the first to demonstrate aberrant expression profiles of the regulatory receptors PD-1 and BTLA on peripheral B cells, as well as within individual B cell subsets, during HIV-1 infection. These receptors were associated with activation, proliferation, and dysfunction in B cells in viremic subjects. Despite this, broad and potent nAbs were produced in some individuals, and their activity was possibly augmented through increased IgG production. The observations reported here provide new insight into peripheral B cell dysfunction in chronic HIV-1 infection, supporting its impact on immune activation and disease progression but revealing a less dramatic effect on nAb activity and breadth.
Supplementary Material
ACKNOWLEDGMENTS
We thank Chris Ibegbu, Kiran Gill, and Deborah Abdul-Ali for their technical assistance. We appreciate the cooperation of all healthy, viremic, and aviremic participants of this study. All Env clone plasmids were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH.
This work was supported by the National Institutes of Health through grants R01-AI58706 to C.A.D. and P30-AI050409 to the Emory University Center for AIDS Research, supporting the Clinical Research, Immunology, and Virology cores.
Footnotes
Published ahead of print 23 May 2012
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Ascher MS, Sheppard HW. 1988. AIDS as immune system activation: a model for pathogenesis. Clin. Exp. Immunol. 73:165–167 [PMC free article] [PubMed] [Google Scholar]
- 2. Barber DL, et al. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682–687 [DOI] [PubMed] [Google Scholar]
- 3. Beirnaert E, et al. 2000. Identification and characterization of sera from HIV-infected individuals with broad cross-neutralizing activity against group M (env clade A-H) and group O primary HIV-1 isolates. J. Med. Virol. 62:14–24 [PubMed] [Google Scholar]
- 4. Beniguel L, et al. 2004. Specific antibody production by blood B cells is retained in late stage drug-naive HIV-infected Africans. Clin. Dev. Immunol. 11:121–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Binley JM, et al. 2008. Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C. J. Virol. 82:11651–11668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blackburn SD, et al. 2009. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 10:29–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Day CL, et al. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350–354 [DOI] [PubMed] [Google Scholar]
- 8. De Milito A, et al. 2004. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood 103:2180–2186 [DOI] [PubMed] [Google Scholar]
- 9. Doria-Rose NA, et al. 2010. Breadth of human immunodeficiency virus-specific neutralizing activity in sera: clustering analysis and association with clinical variables. J. Virol. 84:1631–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Doria-Rose NA, et al. 2009. Frequency and phenotype of human immunodeficiency virus envelope-specific B cells from patients with broadly cross-neutralizing antibodies. J. Virol. 83:188–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dykhuizen M, et al. 1998. Determinants of disease in the simian immunodeficiency virus-infected rhesus macaque: characterizing animals with low antibody responses and rapid progression. J. Gen. Virol. 79(Pt 10):2461–2467 [DOI] [PubMed] [Google Scholar]
- 12. Euler Z, et al. 2012. Longitudinal analysis of early HIV-1-specific neutralizing activity in an elite neutralizer and in five patients who developed cross-reactive neutralizing activity. J. Virol. 86:2045–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Euler Z, et al. 2010. Cross-reactive neutralizing humoral immunity does not protect from HIV type 1 disease progression. J. Infect. Dis. 201:1045–1053 [DOI] [PubMed] [Google Scholar]
- 14. Gray ES, et al. 2011. The neutralization breadth of HIV-1 develops incrementally over four years and is associated with CD4+ T cell decline and high viral load during acute infection. J. Virol. 85:4828–4840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hazenberg MD, et al. 2003. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS 17:1881–1888 [DOI] [PubMed] [Google Scholar]
- 16. Huang KH, et al. 2010. B-cell depletion reveals a role for antibodies in the control of chronic HIV-1 infection. Nat. Commun. 1:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaufmann DE, et al. 2007. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat. Immunol. 8:1246–1254 [DOI] [PubMed] [Google Scholar]
- 18. Kaufmann DE, Walker BD. 2009. PD-1 and CTLA-4 inhibitory cosignaling pathways in HIV infection and the potential for therapeutic intervention. J. Immunol. 182:5891–5897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kroon FP, van Dissel JT, de Jong JC, van Furth R. 1994. Antibody response to influenza, tetanus and pneumococcal vaccines in HIV-seropositive individuals in relation to the number of CD4+ lymphocytes. AIDS 8:469–476 [DOI] [PubMed] [Google Scholar]
- 20. Kroon FP, van Dissel JT, de Jong JC, Zwinderman K, van Furth R. 2000. Antibody response after influenza vaccination in HIV-infected individuals: a consecutive 3-year study. Vaccine 18:3040–3049 [DOI] [PubMed] [Google Scholar]
- 21. Lane HC, et al. 1983. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N. Engl. J. Med. 309:453–458 [DOI] [PubMed] [Google Scholar]
- 22. Li B, et al. 2006. Evidence for potent autologous neutralizing antibody titers and compact envelopes in early infection with subtype C human immunodeficiency virus type 1. J. Virol. 80:5211–5218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li M, et al. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 79:10108–10125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li M, et al. 2006. Genetic and neutralization properties of subtype C human immunodeficiency virus type 1 molecular env clones from acute and early heterosexually acquired infections in Southern Africa. J. Virol. 80:11776–11790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Long EM, Rainwater SM, Lavreys L, Mandaliya K, Overbaugh J. 2002. HIV type 1 variants transmitted to women in Kenya require the CCR5 coreceptor for entry, regardless of the genetic complexity of the infecting virus. AIDS Res. Hum. Retroviruses 18:567–576 [DOI] [PubMed] [Google Scholar]
- 26. Lynch JB, et al. 2011. The breadth and potency of passively acquired human immunodeficiency virus type 1-specific neutralizing antibodies do not correlate with the risk of infant infection. J. Virol. 85:5252–5261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lynch RM, et al. 2011. The B cell response is redundant and highly focused on V1V2 during early subtype C infection in a Zambian seroconverter. J. Virol. 85:905–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Malaspina A, et al. 2006. Appearance of immature/transitional B cells in HIV-infected individuals with advanced disease: correlation with increased IL-7. Proc. Natl. Acad. Sci. U. S. A. 103:2262–2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mizuma H, Litwin S, Zolla-Pazner S. 1988. B-cell activation in HIV infection: relationship of spontaneous immunoglobulin secretion to various immunological parameters. Clin. Exp. Immunol. 71:410–416 [PMC free article] [PubMed] [Google Scholar]
- 30. Moir S, et al. 2008. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J. Exp. Med. 205:1797–1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moir S, et al. 2008. Normalization of B cell counts and subpopulations after antiretroviral therapy in chronic HIV disease. J. Infect. Dis. 197:572–579 [DOI] [PubMed] [Google Scholar]
- 32. Moir S, et al. 2001. HIV-1 induces phenotypic and functional perturbations of B cells in chronically infected individuals. Proc. Natl. Acad. Sci. U. S. A. 98:10362–10367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moir S, et al. 2004. Decreased survival of B cells of HIV-viremic patients mediated by altered expression of receptors of the TNF superfamily. J. Exp. Med. 200:587–599 [PubMed] [Google Scholar]
- 34. Moore JP, et al. 1996. Inter- and intraclade neutralization of human immunodeficiency virus type 1: genetic clades do not correspond to neutralization serotypes but partially correspond to gp120 antigenic serotypes. J. Virol. 70:427–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oballah P, et al. 2011. B cell depletion in HIV-1 subtype A infected Ugandan adults: relationship to CD4 T cell count, viral load and humoral immune responses. PLoS One 6:e22653 doi:10.1371/journal.pone.0022653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Opravil M, Fierz W, Matter L, Blaser J, Luthy R. 1991. Poor antibody response after tetanus and pneumococcal vaccination in immunocompromised, HIV-infected patients. Clin. Exp. Immunol. 84:185–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Piantadosi A, et al. 2009. Breadth of neutralizing antibody response to human immunodeficiency virus type 1 is affected by factors early in infection but does not influence disease progression. J. Virol. 83:10269–10274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rainwater SM, et al. 2007. Cloning and characterization of functional subtype A HIV-1 envelope variants transmitted through breastfeeding. Curr. HIV Res. 5:189–197 [DOI] [PubMed] [Google Scholar]
- 39. Rodriguez-Barradas MC, et al. 1992. Antibody to capsular polysaccharides of Streptococcus pneumoniae after vaccination of human immunodeficiency virus-infected subjects with 23-valent pneumococcal vaccine. J. Infect. Dis. 165:553–556 [DOI] [PubMed] [Google Scholar]
- 40. Rong R, et al. 2007. Role of V1V2 and other human immunodeficiency virus type 1 envelope domains in resistance to autologous neutralization during clade C infection. J. Virol. 81:1350–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rong R, et al. 2009. Escape from autologous neutralizing antibodies in acute/early subtype C HIV-1 infection requires multiple pathways. PLoS Pathog. 5:e1000594 doi:10.1371/journal.ppat.1000594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sather DN, et al. 2009. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J. Virol. 83:757–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schmitz JE, et al. 2003. Effect of humoral immune responses on controlling viremia during primary infection of rhesus monkeys with simian immunodeficiency virus. J. Virol. 77:2165–2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schmitz JE, et al. 2009. Inhibition of adaptive immune responses leads to a fatal clinical outcome in SIV-infected pigtailed macaques but not vervet African green monkeys. PLoS Pathog. 5:e1000691 doi:10.1371/journal.ppat.1000691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Seaman MS, et al. 2010. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J. Virol. 84:1439–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shirai A, Cosentino M, Leitman-Klinman SF, Klinman DM. 1992. Human immunodeficiency virus infection induces both polyclonal and virus-specific B cell activation. J. Clin. Invest. 89:561–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Simek MD, et al. 2009. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J. Virol. 83:7337–7348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tasca S, et al. 2011. Effect of B-cell depletion on coreceptor switching in R5 simian-human immunodeficiency virus infection of rhesus macaques. J. Virol. 85:3086–3094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Titanji K, et al. 2010. Acute depletion of activated memory B cells involves the PD-1 pathway in rapidly progressing SIV-infected macaques. J. Clin. Invest. 120:3878–3890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. van Gils MJ, Euler Z, Schweighardt B, Wrin T, Schuitemaker H. 2009. Prevalence of cross-reactive HIV-1-neutralizing activity in HIV-1-infected patients with rapid or slow disease progression. AIDS 23:2405–2414 [DOI] [PubMed] [Google Scholar]
- 51. Watanabe N, et al. 2003. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat. Immunol. 4:670–679 [DOI] [PubMed] [Google Scholar]
- 52. Zhang JY, et al. 2007. PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors. Blood 109:4671–4678 [DOI] [PubMed] [Google Scholar]
- 53. Zhang Z, et al. 2011. B and T lymphocyte attenuator down-regulation by HIV-1 depends on type I interferon and contributes to T-cell hyperactivation. J. Infect. Dis. 203:1668–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhang ZQ, et al. 2007. Early depletion of proliferating B cells of germinal center in rapidly progressive simian immunodeficiency virus infection. Virology 361:455–464 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.