Abstract
The RV144 trial demonstrated that an experimental AIDS vaccine can prevent human immunodeficiency virus type 1 (HIV-1) infection in humans. Because of its limited efficacy, further understanding of the mechanisms of preventive AIDS vaccines remains a priority, and nonhuman primate (NHP) models of lentiviral infection provide an opportunity to define immunogens, vectors, and correlates of immunity. In this study, we show that prime-boost vaccination with a mismatched SIV envelope (Env) gene, derived from simian immunodeficiency virus SIVmac239, prevents infection by SIVsmE660 intrarectally. Analysis of different gene-based prime-boost immunization regimens revealed that recombinant adenovirus type 5 (rAd5) prime followed by replication-defective lymphocytic choriomeningitis virus (rLCMV) boost elicited robust CD4 and CD8 T-cell and humoral immune responses. This vaccine protected against infection after repetitive mucosal challenge with efficacies of 82% per exposure and 62% cumulatively. No effect was seen on viremia in infected vaccinated monkeys compared to controls. Protection correlated with the presence of neutralizing antibodies to the challenge viruses tested in peripheral blood mononuclear cells. These data indicate that a vaccine expressing a mismatched Env gene alone can prevent SIV infection in NHPs and identifies an immune correlate that may guide immunogen selection and immune monitoring for clinical efficacy trials.
INTRODUCTION
Over 30 million people are infected with human immunodeficiency virus (HIV) worldwide, and 2.5 to 3 million new infections have been estimated to occur yearly. Although effective antiretroviral therapies are available, millions succumb to AIDS every year, especially in sub-Saharan Africa, underscoring the need to develop a vaccine that prevents the spread of this disease. Initial attempts at generating neutralizing antibodies by vaccination with recombinant HIV gp120 protein, analogous to some highly effective licensed vaccines, proved unsuccessful in generating protective immunity (34). In 2009, a large multicenter, double-blind, placebo-controlled clinical study revealed that priming immunization with ALVAC-HIV (a canarypox vector vaccine expressing HIV Env, Gag, and Pro) followed by AIDSVAX B/E (a recombinant HIV Env gp120 vaccine) boosting can reduce the risk of HIV infection among heterosexuals by 31% (38). The vaccine induced short-term protection, and a specific antibody response to the V1V2 region of HIV Env protein correlates with protection against infection (21). It remains possible that other mechanisms, such as T-cell immunity or a proinflammatory response, may have contributed to protection. For example, secretion of RANTES, MIP-1α, or MIP-1β independent of HIV antigens from proinflammatory signals could lead to occupation of the CCR5 coreceptor, which is critical for viral entry. Elaboration of cytokines, such as alpha interferon (IFN-α), could similarly exert antiviral effects. A recent study of simian immunodeficiency virus (SIV) infection in rhesus macaques showed effective control of viremia in the absence of neutralizing antibodies due to the generation of CD8 effector cells (20). Another study showed that concerted cellular and humoral immune responses mediated protection against a simian-human immunodeficiency virus (SHIV) challenge (36), and a recent paper suggested that the inclusion of Env, in addition to Gag and Pol components, increased protection against SIV (4).
Gene-based viral vaccine vectors provide a major advantage over protein vaccines in their ability to induce strong T-cell responses in addition to antibody responses. Specifically, the ability of prime-boost immunization with heterologous vectors to elicit robust cellular and humoral immune responses has been well documented (7, 18, 23). In this study, we first evaluated the potential of different prime-boost combinations involving replication-defective lymphocytic choriomeningitis virus (rLCMV), a gene-based viral vector that elicits potent CD8 immune responses (17). This vector was examined in combination with plasmid DNA or adenoviral vectors. We tested the efficacy of the optimal combination in a repetitive mucosal challenge in nonhuman primates (NHP) and have used this model to explore potential correlates of immunity that may be informative for future human efficacy trials.
MATERIALS AND METHODS
Animals.
We used 6- to 10-week-old female BALB/c mice ordered from NCI/DCT, Jackson, or Charles River. They were housed in the animal facility of the Vaccine Research Center (VRC), NIAID, NIH, Bethesda, MD. Three- to 5-year-old male Macaca mulatta animals of Indian origin with an average body weight of 4.8 kg were used in the NHP study. All animal experiments were reviewed and approved by the Animal Care and Use Committee of the Vaccine Research Center, NIAID, NIH, and all animals were housed and cared for in accordance with local, state, federal, and institute policies in an American Association for Accreditation of Laboratory Animal Care (AAALAC)-accredited facility at the NIH.
SIV challenge.
To evaluate the infectibility of immunized and control animals, 10 vaccinated Macaca mulatta animals of Indian origin and 10 controls were challenged intrarectally with SIVsmE660 at the dose of one median animal infectious dose (AID50) 6 weeks after the rLCMV boost as recently described for the same virus stock (28).
TRIM5 genotyping.
Genomic DNA was isolated from lymphocytes of monkeys using the QIAamp DNA kit (Qiagen) and sequenced for TRIM5 exons as previous described (28).
Vaccine vectors.
The rLCMV vectors were generated and titrated as described previously (17). Briefly, the HIV clade B gp145ΔCFIΔV1V2 or gp140ΔCFIΔV1V2 or SIVmac239 gp140ΔCFI (9, 28) was inserted into a GP-deleted S segment under the control of a murine pol I promoter, and viral vectors were recovered using a pol I/pol II rescue system as previously described (16). Recombinant adenovirus type 5 (rAd5) vectors are replication-defective E1-, E3-, and E4-deleted human Ads. rAd28 vectors are E1- and E3-deleted replication-defective vectors, and all have been previously described (44). DNA plasmids have been extensively described and used in clinical trials (8, 9). We used the truncated V1V2-deleted HIV-1 Env gp140ΔCFI because previous experiments have shown that it generates improved antibody responses without reducing the potency of the cytotoxic-T-lymphocyte (CTL) response (9, 45). The V1V2 region of the clade B immunogen was removed because this sequence significantly reduced rAd vector productivity. Previous experiments with gp140 and gp145 DNA plasmids have revealed comparable immunogenicity. rAd5 and rLCMV can therefore be considered to express essentially the same antigen. All animals were injected intramuscularly (i.m.) with recombinant adenoviral vectors or DNA and intravenously (i.v.) with rLCMV vectors unless noted.
ICS, MHC tetramer staining, and enzyme-linked immunosorbent spot assay (ELISpot assay).
Splenic lymphocytes from individual mice were used for tetramer staining and intracellular cytokine staining (ICS). A detailed description of the major histocompatibility complex (MHC) tetramer and ICS stimulation has been published previously (22). For nonhuman primates, a qualified ICS assay was performed in batch on cryopreserved peripheral blood mononuclear cells (PBMC). Cryopreserved PBMC were thawed in a 37°C water bath, washed, resuspended at 1 million to 2 million cells/ml in R10 (RPMI, 10% fetal bovine serum [FBS], and 1% penicillin-streptomycin), and rested overnight in a 37°C, 5% CO2 incubator. In vitro stimulations were performed the following morning. Cells were transferred to a 96-well V-bottom plate at 1 million to 3 million cells/well and stimulated with the SIV Env peptide pool (15-mers overlapping by 11 amino acids spanning SIVmac239 Env; provided by the NIH AIDS Research and Reference Reagent Program, Germantown, MD) in the presence of brefeldin A at a final concentration of 2 μg/ml or 10 μg/ml for 6 h. Negative controls received an amount of dimethyl sulfoxide (DMSO) (the peptide diluent) equal to that for the peptide-stimulated cells. At the end of the incubation, the plate was placed at 4°C overnight. Staining for cell surface and intracellular molecules was performed the next morning. Cells were surface stained with CD4-QD605 (clone MT477; Invitrogen), CD28-Cy5-phycoerythrin (PE) (clone 28.2; BD Biosciences), and CD45RA-Cy7-PE (clone L48; BD Biosciences), fixed and permeabilized with Cytofix/Cytoperm (BD Biosciences), and then intracellularly stained with CD3-Cy7-allophycocyanin (APC) (clone SP34.2, BD Biosciences), IFN-γ-APC (clone B27; BD Biosciences), interleukin-2 (IL-2)-PE (clone MQ1-17H12; BD Biosciences), and tumor necrosis factor alpha (TNF-α)-fluorescein isothiocyanate (FITC) (clone Mab11; BD Biosciences). An Aqua LIVE/DEAD kit (Invitrogen, Carlsbad, CA) was used to exclude dead cells. The titers of all antibodies were determined to determine the saturating dilution. Samples were acquired on an LSR II flow cytometer and analyzed using FlowJo software (Treestar, Inc., Ashland, OR) and SPICE 5.2 software (http://exon.niaid.nih.gov).
For ELISpot assay, multiscreen 96-well Immobilon-P plates (Millipore) were coated with purified mouse anti-human IFN-γ (BD Pharmingen) at 5 μg/ml for 2 h at 37°C, washed with 0.1% Tween 20–phosphate-buffered saline (PBS), and blocked with R10 for 1 h. Cells were plated in triplicate, and 2 × 105 cells/well were stimulated with the SIV Env peptide pool (15-mers overlapping by 11 amino acids spanning SIVmac239 Env; provided by the NIH AIDS Research and Reference Reagent Program, Germantown, MD) at 1 μg/ml and incubated for 18 h at 37°C. Wells were washed with 0.1% Tween 20–PBS 9 times, followed by one wash with double-distilled water (ddH2O). Next, wells were incubated with rabbit polyclonal anti-human IFN-γ–biotin (U-Cytech) at 100 μg/ml for 2 h at room temperature (RT). After 2 h of incubation, wells were washed with 0.1% Tween–PBS six times and incubated with streptavidin-alkaline phosphatase diluted at 1:500 (Southern Biotechnology) for 2 h at RT. Wells were washed five times with 0.1% Tween–PBS and three times with PBS. Wells were then incubated for 10 min with nitroblue tetrazolium chloride–5-bromo-4-chloro-3′-indolylphosphate p-toluidine salt chromogen (Pierce) and washed thoroughly with tap water. Plates were allowed to dry overnight and read with an ELISpot reader (Cellular Technology Limited). The number of spot-forming cells (SFC) per million PBMC was calculated and graphed using SPICE 5.2.
ELISA.
Levels of antigen-specific IgG in the sera were assessed using an enzyme-linked immunosorbent assay (ELISA) described previously (24, 28). The ELISA plate was coated with either SIVmac239 gp140 or HIV gp120B (Immune Technology, NY). Absorbance at 450 nm was determined with a Spectra Max instrument (Molecular Devices).
Statistical analysis.
For statistical analysis, we used an unpaired two-tailed t test with a confidence interval of 95% from Prism 5 for Mac OS X. P values of less than 0.05 were considered significant and P values of less than 0.01 highly significant. Presentation and statistical comparison of ICS functional and memory phenotype distributions were performed using SPICE version 5.2 (40).
MACS.
We used the following Miltenyi magnetically activated cell sorting (MACS) kits according to the manufacturer's instructions: CD4+ dendritic cell (DC) isolation kit (130-091-262), plasmacytoid dendritic cell (pDC) isolation kit II (130-092-786), and CD8+ dendritic cell isolation kit (130-091-169). For CD4+ DC and CD8+ DC isolation, negative selection was followed by positive selection, while for pDC isolation, only negative selection was used. CD4 and CD11c were stained for CD4− DCs, CD8 and CD11c were stained for CD8+ DCs, and Ly-6c and mPDCA-1 were stained for pDCs. The purity for all three DC subsets was 88 to 92%.
Neutralization assays.
Neutralizing antibody responses against SIVsmE660 Env pseudoviruses were measured with a luciferase-based assay in TZM-bl cells (obtained from J. C. Kappes, X. Wu, and Tranzyme Inc. through the National Institutes of Health AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, NIH) as previously described (29). PBMC-based neutralization assays were performed as described previously (28), using healthy human donors instead of monkey PBMC due to more consistent availability and reproducibility of the neutralization assay on lymphocytes from human blood. Human PBMC (hPBMC) were purified from 50 ml of blood with Ficoll-Hypaque centrifugation, and CD8 T cells were depleted.
RESULTS
rLCMV targets dendritic cells and efficiently induces Env- and Gag-specific immune responses in mice after a single immunization.
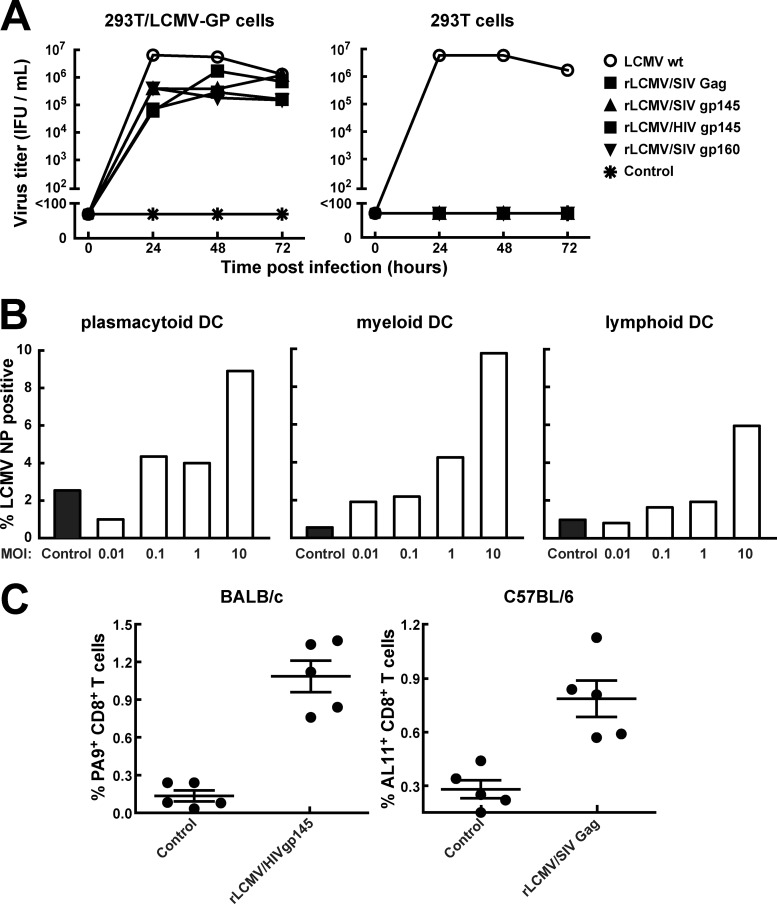
Using a previously described pol I/pol II-based four-plasmid transfection system, we generated rLCMV vectors expressing either HIV/SIV Env or Gag (16). All the vectors were replication incompetent and could be productively grown only on producer cell lines complementing the vector with the LCMV glycoprotein (Fig. 1A), a protein necessary for the infectivity of rLCMV vector particles. Dendritic cells (DCs) are critical for the priming of both CD4+ and CD8+ T cells (35). To assess the ability of rLCMV to infect dendritic cells, we purified mouse myeloid, plasmacytoid, and lymphoid DCs from mouse spleens and infected them with rLCMV vectors encoding the truncated HIV envelope in vitro. The different DC subsets were transduced at multiplicities of infection (MOIs) of 10, with an infection rate from 6 to 10% measured by anti-LCMV NP fluorochrome-stained cells at 36 h after infection (Fig. 1B). We then immunized BALB/c or C57BL/6 mice with rLCMV vector (2 × 105 immunofocus-forming units [IFU]) to assess T-cell priming in vivo. Both rLCMV vectors (rLCMV/HIVgp145 and rLCMV/SIVgag) induced CD8+ T-cell responses as measured by H-2Dd/PA9 or H-2Kb/AL11 tetramer staining, respectively, 14 days after a single immunization in mice (Fig. 1C).
Fig 1.
rLCMV vectors are replication defective in cell culture, transduce murine DCs, and elicit CD8 T cells. (A) LCMV GP-expressing 293T/LCMV-GP or wild-type 293T cells were infected either with wild type LCMV (LCMV wt) or with rLCMV expressing several HIV and SIV antigens (rLCMV/SIVgag, rLCMV/SIVgp160, rLCMV/SIVgp145, and rLCMV/HIVgp145) at an MOI of 0.01, and viral propagation was measured over time as immunofocus-forming units (IFU) in the supernatant. (B) Murine plasmacytoid, myeloid, and lymphoid DCs were isolated by MACS from spleens of BALB/c mice and infected with rLCMV/HIVgp145 at an MOI of 0.01, 0.1, 1, or 10, and infectivity was determined at 36 h postinfection by intracellular staining of LCMV nucleoprotein with monoclonal PE-labeled anti-NP antibody VL-4 using flow cytometry. (C) BALB/c (left graph) and C57BL/6 (right graph) mice were immunized with either rLCMV/HIVgp145 or rLCMV/SIVgag vaccine vectors, and the antiretroviral CD8 T-cell responses in PBMC were measured by tetramer staining.
DNA or rAd5 priming followed by rLCMV boosting induces cellular and humoral immunity.
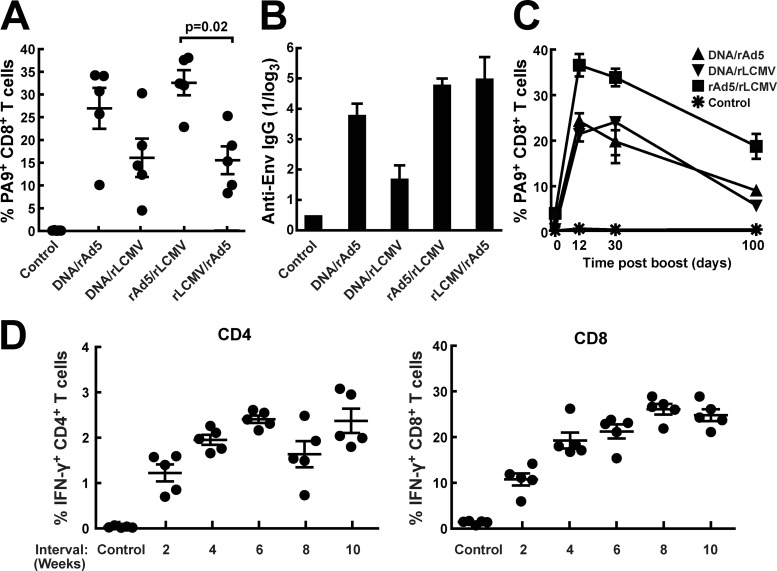
To establish an optimal heterologous prime-boost vector combination, we compared different prime and boost combinations using rLCMV/HIVgp145, rAd5, and plasmid DNA as immunogens. Plasmid DNA priming followed by rAd5 boosting was used for comparison, since this combination has been evaluated extensively in nonhuman primate studies and formed the basis for a current clinical trial (27). This combination has been shown to increase survival in an SIV challenge model in nonhuman primates and has also proven to be effective in blocking acquisition in a recent NHP study, though the relative contributions of Gag and Env inserts were not evaluated (28). When testing different combinations, we found that both rLCMV/HIVgp145 and rAd5/HIVgp140 were comparably immunogenic when used as a boost after plasmid DNA priming. However, when rAd5 priming was followed by rLCMV boosting, significantly more potent T-cell immunity was elicited, particularly in CD8+ T cells (Fig. 2A). All immunization protocols elicited substantial antibody responses as detected by Env-specific ELISA (Fig. 2B). rAd5-rLCMV/HIVgp145 immunization elicited CD8+ T-cell responses that could be detected for 100 days at a frequency of approximately 20% of total CD8+ T cells (Fig. 2C). With this vector combination, we did not see a rapid contraction phase, as previously described for immunization with rAd5-rAd5 (14). To determine whether the prime-boost interval could affect immunogenicity, we varied the time of boosting over a range of 2 to 10 weeks. These experiments showed that 2 weeks was not a sufficient prime-boost interval for optimal CD4+ and CD8+ T-cell responses; at 4 weeks or longer, increases in the magnitude of the boosting effect were observed (Fig. 2D).
Fig 2.
Prime-boost regimens using DNA, adenoviral, and LCMV vaccine vectors. BALB/c mice were immunized using different combinations of DNA, adenoviral, and LCMV vectors encoding either gp140 or gp145 of the HIV envelope gene. The interval between the two immunizations was 4 weeks. (A) Two weeks after the boost immunization, cellular immune responses in PBMC were determined using PA9 (Dd-6433) MHC-I tetramer staining. (B) Anti-HIV-1 Env antibody responses were measured by ELISA (HIV gp coated) at 2 weeks after the boost for the same immunization groups. (C) The time kinetics of the cellular immune response was monitored by measuring tetramer-specific T cells in PBMC after immunization. (D) We tested time intervals of 2, 4, 6, 8, or 10 weeks between an adenovirus prime and an LCMV boost. Immunogenicity was assessed by IFN-γ production after peptide pool stimulation at 3 weeks after the last immunization.
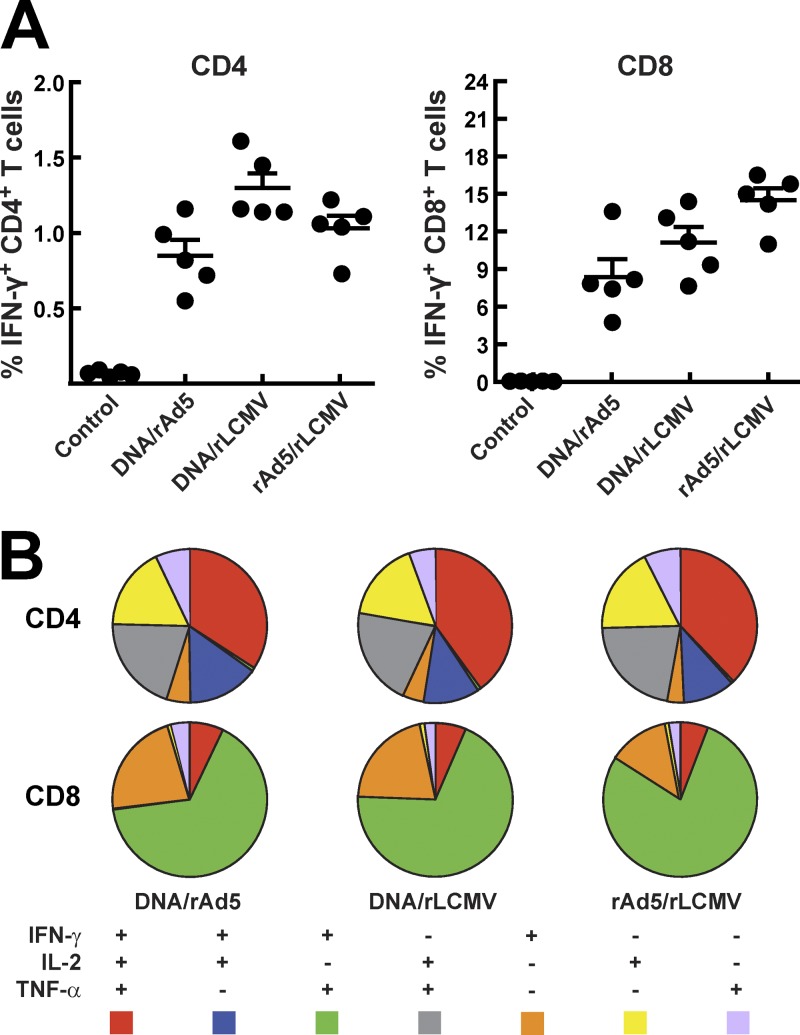
The ability of T cells to simultaneously produce a number of effector cytokines upon antigen stimulation is relevant for the control of HIV-1 replication by T cells (2). We therefore analyzed the polyfunctionality of rLCMV-induced T cells following priming with either DNA or rAd5 and compared it with that for DNA-rAd5. We found no significant difference in terms of CD8+ and CD4+ T-cell polyfunctionality among the immunization groups. All three groups displayed similar CD4+ and CD8+ T-cell intracellular cytokine staining (ICS) patterns, with the majority of cells producing two or three cytokines simultaneously upon stimulation with cognate antigen (Fig. 3A and B).
Fig 3.
Intracellular cytokine staining and polyfunctionality of T-cell responses at 2 weeks after the boost. Spleens from mice were harvested and crushed, and then single-cell suspensions were pulsed with the HIV envelope peptide pool. (A) Intracellular cytokine staining (IFN-γ, TNF-α, and IL-2) was performed, and respective frequencies for CD4+ and CD8+ T cells were determined. (B) Using SPICE software, we calculated and displayed polyfunctionality of the T-cell responses.
An alternative serotype D rAd, rAd28, can also prime for an rLCMV/HIVgp145 boost.
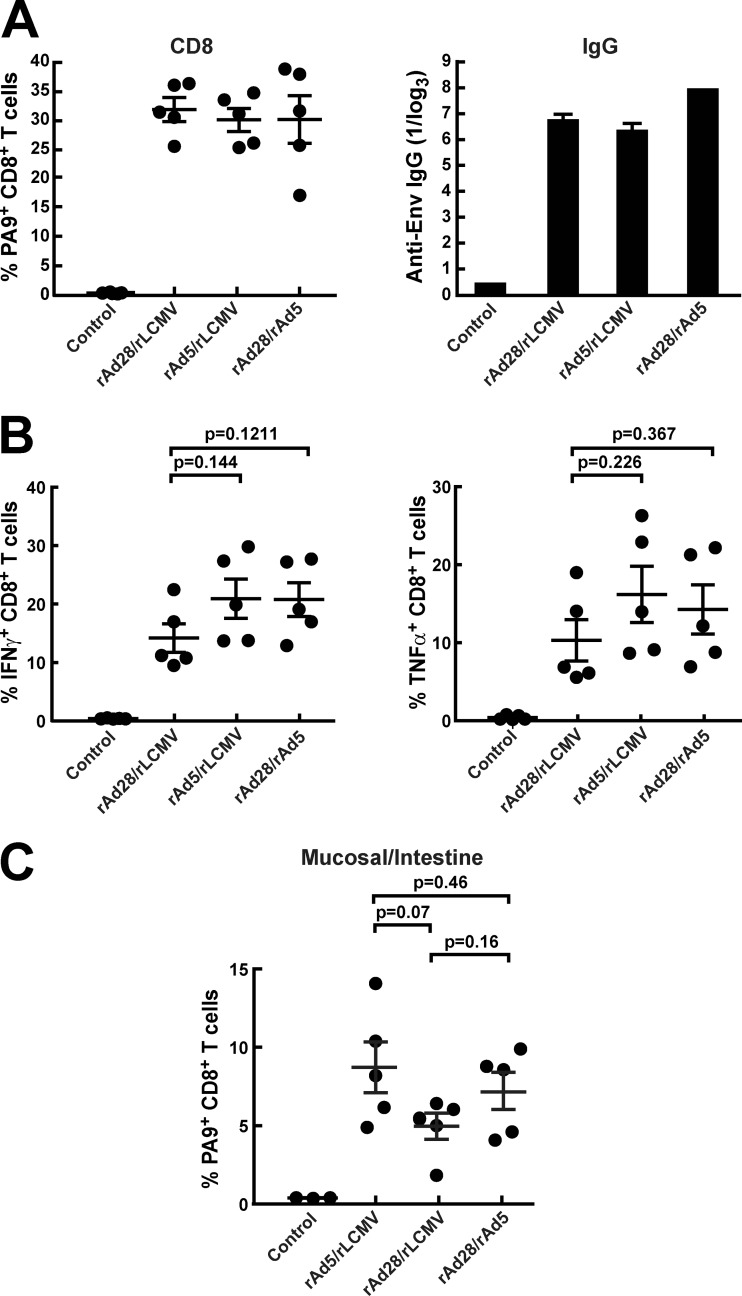
Although the DNA-rLCMV/HIVgp145 combination was immunogenic, it was unclear whether alternative rAd vectors could substitute for DNA or rAd5 and improve priming. Since D serotype vectors such as rAd26 or rAd28 have been shown to function well for priming in vivo (1), we asked whether they might also prime for the rLCMV boost (25). There was no significant difference among CD8+ MHC tetramer-binding T-cell frequencies elicited by rAd28-rAd5, rAd5-rLCMV, and rAd28-rLCMV encoding HIV Env (Fig. 4A). Likewise, the three immunization protocols elicited similar levels of Env antibodies by ELISA (Fig. 4A). ICS analysis revealed no statistically significant differences in magnitude between rAd5- and rAd28-primed animals (Fig. 4B). These prime-boost combinations also elicited similar cellular immune responses localized to the gut (Fig. 4C).
Fig 4.
rLCMV in combination with alternative adenoviral vector rAd28. (A) PA9 (Dd6433) MHC-I tetramer staining of PBMC and antibody assessment by ELISA were performed after immunizing BALB/c mice with a prime-boost setting using a 4-week interval. (B) Intracellular cytokine staining for IFN-γ and TNF-α was performed with peptide-pulsed splenocytes from the same animals. (C) T cells were extracted from the gut, and CD8 T cells were stained using PA9 (Dd6433) MHC-I tetramers.
Induction of humoral and cellular immune responses in nonhuman primates following immunization with rAd5/rLCMV.
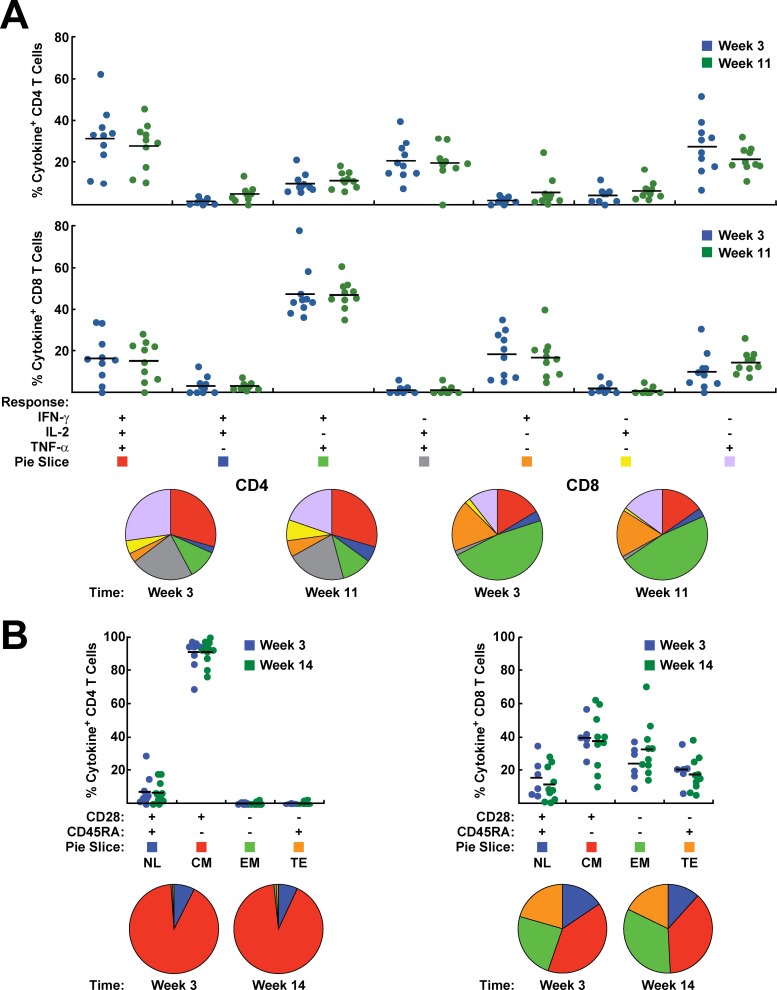
Based on our mouse data, we sought to assess the immunogenicity of the rAd5/rLCMV 8 week prime-boost vector combination in primates (Fig. 5A). Empty vectors were used in control animals. There was an increase in the magnitude of the cellular immune response at 3 weeks after rLCMV injection (Fig. 5B and C). The antibody response measured by ELISA remained unchanged from week 3 to week 8, and there was a 10-fold increase after the boost at week 11 (Fig. 5C) (P < 0.0001). Similarly, although antigen-specific ELISpot and cytokine-producing T cells decreased after administration of rAd5 from week 3 to week 8, an increase was observed following rLCMV immunization (Fig. 5B). There was no significant difference in the polyfunctionality of CD8+ or CD4+ T cells before and after boosting (weeks 3 and 11, respectively) (Fig. 6A). Memory phenotyping of these T cells defined by antibodies to CD28 and CD45RA revealed that the rLCMV/SIVgp140 boosting marginally altered the proportions of central memory, terminal effector, and effector memory CD8+ T cells (Fig. 6B). Before and after boosting, most SIV-specific CD4+ T cells were CD28+ CD45RA−, which is characteristic of central memory T cells. For SIV-specific CD8+ T cells, we observed a more balanced distribution between central and effector memory T cells (Fig. 6B). The percentage of transitional memory T cells decreased, while the effector memory CD8+ T cells slightly increased after the boost.
Fig 5.
Vaccination and challenge schema and humoral and cellular immune responses in the nonhuman primate study. (A) Animals were divided into null and vaccine groups and were immunized at week 0 with rAd5 null or rAd5 SIV Env gp145 at 1 × 1011 viral particles intramuscularly and boosted with LCMV null or LCMV SIV Env gp140 at 1 × 108 PFU i.v., respectively, at week 8. Animals in the null and vaccine groups were challenged weekly from week 14 to week 25 with one AID50 of SIV E660 virus. (B and C) Humoral immune responses were measured by ELISA and cellular immune responses in PBMC were measured by ELISpot or intracellular cytokine staining after stimulation with SIV Env peptide pool. Env-specific cellular immune responses were determined by performing intracellular cytokine staining (B) and IFN-γ ELISpot (C) assays on PBMC at various time points postvaccination. Lines are drawn at the mean, and P values were determined using the Wilcoxon matched-pairs test. Cytokine+ represents the frequency of cells making any of the measured cytokines (IFN-γ, IL-2, or TNF-α).
Fig 6.
Polyfunctionality and phenotypes of T-cell responses after prime and boost immunization. The frequency of cytokine production after stimulation with a peptide pool for Env was determined by intracellular cytokine staining for IFN-γ, IL-2, and TNF-α at weeks 3 and 11. The results are shown in bar charts and pie charts. (A) CD4+ and CD8+ T-cell responses are shown separately for each of the 7 functional subsets. Red, 3 functions; blue, green, and gray, 2 functions; orange, yellow, and purple, 1 function. (B) Analysis of cytokine production based on the expression of CD28 and CD45RA. NL, naïve-like; CM, central memory; EM, effector memory; TE, terminal effector.
Vaccination with rAd5/rLCMV protects nonhuman primates against acquisition of SIVsmE660 infection.
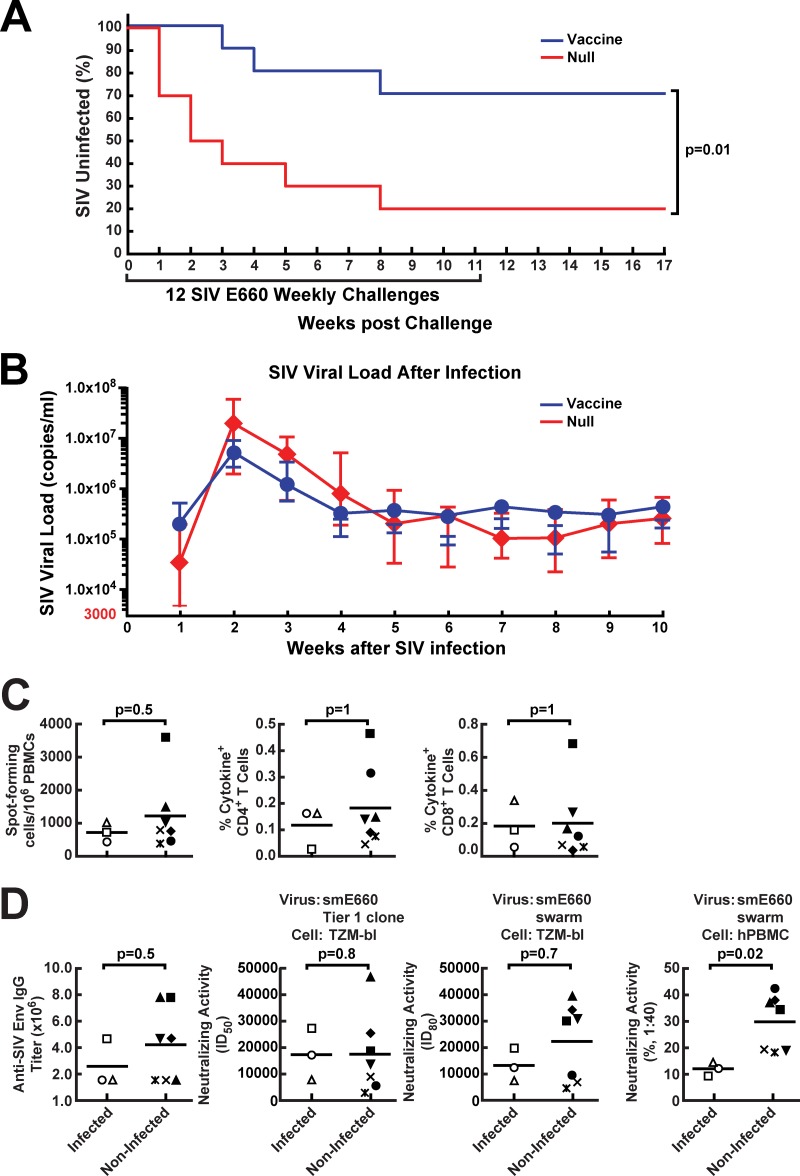
To determine whether prime-boost vaccination with a mismatched Env could confer protection against lentiviral infection, we administered 12 consecutive weekly intrarectal challenges of SIVsmE660 to the animals (10 vaccinees and 10 null-vaccinated controls) at 6 weeks after the rLCMV boost, with SIV plasma viral load as an endpoint as recently described for DNA/rAd Gag Pol Env vaccines (28). Once an animal became infected, no additional challenges were performed, and the rAd5/LCMV Env vaccine was compared to the same vectors without inserts as negative controls. We observed a substantial reduction in acquisition in the SIV Env-immunized animals (Fig. 7A) (P = 0.01 by log rank test). In the vaccinated group, three of the 10 monkeys became infected after 99 challenges (3% infection rate per challenge), while in the control null group, eight of the 10 monkeys were infected after 47 challenges (17% infection rate per challenge), indicating a protective efficacy of 82% per challenge. Over the course of 12 exposures, three animals in the vaccinated group and eight in the control arm became infected, indicating a cumulative protective efficacy of 62%. In contrast, the vaccine had no significant effect on either peak or set-point plasma viremia in infected monkeys, although viral load at weeks 2, 3, and 4 postinfection trended lower in the vaccine group than in the null group (Fig. 7A and B). In terms of genetic factors that may have affected infectivity in these groups, no animals expressed the previously identified SIV-restrictive MHC alleles, including Mamu A*O1, B*08, and B*17, and the restrictive or sensitive TRIM alleles were randomly distributed among groups. They showed no correlation with vaccine-induced protection (P = 0.3 by log rank test). None of the three infected monkeys in the vaccine group had the restrictive TRIM5TFP/TFP alleles, while all of the monkeys with the restrictive TRIM5TFP/TFP alleles in the control group were infected (see Table S1 in the supplemental material).
Fig 7.
Protection of immunized monkeys against acquisition of SIV smE660 infection and correlates of protection in vaccinated animals. (A) Kaplan-Meier curves for SIV acquisition are shown for the 10 monkeys which were vaccinated using rAd5 encoding SIV Env and then boosted 8 weeks later with an LCMV vector encoding SIV Env in comparison with mock (null vector)-vaccinated animals. Weekly challenges were discontinued in infected animals upon detection of SIV loads in plasma. The reduction in protection per challenge was 82%, while cumulative protection over the course of the study was 62% (P = 0.01 by the log rank test). (B) For animals that acquired SIV infection during the challenge period independent of whether vaccinated or not, peak plasma SIV viral loads were recorded each week upon detection. Geometric means with standard errors of the means (SEMs) are plotted for 8 control animals and 3 vaccinated animals that were infected. (C and D) At week 11, whole blood in EDTA or serum samples were obtained from vaccinated animals. SIV-specific cellular immune responses in PBMC were quantified after in vitro stimulation with Env peptide pools and were analyzed by either ELISpot formation or cytokine production of CD4+ and CD8+ T cells. (C) Cellular immune responses of infected and noninfected vaccinated monkeys were analyzed. (D) The serum samples were tested for the presence of SIV Env binding antibodies (ELISA), or neutralizing activity against an SIV smE660 tier 1 clone or smE660 swarm was assessed using TZM-bl cells or human PBMC. The two groups were then compared using statistical analysis. In panels C and D, each symbol represents one individual animal.
Identification of an immune correlate of protection.
To determine whether the immune responses elicited by vaccination correlated with the protection seen after challenge, we compared the prechallenge immune responses induced by immunization in monkeys that were infected with those in monkeys that were not infected in the vaccine group. There were no significant differences in the frequencies of T-cell ELISpot responses or percentages of T cells that produced cytokines after SIV antigen stimulation in the uninfected monkeys compared with the infected ones (Fig. 7C) (ELISpot, P = 0.5; CD4+ cell response, P = 1; CD8+ cell response, P = 1). This was also true for the anti-SIV Env binding IgG ELISA titers or the neutralizing titers against an smE660 tier 1 clone (Fig. 7D) (ELISA, P = 0.5; tier 1 clone, P = 0.8) or against an smE660 swarm assayed in TZM-bl cells (Fig. 7D) (P = 0.7). Strikingly, however, we found a highly significant difference when the neutralizing activity of sera from vaccinated-protected animals was compared to that from unprotected animals using the SIVsmE660 swarm used for the challenge upon assay in PBMC (Fig. 7D) (SIVsmE660 swarm, P = 0.02). Such neutralization differences were not due to anti-human CD4 antibodies, as anti-human CD4 antibodies were not detectable in any of the monkeys (see Fig. S1A in the supplemental material), and there were no significant differences between the null group and the vaccine group for antibodies generated to human 293 expressing human CD4 (see Fig. S1B in the supplemental material).
DISCUSSION
In this study, we analyzed the immunogenicity and protective efficacy of a mismatched Env vaccine delivered by heterologous prime-boost vaccination. We found that rLCMV could be used in alternative prime-boost combinations with plasmid DNA and adenoviral vaccine vectors. Importantly, these data provide the first evidence that unmatched Env alone is sufficient to confer protection against a heterologous SIV swarm in a repetitive mucosal NHP challenge. Vaccination with this vector combination stimulates multiple arms of the immune system, including antigen-specific CD4+ and CD8+ T cells in the blood, spleen, and gut-associated tissue, along with HIV-1 Env-specific antibodies. It was not unexpected that rLCMV would be effective in boosting antibody responses to Env in the NHP, since it stimulates dendritic cells to provide T-cell help, especially during the boost phase of the immune response.
Although rLCMV-based vaccine vectors have been only recently described, wild-type LCMV has been a broadly used tool to study T-cell immunology in mice (46). As detailed here and published previously in regard to wild-type LCMV, the glycoprotein of this prototypic arenavirus efficiently targets antigen-presenting cells, especially DEC205+ CD11c+ dendritic cells, and activates them, resulting in the priming of B and T cells (41). This particular targeting of DCs may explain why even low doses of rLCMV elicit a substantial immune response after boosting, whereas higher doses of Ad5 (up to several log units higher) are necessary to reach similar potency. rAd5 is known to infect cells ubiquitously, and only the rAd5 fiber shaft and penton base interact with the DC and mononuclear subsets necessary for T-cell priming (11). Although the NHP prime-boost regimen confirms the mouse data, the effect of the boost on the cellular immune response was less than that observed in mice. This difference was likely due to the relatively lower LCMV vector dose per kilogram, a problem that may be remedied when large-scale production of the vector is achieved.
One of the major problems with rAd5 is the high seroprevalence of Ad5 in the human population, particularly in sub-Saharan Africa, where an HIV vaccine is urgently needed (42). Recently, several groups have proposed simian, great ape, or human alternative serotype adenoviral vectors and that a combination with another potent vector would be ideal. While rAd28 or rAd26 are representatives from one such alternative serotype, it has become apparent that seropositivity to these vectors is markedly increased in Africa, where AIDS is endemic (10, 31). As an alternative, the simian or great ape adenoviruses, particularly the serotype C chimpanzee Ad3 vector, may represent an alternative vector with similar immunogenicity but low seropositivity that could be used in such settings (33). In contrast, replication-competent LCMV can replicate in human cells but rarely infects immunocompetent individuals (15), as evidenced by its low (<5%) seropositivity worldwide (3, 12, 30, 32, 39). In addition, even if preexisting antibodies exist, they are rarely neutralizing (19).
The immunogenicity of one of these vector combinations was confirmed in NHP and used to evaluate its ability to protect against a tier 2-like SIV strain, SIVsmE660, in a mucosal challenge model. This challenge model was established to recapitulate human mucosal infection by HIV-1. It has been shown previously that DNA/Ad5 (26, 28) or DNA/modified vaccinia virus Ankara (MVA) vaccines expressing Env in addition to three antigens, including Gag, Pol, and Nef or Tat, protected in this challenge model. In the case of DNA/MVA vaccines, protective efficacy required the coexpression of granulocyte-macrophage colony-stimulating factor (GM-CSF). In these studies, it is unclear whether other immunogens aside from Env contributed to the protection. A more recent study showed that the addition of Env to Gag and Pol in the vaccine was required to delay SIVmac251 infection (4). While the protective effect of a vaccine regimen consisting of Env alone was not demonstrated in this study, antibody responses to Env as assayed by ELISA, neutralization activity against a tier 1 clone, and Env V2 peptide binding antibodies also correlated with protection. The difference in efficacy in these studies may be due to one of several factors, including the specific challenge virus, the dose used for challenge, the choice of vaccine vectors, or the specific vaccine antigen that was used to establish immunity. Importantly, Barouch et al. suggested that addition of Env improved vaccine-induced protection, consistent with our conclusion, but they did not demonstrate that Env alone prevents acquisition (4). Although other multivalent vaccines have been previously shown to protect against challenges with a defined single SIV or SHIV strain (4), we show here for the first time that a vaccine expressing only mismatched Env reduced the risk of infection by 82% and clearly demonstrate that Env alone is necessary and sufficient to protect against lentiviral infection by a heterologous quasispecies. The sequence distance between the vaccine Env (SIVmac239) and the Env of the challenge viral strain (SIVsmE660) is comparable to that between two sequence-divergent isolates of clade B viruses (28). The timing of the challenge relative to the boost can affect efficacy and does vary among published studies in the literature. In this study, the 6-week interval was chosen to take advantage of the high level of immunity observed at that time. Since the immune correlates are measured both on the day of challenge and at the peak of immune response, unless the mechanism of protection changes with time, this difference in timing is unlikely to change the immune correlate. In support of this notion, we have previously shown that the correlates of protection with a DNA/Ad vaccine regimen with challenge 16 weeks after boost agree with the present study (28).
A previous study showed that inactivated SIV grown in human T cells generated antibodies to human cell surface proteins (5). Specifically, antibodies to human CD4 in monkeys conferred protection against SHIV infection. The vectors used in the present study were purified from human HEK 293-derived vector packaging cell lines and did not generate detectable anti-CD4 immune responses as determined by ELISA or cell surface staining (see Fig. S1 in the supplemental material). This result suggests that anti-CD4 antibodies or antibodies to the producer 293 cells were not responsible for the vaccine protective effect seen in previous studies (5, 37). In addition, because antibody responses to viral Env were shown to correlate with protection, it is likely that antibodies to HIV-1 Env mediate this effect. Further, a previous study indicated that antibody-dependent cell-mediated cytotoxicity (ADCC) activity did not correlate with protection (28). Antibodies that neutralize the challenge virus swarm when tested in PBMC, but not in TZM-bl cells, appear to identify the correlate, highlighting the importance of subtleties in different neutralization assays for assessing biologically meaningful correlates. This study confirmed the neutralizing activity assayed on PBMC as the correlate of protection revealed by a previous study using a larger cohort, 89 animals (28). The fact that we did not observe more than 50% neutralization in this assay suggests that the challenge swarm is more similar to that of a tier 2 virus when tested on human PBMC. These data together provide a cautionary note about differences in alternative methods for measuring antibody neutralization. In particular, differences in expression levels of CD4, CCR5, or other cofactors between PBMC and transformed cell lines may affect viral entry or replication and affect the sensitivity or specificity of virus neutralization, which may hinder the ability to detect a correlate of protection. The sensitivity of detection for neutralizing activity targeted to particular epitopes may differ in TZM-bl cells and PBMC, as documented in the case of antibodies to HIV gp41 (6, 13, 43). It will be important to evaluate such alternative assay formats and natural viral isolates to define these correlates in future clinical efficacy trials.
In summary, this study suggests that immunization with a mismatched Env is necessary and sufficient to protect against a tier 2-like SIV challenge model, and this protection correlated with the ability to generate a neutralizing antibody response to the infecting viral swarm. Furthermore, we have identified two viral vectors that together elicit both humoral and cellular immunity. In clinical studies, it is possible that nonhuman rAd vectors such as chimpanzee Ad3 might substitute for rAd5. Because such a combination provides substantial protection against mucosal lentiviral challenge, this finding suggests that attention should be focused on this HIV gene product, an approach that would facilitate the development of an effective AIDS vaccine.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the Nabel lab for suggestions and discussions, Steve Perfetto, Richard Nguyen, and David Ambrozak for advice and help with flow cytometry and intracellular cytokine staining, Brenda Hartman for help with figure preparation, Mirko Paiardini for critical reading of the manuscript, Ati Tislerics for support with manuscript editing, Martha Nason for providing advice on statistical analysis, Jugamul Noor for the in vivo aspects of the NHP study, and Corrine Luedemann and Sophie Lane for assistance in performing the PBMC neutralization assays.
This research was supported in part by the Intramural Research Program of the Vaccine Research Center, NIAID, National Institutes of Health. L.F. was supported by the Mayenfisch Foundation.
L.F. and D.D.P. are listed as coinventors on a patent on arenavirus vectors. The other authors declare no competing financial interests.
Footnotes
Published ahead of print 16 May 2012
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Abbink P, et al. 2007. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 81:4654–4663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Almeida JR, et al. 2007. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J. Exp. Med. 204:2473–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Al-Zein N, Boyce TG, Correa AG, Rodriguez V. 2008. Meningitis caused by lymphocytic choriomeningitis virus in a patient with leukemia. J. Pediatr. Hematol. Oncol. 30:781–784 [DOI] [PubMed] [Google Scholar]
- 4. Barouch DH, et al. 2012. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 482:89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bergmeier LA, Walker J, Tao L, Cranage M, Lehner T. 1994. Antibodies to human and non-human primate cellular and culture medium components in macaques vaccinated with the simian immunodeficiency virus. Immunology 83:213–220 [PMC free article] [PubMed] [Google Scholar]
- 6. Binley JM, et al. 2004. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 78:13232–13252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Capone S, et al. 2010. Immune responses against a liver-stage malaria antigen induced by simian adenoviral vector AdCh63 and MVA prime-boost immunisation in non-human primates. Vaccine 29:256–265 [DOI] [PubMed] [Google Scholar]
- 8. Catanzaro AT, et al. 2007. Phase I clinical evaluation of a six-plasmid multiclade HIV-1 DNA candidate vaccine. Vaccine 25:4085–4092 [DOI] [PubMed] [Google Scholar]
- 9. Chakrabarti BK, et al. 2002. Modifications of the human immunodeficiency virus envelope glycoprotein enhance immunogenicity for genetic immunization. J. Virol. 76:5357–5368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen H, et al. 2010. Adenovirus-based vaccines: comparison of vectors from three species of adenoviridae. J. Virol. 84:10522–10532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng C, et al. 2007. Mechanism of Ad5 vaccine immunity and toxicity: fiber shaft targeting of dendritic cells. PLoS Pathog. 3:e25 doi:10.1371/journal.ppat.0030025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Lamballerie X, Fulhorst CF, Charrel RN. 2007. Prevalence of antibodies to lymphocytic choriomeningitis virus in blood donors in southeastern France. Transfusion 47:172–173 [DOI] [PubMed] [Google Scholar]
- 13. Fenyo EM, et al. 2009. International network for comparison of HIV neutralization assays: the NeutNet report. PLoS One 4:e4505 doi:10.1371/journal.pone.0004505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Finn JD, et al. 2009. Persistence of transgene expression influences CD8+ T-cell expansion and maintenance following immunization with recombinant adenovirus. J. Virol. 83:12027–12036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fischer SA, et al. 2006. Transmission of lymphocytic choriomeningitis virus by organ transplantation. N. Engl. J. Med. 354:2235–2249 [DOI] [PubMed] [Google Scholar]
- 16. Flatz L, Bergthaler A, de la Torre JC, Pinschewer DD. 2006. Recovery of an arenavirus entirely from RNA polymerase I/II-driven cDNA. Proc. Natl. Acad. Sci. U. S. A. 103:4663–4668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Flatz L, et al. 2010. Development of replication-defective lymphocytic choriomeningitis virus vectors for the induction of potent CD8+ T cell immunity. Nat. Med. 16:339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guimaraes-Walker A, et al. 2008. Lessons from IAVI-006, a phase I clinical trial to evaluate the safety and immunogenicity of the pTHr.HIVA DNA and MVA.HIVA vaccines in a prime-boost strategy to induce HIV-1 specific T-cell responses in healthy volunteers. Vaccine 26:6671–6677 [DOI] [PubMed] [Google Scholar]
- 19. Hangartner L, Zinkernagel RM, Hengartner H. 2006. Antiviral antibody responses: the two extremes of a wide spectrum. Nat. Rev. Immunol. 6:231–243 [DOI] [PubMed] [Google Scholar]
- 20. Hansen SG, et al. 2009. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat. Med. 15:293–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haynes BF, et al. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 366:1275–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Honda M, et al. 2009. Different vaccine vectors delivering the same antigen elicit CD8+ T cell responses with distinct clonotype and epitope specificity. J. Immunol. 183:2425–2434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jaoko W, et al. 2010. Safety and immunogenicity study of multiclade HIV-1 adenoviral vector vaccine alone or as boost following a multiclade HIV-1 DNA vaccine in Africa. PLoS One 5:e12873 doi:10.1371/journal.pone.0012873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ko SY, et al. 2009. Enhanced induction of intestinal cellular immunity by oral priming with enteric adenovirus 41 vectors. J. Virol. 83:748–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ko SY, et al. 2009. Development of recombinant adenovirus 28 vectors for HIV vaccines. Retrovirology 6(Suppl. 3):P309 doi:10.1186/1742-4690-6-S3-P309 [Google Scholar]
- 26. Lai L, et al. 2011. Prevention of infection by a granulocyte-macrophage colony-stimulating factor co-expressing DNA/modified vaccinia Ankara simian immunodeficiency virus vaccine. J. Infect. Dis. 204:164–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Letvin NL, et al. 2006. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science 312:1530–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Letvin NL, et al. 2011. Immune and genetic correlates of vaccine protection against mucosal infection by SIV in monkeys. Sci. Transl. Med. 3:81ra36 doi:10.1126/scitranslmed.3002351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li M, et al. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 79:10108–10125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marrie TJ, Saron MF. 1998. Seroprevalence of lymphocytic choriomeningitis virus in Nova Scotia. Am. J. Trop. Med. Hyg. 58:47–49 [DOI] [PubMed] [Google Scholar]
- 31. Mast TC, et al. 2010. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine 28:950–957 [DOI] [PubMed] [Google Scholar]
- 32. Palacios G, et al. 2008. A new arenavirus in a cluster of fatal transplant-associated diseases. N. Engl. J. Med. 358:991–998 [DOI] [PubMed] [Google Scholar]
- 33. Peruzzi D, et al. 2009. A novel chimpanzee serotype-based adenoviral vector as delivery tool for cancer vaccines. Vaccine 27:1293–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pitisuttithum P, et al. 2006. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J. Infect. Dis. 194:1661–1671 [DOI] [PubMed] [Google Scholar]
- 35. Pulendran B, Tang H, Denning TL. 2008. Division of labor, plasticity, and crosstalk between dendritic cell subsets. Curr. Opin. Immunol. 20:61–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rasmussen RA, Lakhashe SK, Ruprecht RM. 2010. Bimodal AIDS vaccine approach: induction of cellular as well as humoral immunity can protect from systemic infection. Vaccine 28(Suppl. 2):B25–B31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Reimann KA, et al. 1995. In vivo administration of CD4-specific monoclonal antibody: effect on provirus load in rhesus monkeys chronically infected with the simian immunodeficiency virus of macaques. AIDS Res. Hum. Retroviruses 11:517–525 [DOI] [PubMed] [Google Scholar]
- 38. Rerks-Ngarm S, et al. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361:2209–2220 [DOI] [PubMed] [Google Scholar]
- 39. Riera L, et al. 2005. Serological study of the lymphochoriomeningitis virus (LCMV) in an inner city of Argentina. J. Med. Virol. 76:285–289 [DOI] [PubMed] [Google Scholar]
- 40. Roederer M, Nozzi JL, Nason MC. 2011. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry 79A:167–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sevilla N, et al. 2000. Immunosuppression and resultant viral persistence by specific viral targeting of dendritic cells. J. Exp. Med. 192:1249–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sumida SM, et al. 2005. Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J. Immunol. 174:7179–7185 [DOI] [PubMed] [Google Scholar]
- 43. Tomaras GD, et al. 2011. Polyclonal B cell responses to conserved neutralization epitopes in a subset of HIV-1-infected individuals. J. Virol. 85:11502–11519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang L, et al. 2009. Delivery of adenovirus HIV vaccine vectors to the intestine induces enhanced mucosal cellular immunity. J. Virol. 83:7166–7175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yang ZY, et al. 2004. Selective modification of variable loops alters tropism and enhances immunogenicity of human immunodeficiency virus type 1 envelope. J. Virol. 78:4029–4036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zinkernagel RM. 2002. Lymphocytic choriomeningitis virus and immunology. Curr. Top. Microbiol. Immunol. 263:1–5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.