Abstract
The DNA damage response to infection with minute virus of mice (MVM) leads to activated p53; however, p21 levels are reduced via a proteasome-mediated mechanism. This loss was sustained, as virus replicated in infected cells held at the G2/M border. Addition of the cyclin-dependent kinase (CDK) inhibitor roscovitine after S-phase entry reduced MVM replication, suggesting that CDK activity was critical for continued viral replication and virus-induced reduction of p21 may thus be necessary to prevent inhibition of CDK.
TEXT
Following infection of a variety of both murine and human cell types, minute virus of mice (MVM) induces a cellular DNA damage response (DDR), which positively contributes to viral replication (3). A number of kinases and effector proteins that are activated and recruited to MVM replication compartments, in particular ATM (ataxia-telangiectasia mutated) and members of the MRN (Mre11-Rad50-Nbs1) complex, initiate a signaling cascade that results in activation of a number of cellular targets, including Nbs1, Chk2, and p53 (3, 26). p53, a critical node upon which various DNA damage stimuli converge (28), was shown to be phosphorylated and stabilized during MVM infection.
A major consequence of the DDR induced by MVM and other parvoviruses is cell cycle arrest (3, 7, 8, 21), which may aid viral replication by providing an environment conducive to continuing replication (6, 11, 25). Following induction of cellular stresses, including the DNA damage response pathway, p53 becomes activated via phosphorylation and acetylation (20, 27). Downstream targets of p53 are then upregulated to effect cell cycle arrest and in some cases apoptosis (20). While DNA tumor viruses typically employ various strategies to inactivate p53 (19), in this study, we have shown that MVM replication leads, in addition to p53 phosphorylation, to its abundant acetylation and significant activation. One of the critical effector proteins targeted by p53 is p21 (12, 13), which plays important roles in cell cycle arrest, apoptosis, and senescence (1). Surprisingly, although p21 RNA levels remained high during MVM infection, we observed a proteasome-mediated loss of p21 that was sustained throughout the course of viral replication. This loss was not due directly to effects of the viral replicator protein NS1. Our results suggest that while p21 levels may not directly govern MVM-induced cell cycle arrest, its loss may be essential for prolonged viral replication in arrested cells.
p53 is activated and transcriptionally active following MVM infection.
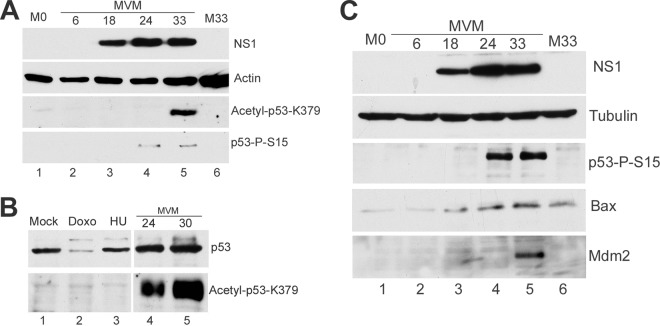
We have previously shown that during the MVM infection-induced DDR in murine cells, and soon after expression of the main viral replication protein NS1 begins, p53 is stabilized and phosphorylated on serine 18 (3), (serine 15 in humans) (shown in Fig. 1A, lanes 4 and 5). During MVM infection, we also observed a strong acetylation of p53 at Lys 379 (Lys 382 in humans) soon after NS1 expression (Fig. 1A, lane 5), a modification known to also contribute to p53 stabilization and activation (16, 27). These and subsequent immunoblots were done as previously described (3). We could not detect acetylation of p53 following expression of NS1 alone (data not shown), consistent with our previous finding that the MVM DDR was dependent on viral replication (3). Notably, acetylation of p53 following MVM infection of murine cells was particularly strong and significantly higher than that seen following treatment with DNA-damaging drugs doxorubicin (catalog no. D1515; Sigma) and hydroxyurea (catalog no. H-8627; Sigma) under conditions where the levels of phosphorylated p53 were similarly induced (Fig. 1B, compare lanes 4 and 5 with lanes 2 and 3).
Fig 1.
(A) MVM infection results in acetylation of p53. A9 cells parasynchronized by isoleucine deprivation (3) were released into complete medium and at the same time mock infected or infected with MVM at a multiplicity of infection (MOI) of 10. Cells were harvested at the indicated time points after release into complete medium and lysed in modified radioimmunoprecipitation assay (RIPA) buffer (3), and the amounts of protein were quantitated by the Bradford assay, and equal amounts of protein were loaded in each well. Western blot analyses were performed using antibodies against NS1 (10), actin (catalog no. MA515739; Pierce), phosphorylated p53 S15 (catalog no. 9284S; Cell Signaling) (p53-P-S15), and p53 acetylated on Lys379 (catalog no. 2570S; Cell Signaling) (acetyl-p53). Lanes M0 and M33 contain mock-treated cells at the time of release and 33 h postrelease, respectively. (B) p53 acetylation is significantly higher in MVM-infected cells. A9 cells were infected with MVM at the indicated time points as described above for panel A. Control cells were mock treated or treated with 1 mM hydroxyurea (HU) or 100 nM doxorubicin (Doxo) for 24 h. Western blots were performed against total p53 (catalog no. sc-6243; Santa Cruz Biotechnology) (phosphorylated p53 migrates as a slower running band), and acetyl-p53. (C) MVM infection results in upregulation of Bax and Mdm2. Cells were mock infected or infected with MVM as described above for panel A. Western blotting was performed using antibodies directed against NS1, tubulin (catalog no. T4026; Sigma), p53-S15, Bax (catalog no. 2772; Cell Signaling), and Mdm2 (catalog no. MAB3776; Millipore).
p53 activity is typically associated with upregulation of a number of target genes that are important in apoptosis and cell cycle arrest (20). During the MVM-induced DDR, we found a modest but significant increase in the levels of the known p53 targets Bax (Fig. 1C, lanes 4 and 5) and Mdm2 (Fig. 1C, lane 5), consistent with p53 activation; however, we found that levels of the cell cycle regulator p21, normally also a target of activated p53, were reduced and, surprisingly, remained so for an extended period of time.
Although p53 is activated, p21 is proteasomally downregulated for extended times following MVM infection.
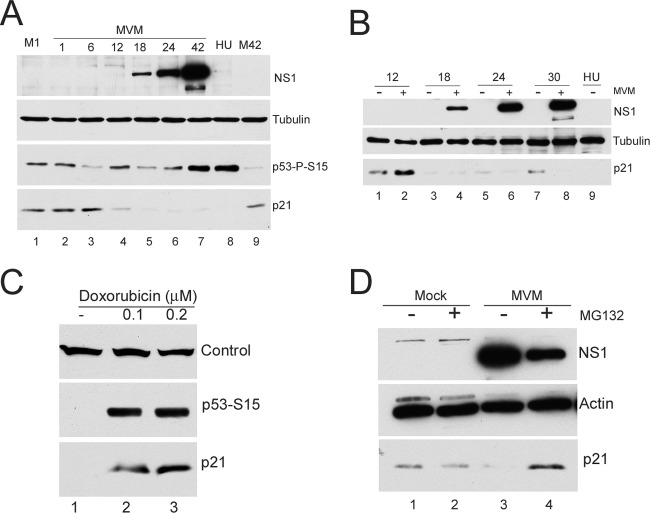
p21 has been shown to be involved in cell cycle arrest both in G1 and G2. It has recently become clear that upon S-phase entry in normal cycling cells, and following certain types of DNA damage, p21 levels are diminished via the activity of the CRL4Cdt2 ubiquitin ligase, which targets p21 for degradation in a manner that depends on its interaction with PCNA associated with chromatin (2, 17, 23). Upon S-phase exit/G2 entry, p21 expression is then typically restored to basal levels. During MVM infection of parasynchronous A9 cells, p21 levels were, as expected, reduced as cells cycled into S phase (approximately 8-fold [Fig. 2A, lane 4]). However, surprisingly, even though p53 was activated, p21 levels remained diminished for at least 42 h (Fig. 2A, lanes 5 to 7). Mock-infected cells showed recovery of p21 levels at this late time (approximately 18-fold [Fig. 2A, compare lanes 7 and 9]). A more detailed analysis of p21 levels across the cell cycle for both mock-infected and infected cells showed a greater than 10-fold reduction at 18 h following release from isoleucine block into complete medium in both cases (Fig. 2B, compare lanes 1 and 2 to lanes 3 and 4). However, as early as 24 h after release into complete medium, the levels of p21 had begun to be restored in mock-infected cells as they asynchronously exited S phase into G2/M (approximately 2-fold at 24 h [Fig. 2B, lane 5] and up to 7-fold at 30 h [Fig. 2B, lane 7]). The levels of p21 in MVM-infected cells (which featured activated p53) remained very low (Fig. 2B, lanes 4, 6, and 8). Notably, virus replication was proceeding during this time.
Fig 2.
(A) MVM infection results in loss of p21 in the presence of activated p53. A9 cells parasynchronized as described in the legend to Fig. 1 were mock infected or infected with MVM at an MOI of 10. Western blot analyses were performed using antibodies against NS1, tubulin, phosphorylated p53 S15 and p21 (catalog no. sc-271532; Santa Cruz Biotechnology). Lanes M1 and M42 contain mock-treated cells harvested at 1 h and 42 h after release from isoleucine block into complete medium, respectively. Treatment of A9 cells with hydroxyurea (HU)—which induces an ATR-mediated DNA damage response and blocks cells in S phase—also reduced levels of p21 (lane 8) as previously documented by others (14). (B) G2/M restoration of p21 levels does not occur in MVM-infected cells. Mock- and MVM-infected A9 cells were harvested at the indicated time points after release from isoleucine-deprived medium. Western blot analyses were performed as described above for panel A. (C) Agents that block cells in G2/M result in increased levels of p21. A9 cells were treated with doxorubicin at the indicated concentrations for 24 h or were not treated with doxorubicin (−). Cells were harvested and processed for Western blotting. The band labeled Control in the top blot is a cellular cross-reacting band to the p21 antibody, which was used as a loading control. (D) p21 loss is proteasome mediated. Mock- and MVM-infected A9 cells, parasynchronized as described in the legend to Fig. 1, were treated with the proteasome inhibitor MG132 or DMSO vehicle for 6 h at 26 hours postinfection (hpi). Cells were harvested at 32 hpi, and Western blotting was performed as described in the legend to Fig. 1.
MVM infection blocks cells at the G2/M border. Could sustained low levels of p21 merely be a consequence of holding cells at this point in the cell cycle? Doxorubicin is a DNA-damaging agent that, similar to MVM, causes cell cycle arrest at the G2/M phase border which is accompanied by phosphorylation of p53 (31). However, in contrast to MVM infection, doxorubicin treatment at concentrations (100 nM and 200 nM) that resulted in cell cycle arrest (as monitored by flow cytometry analysis [data not shown]) and levels of p53 phosphorylation similar to those seen during MVM infection resulted in a marked increase in p21 levels (Fig. 2C, lanes 2 and 3). Similar results were also observed when cells where treated with etoposide (1 μM and 2 μM) (catalog no. E1383; Sigma), another DNA-damaging agent that causes cell cycle arrest (data not shown). Thus, the loss of p21 following MVM infection is likely to be specific and not merely a consequence of cell cycle arrest.
MVM-induced loss of p21 was found to be via proteasomal degradation. Initial experiments revealed no significant differences in p21 RNA levels by semiquantitative reverse transcription-PCR (RT-PCR) throughout MVM infection (data not shown), suggesting that p21 reduction happened posttranscriptionally. To determine whether p21 loss occurred via the proteasome, parasynchronized cells either infected with MVM or mock infected at the time of release into complete medium, were treated with the proteasome inhibitor MG132 (10 μM) (catalog no. 474791; Calbiochem) for 6 h before the assay at 32 h postrelease (Fig. 2D). At this time, mock-infected cells had cycled out of S phase and p21 levels had recovered on their own, while p21 levels in infected cells were substantially restored by MG132 (at least 25-fold [Fig. 2D, compare lanes 3 and 4]). Adeno-associated virus type 2 (AAV2) has previously been shown to increase proteasome-dependent degradation of p21 in human papillomavirus-positive cell lines (4).
Reduction of p21 levels occurs independently of MVM NS1 and requires viral replication.
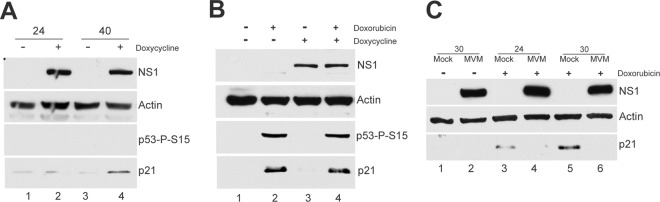
Transient transfection of murine cells, done as previously described (3), with increasing amounts of MVM NS1 did not result in significant reduction in the levels of p21 (data not shown). To examine this point further, we developed murine A9 cell lines expressing NS1 under the control of a doxycycline-responsive inducible promoter, using the pINDUCER lentivirus system (22). At 24 h after induction of NS1 in these cell lines, no significant reduction in p21 levels was observed (Fig. 3A, lanes 1 and 2), and by 40 h after induction, instead of a decrease, a modest increase was consistently observed (approximately 15-fold [Fig. 3A, compare lanes 3 and 4]), as previously seen by others (24, 30). Thus, MVM NS1 alone did not result in reduced p21, and in fact, MVM NS1 alone led to an increase in p21 levels in a p53-independent manner. Whether p21 was actively induced by NS1 or was stabilized as consequence of the expected NS1-induced cell cycle block is under investigation.
Fig 3.
(A) NS1 leads to stabilization of p21. Stable A9 cell lines that inducibly express NS1 under a doxycycline-responsive promoter (A9-NS1) were generated using the pINDUCER lentivirus system (22) as described in the text. Cells were treated with doxycycline (500 ng/ml) (+) or vehicle (−) for 24 and 40 h and harvested, and the levels of NS1, actin, p53 phosphorylated on serine 15, and p21 were assayed by Western blotting. (B) NS1 expression does not lead to degradation of p21 in the context of DNA damage. A9 cells that were induced to express NS1 were treated with doxorubicin or vehicle control as indicated at a final concentration of 150 nM. Shortly thereafter, doxycycline (500 ng/ml) or vehicle control was added for 24 h to induce NS1 expression. Cells were then harvested and assayed via Western blotting as described above for panel A. (C) MVM infection prevents p21 upregulation by doxorubicin treatment. Parasynchronized A9 cells were released into complete medium and at the same time mock infected or infected with MVM at an MOI of 10. At 18 h postrelease, doxorubicin was added to mock- and MVM-infected cells at a final concentration of 150 nM. Cells were harvested at the indicated time points and assayed via Western blotting as described above for panel A.
In these experiments, p53 was not phosphorylated; we have previously reported that the MVM-induced DDR as well as p53 activation required viral replication and was not a result of NS1 expression alone (3). Thus, might NS1 have an effect on p21 only in the presence of an ongoing DDR? To test this possibility, we first treated the NS1-inducible cell lines with doxorubicin (which mimics an ATM-mediated DDR [18]) and then induced NS1 expression. NS1 expression did not detectably reduce the levels of p21 under these conditions either (Fig. 3B, compare lanes 2 and 4).
These experiments indicated that NS1 alone, either in the presence or absence of an ongoing DDR, did not affect reduction of p21 levels, and suggested further that p21 reduction was a consequence of events induced by viral infection and that could function in the presence of large amounts of NS1 generated during infection. If this were the case, viral infection might be expected to cause the loss of p21 induced by other DDR-inducing agents. Thus, 18 h after release from an isoleucine block, infected and mock-infected cells were treated with 150 nM doxorubicin for 6 h (Fig. 3C, lanes 3 and 4) or 12 h (Fig. 3C, lanes 5 and 6) and assayed for p21 levels. As expected, in the absence of infection, doxorubicin treatment resulted in increased levels of p21; however, in infected cells, p21 levels remained low (Fig. 3C, compare lanes 3 and 4 and lanes 5 and 6). Thus, MVM infection downregulated p21 levels induced by exogenous agents that stabilized or induced overexpression of p21.
Inhibition of CDK activity following S-phase entry reduces MVM replication.
Upon entering S phase, p21 levels are normally downregulated to allow cellular replication to proceed. p21 has at least two critical interacting cellular partners that affect DNA replication, a cyclin-dependent kinase (CDK) binding site in the N terminus and a C-terminal PCNA interaction motif (9, 29). It has been suggested that MVM utilizes the host DNA polymerase δ-mediated replication machinery—which can be inhibited by p21 binding to the polymerase cofactor PCNA—to replicate its genome in infected cells (5). Thus, it seemed possible that the loss of p21 and consequent effects may have been necessary for efficient MVM replication. Indeed, in in vitro assays, the addition of p21 has been shown to inhibit the conversion of the incoming single-stranded genome to subsequent replicative intermediates and subsequent gene expression (5). This inhibition could be relieved by the addition of excess PCNA, suggesting that p21 could inhibit MVM replication in a PCNA-dependent manner (5).
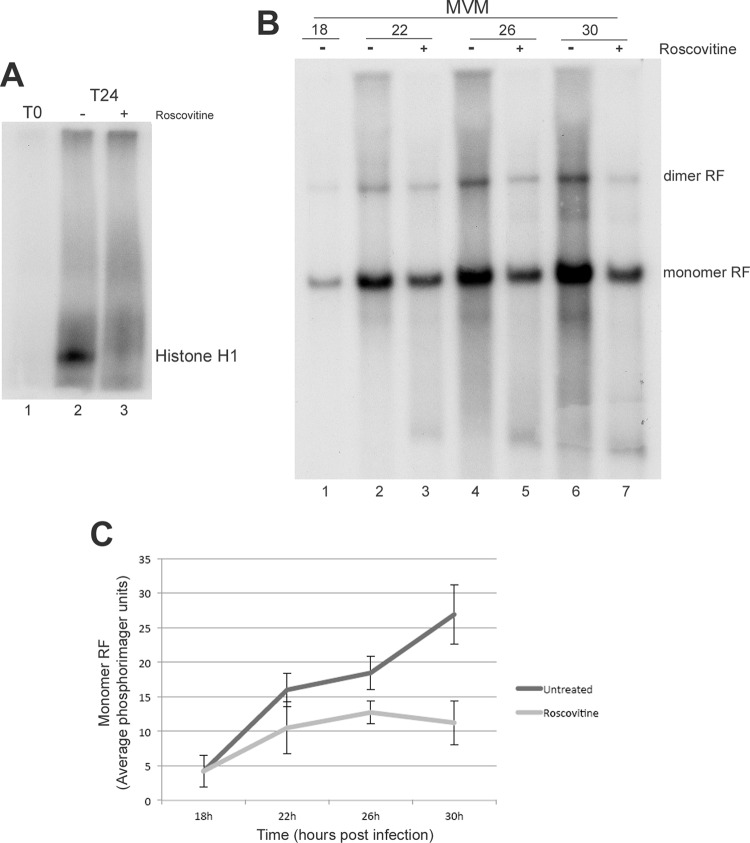
Attempts to directly test the role that depletion of p21 plays in MVM infection by transient overexpression of p21 were complicated by the expectation that such expression would inhibit cell cycle progression and entry into S phase (15), which itself would be predicted to limit MVM replication. However, the effects of p21 are primarily mediated by its inhibition of CDKs (1, 32), which thus presented an alternative target for our analysis. Cdk2 activity is particularly critical during S phase and is required for efficient cellular DNA replication. Indeed in MVM infection, onset of DNA synthesis was shown to correlate with an increase in cyclin A-associated kinase activity (5). Therefore, as an alternative approach, we utilized roscovitine, a drug that has been shown to be a specific inhibitor of cyclin-dependent kinases, to mimic effects due to overexpression of p21. Cells were treated with 37.5 μM roscovitine (catalog no. R7772; Sigma) or dimethyl sulfoxide (DMSO) vehicle 18 h after release from an isoleucine block (at which time cells had entered S phase and viral replication had begun to take place [Fig. 4B, lane 1]). We first confirmed that Cdk2 was inhibited via roscovitine treatment using an established immunoprecipitation kinase assay (5), in which Cdk2 was immunoprecipitated (antibody catalog no. sc-163; Santa Cruz Biotechnology) from 80 μg of cell extract. Kinase reactions were performed using 1 μg histone H1 (catalog no. 14-155; Millipore) as the substrate and indicated significant inhibition (Fig. 4A, compare lanes 2 and 3). Cells were then harvested at 4, 8, and 12 h after treatment (that is 22, 26, and 30 h after release [Fig. 4B]) and assayed via Southern blotting as previously described (10). In the presence of roscovitine, there was a significant reduction in the rate of accumulation of viral replication intermediates (Fig. 4B, lanes 3, 5, and 7) compared to control treated infected cells (Fig. 4B, lanes 2, 4, and 6). Quantifications of three separate experiments are shown in Fig. 4C. These results suggested that CDK activity is critical for viral replication and that reduction of p21 may be necessary to prevent inhibition of CDK activity.
Fig 4.
(A) Inhibition of Cdk2 activity by roscovitine treatment. A9 cells parasynchronized by isoleucine deprivation were harvested at the time of release (T0) and 24 h after release (T24) (lanes 2 and 3). In the sample shown in lane 3, roscovitine was added for 6 h at 18 h after release. Lysates were quantified, immunoprecipitated, and used for kinase reactions as described in the text. Samples were run on 12% SDS-polyacrylamide gels, dried, and used for autoradiography. (B) Roscovitine treatment affects MVM replication following S-phase entry. A9 cells parasynchronized by isoleucine deprivation were infected with MVM at the time of release at an MOI of 1. At 18 hpi, cells were treated with vehicle or roscovitine for 4, 8, or 12 h and harvested at 22, 26, or 30 hpi, respectively. Cell lysates were used for Southern blotting. Equivalent loading was confirmed by measurements using a nanodrop spectrophometer as well as via ethidium bromide staining of cellular DNA. The positions of monomers and dimers of the 5-kb double-stranded replicative form (RF) are shown to the right of the blot. A representative blot is shown. (C) Quantification of the replication. The monomer double-stranded replicative forms from the Southern blots (an example of which is shown in panel B above) were quantitated using phosphorimager analysis (10). Data points represent averages from three independent experiments. The error bars represent the standard deviations. At both 26 and 30 h, the values for the treated samples were significantly different from the values for the untreated samples, with P values of <0.05.
ACKNOWLEDGMENTS
We thank Lisa Burger for excellent technical assistance, Guang Hu at NIH/NIEHS for pINDUCER reagents, and Matt Fuller and Olufemi Fasina for helpful discussions and participation in portions of this work.
This work was supported by PHS grants AI21302 and AI46458 to D.J.P.
Footnotes
Published ahead of print 23 May 2012
REFERENCES
- 1. Abbas T, Dutta A. 2009. p21 in cancer: intricate networks and multiple activities. Nat. Rev. Cancer 9:400–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abbas T, et al. 2008. PCNA-dependent regulation of p21 ubiquitylation and degradation via the CRL4Cdt2 ubiquitin ligase complex. Genes Dev. 22:2496–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adeyemi RO, Landry S, Davis ME, Weitzman MD, Pintel DJ. 2010. Parvovirus minute virus of mice induces a DNA damage response that facilitates viral replication. PLoS Pathog. 6:e1001141 doi:10.1371/journal.ppat.1001141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alam S, Sen E, Brashear H, Meyers C. 2006. Adeno-associated virus type 2 increases proteosome-dependent degradation of p21WAF1 in a human papillomavirus type 31b-positive cervical carcinoma line. J. Virol. 80:4927–4939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bashir T, Horlein R, Rommelaere J, Willwand K. 2000. Cyclin A activates the DNA polymerase delta-dependent elongation machinery in vitro: a parvovirus DNA replication model. Proc. Natl. Acad. Sci. U. S. A. 97:5522–5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chaurushiya MS, Weitzman MD. 2009. Viral manipulation of DNA repair and cell cycle checkpoints. DNA Repair (Amst.) 8:1166–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen AY, Luo Y, Cheng F, Sun Y, Qiu J. 2010. Bocavirus infection induces mitochondrion-mediated apoptosis and cell cycle arrest at G2/M phase. J. Virol. 84:5615–5626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen AY, Qiu J. 2010. Parvovirus infection-induced cell death and cell cycle arrest. Future Virol. 5:731–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen J, Jackson PK, Kirschner MW, Dutta A. 1995. Separate domains of p21 involved in the inhibition of Cdk kinase and PCNA. Nature 374:386–388 [DOI] [PubMed] [Google Scholar]
- 10. Choi EY, Newman AE, Burger L, Pintel D. 2005. Replication of minute virus of mice DNA is critically dependent on accumulated levels of NS2. J. Virol. 79:12375–12381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davy C, Doorbar J. 2007. G2/M cell cycle arrest in the life cycle of viruses. Virology 368:219–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dulic V, et al. 1994. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell 76:1013–1023 [DOI] [PubMed] [Google Scholar]
- 13. el-Deiry WS, et al. 1993. WAF1, a potential mediator of p53 tumor suppression. Cell 75:817–825 [DOI] [PubMed] [Google Scholar]
- 14. Gottifredi V, McKinney K, Poyurovsky MV, Prives C. 2004. Decreased p21 levels are required for efficient restart of DNA synthesis after S phase block. J. Biol. Chem. 279:5802–5810 [DOI] [PubMed] [Google Scholar]
- 15. Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. 1993. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75:805–816 [DOI] [PubMed] [Google Scholar]
- 16. Ito A, et al. 2001. p300/CBP-mediated p53 acetylation is commonly induced by p53-activating agents and inhibited by MDM2. EMBO J. 20:1331–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim Y, Starostina NG, Kipreos ET. 2008. The CRL4Cdt2 ubiquitin ligase targets the degradation of p21Cip1 to control replication licensing. Genes Dev. 22:2507–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kurz EU, Douglas P, Lees-Miller SP. 2004. Doxorubicin activates ATM-dependent phosphorylation of multiple downstream targets in part through the generation of reactive oxygen species. J. Biol. Chem. 279:53272–53281 [DOI] [PubMed] [Google Scholar]
- 19. Levine AJ. 2009. The common mechanisms of transformation by the small DNA tumor viruses: the inactivation of tumor suppressor gene products: p53. Virology 384:285–293 [DOI] [PubMed] [Google Scholar]
- 20. Levine AJ. 1997. p53, the cellular gatekeeper for growth and division. Cell 88:323–331 [DOI] [PubMed] [Google Scholar]
- 21. Luo Y, Chen AY, Qiu J. 2011. Bocavirus infection induces a DNA damage response that facilitates viral DNA replication and mediates cell death. J. Virol. 85:133–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meerbrey KL, et al. 2011. The pINDUCER lentiviral toolkit for inducible RNA interference in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A. 108:3665–3670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nishitani H, et al. 2008. CDK inhibitor p21 is degraded by a proliferating cell nuclear antigen-coupled Cul4-DDB1Cdt2 pathway during S phase and after UV irradiation. J. Biol. Chem. 283:29045–29052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Op De Beeck A, et al. 2001. NS1- and minute virus of mice-induced cell cycle arrest: involvement of p53 and p21(cip1). J. Virol. 75:11071–11078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Orba Y, et al. 2010. Large T antigen promotes JC virus replication in G2-arrested cells by inducing ATM- and ATR-mediated G2 checkpoint signaling. J. Biol. Chem. 285:1544–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ruiz Z, Mihaylov IS, Cotmore SF, Tattersall P. 2011. Recruitment of DNA replication and damage response proteins to viral replication centers during infection with NS2 mutants of minute virus of mice (MVM). Virology 410:375–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sakaguchi K, et al. 1998. DNA damage activates p53 through a phosphorylation-acetylation cascade. Genes Dev. 12:2831–2841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sengupta S, Harris CC. 2005. p53: traffic cop at the crossroads of DNA repair and recombination. Nat. Rev. Mol. Cell Biol. 6:44–55 [DOI] [PubMed] [Google Scholar]
- 29. Waga S, Hannon GJ, Beach D, Stillman B. 1994. The p21 inhibitor of cyclin-dependent kinases controls DNA replication by interaction with PCNA. Nature 369:574–578 [DOI] [PubMed] [Google Scholar]
- 30. Wan Z, et al. 2010. Human parvovirus B19 causes cell cycle arrest of human erythroid progenitors via deregulation of the E2F family of transcription factors. J. Clin. Invest. 120:3530–3544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xiao Z, et al. 2003. Chk1 mediates S and G2 arrests through Cdc25A degradation in response to DNA-damaging agents. J. Biol. Chem. 278:21767–21773 [DOI] [PubMed] [Google Scholar]
- 32. Xiong Y, et al. 1993. p21 is a universal inhibitor of cyclin kinases. Nature 366:701–704 [DOI] [PubMed] [Google Scholar]