Abstract
La Crosse virus (LACV) is a leading cause of pediatric encephalitis and aseptic meningitis in the midwestern and southern United States, where it is considered an emerging human pathogen. No specific therapies or vaccines are available for LACV or any other orthobunyaviruses. Inhibition of LACV entry into cells is a potential target for therapeutic intervention, but this approach is limited by our current knowledge of the entry process. Here, we determined that clathrin-mediated endocytosis is the primary mechanism of orthobunyavirus entry and identified key cellular factors in this process. First, we demonstrated that LACV colocalized with clathrin shortly after infection in HeLa cells; we then confirmed the functional requirement of dynamin- and clathrin-mediated endocytosis for orthobunyavirus entry using several independent assays and, importantly, extended these findings to primary neuronal cultures. We also determined that macropinocytosis and caveolar endocytosis, both established routes of virus entry, are not critical for cellular entry of LACV. Moreover, we demonstrated that LACV infection is dependent on Rab5, which plays an important regulatory role in early endosomes, but not on Rab7, which is associated with late endosomes. These findings provide the first description of bunyavirus entry into cells of the central nervous system, where infection can cause severe neurological disease, and will aid in the design and development of antivirals and therapeutics that may be useful in the treatment of LACV and, more broadly, arboviral infections of the central nervous system.
INTRODUCTION
In the past decade, arthropod-borne viruses (arboviruses) have been responsible for approximately 30% of all emerging infectious diseases, highlighting their potential global impact as human and veterinary pathogens (21, 28, 60). The Bunyaviridae are the largest family of viruses, comprising five genera (Hantavirus, Orthobunyavirus, Nairovirus, Phlebovirus, and Tospovirus), each of which contains important human and/or agricultural pathogens (9); all but the Hantaviruses are arboviruses. La Crosse virus (LACV) is a member of the California serogroup of orthobunyaviruses that also includes California encephalitis virus and snowshoe hare virus (9). In addition to being a major cause of pediatric encephalitis and aseptic meningitis in the midwestern United States, LACV has established itself as an emerging pathogen in the southern United States because of its introduction through an invasive mosquito species (16, 18). LACV infection in humans is usually asymptomatic or results in a nonspecific febrile illness; however, a small proportion of children infected with LACV develop acute and sometimes fatal encephalitis. Approximately half of those children have seizures during the acute illness, and a subset develops chronic epilepsy (∼10%) and/or neurobehavioral sequelae (∼2%) (6, 37, 48). Currently, there are no effective antivirals against LACV or other arboviral encephalitides, underscoring the importance of enhancing our understanding of the viral and cellular factors determining virus entry, fusion, replication, tropisms, and pathogenesis.
Virus entry into host cells is a complex, early event in infection, and several major routes of virus entry have been described. Clathrin-mediated endocytosis (CME) is one of the most common cellular endocytic pathways and is a mechanism of entry for enveloped viruses into mammalian cells. Among the many important pathogens that enter cells through this pathway are flaviviruses (West Nile virus and dengue virus), alphaviruses (Semliki Forest virus and Sindbis virus), and other bunyaviruses (Hantaan virus, Crimean-Congo hemorrhagic fever virus [CCHFV], and Oropouche virus) (1, 12, 27, 35, 51, 54). CME is a dynamic process that uses numerous regulatory and accessory proteins to assemble clathrin-coated vesicles (CCVs) in ∼1 min (14, 35). In this process, a virus binds to its receptor, inducing a clathrin-coated pit (CCP) to form on the cytoplasmic side of the plasma membrane and concomitantly invaginate as the clathrin lattice assembles. In addition to CME, two major alternative pathways of virus entry have also been described: caveolar/raft-dependent endocytosis and macropinocytosis. Caveolae are flask-shaped invaginations at the plasma membrane associated with cholesterol-rich lipid rafts and are smaller (60 to 80 nm in diameter) than CCVs (∼120 nm). In contrast, macropinocytosis can take in larger cargo, as there are macropinosomes that range in size from 200 to 10,000 nm in diameter. Macropinocytosis occurs when membrane ruffles fold back upon themselves with the distal end fusing with the plasma membrane to form a cavity known as a macropinosome. As with caveolar endocytosis, macropinocytosis is dependent on cholesterol-rich lipid rafts. Although these are the most well-characterized routes of virus entry, other clathrin- and caveola-independent endocytic routes have also been described (reviewed in references 31 and 36).
Current understanding of LACV entry is based solely on its sensitivity to lysosomotropic agents (46) and, as with many other neurotropic viruses, including Rift Valley fever virus (RVFV) and West Nile virus, the route of cellular entry has only been examined in cell lines (12, 33, 46) and not in primary cells of the central nervous system (CNS). Moreover, the functional requirement for the cellular factors involved in entry and trafficking of LACV has not been determined. In this study, we used a multifaceted approach to determine the route of LACV entry and early trafficking events leading to LACV membrane fusion. Here, each of the major endocytic pathways is examined by known chemical inhibitors of these pathways to determine their effect on the infection of LACV and other orthobunyaviruses. Our data indicated that LACV enters and infects HeLa cells, BHK-21 cells, and, notably, primary neuronal cultures through a dynamin- and clathrin-dependent process. Furthermore, LACV enters and infects cells independently of macropinocytosis or caveolar endocytosis. Subsequent to endocytosis, LACV requires trafficking to early endosomes for infection, as indicated by its dependence on Rab5. Because the orthobunyaviruses share a high degree of sequence similarity in the attachment protein (Gc) and have similar requirements for fusion and entry in mammalian cells, these results can be extended to other members of this genus (15, 45). Importantly, this study provides the first specific insight into the neuronal entry of bunyaviruses and lays the foundation for future studies focused on inhibiting neuronal entry during CNS infection, potentially limiting the severity of disease and attenuating or preventing neurological sequelae by inhibiting the spread of virus to uninfected neuronal cells.
MATERIALS AND METHODS
Cells and viruses.
BHK-21 (baby hamster kidney), HeLa, CHME-5 (human fetal microglia), and Vero (African green monkey kidney) cells were grown at 37°C and 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals, GA), 1% (vol/vol) penicillin-streptomycin (Invitrogen), 1% (vol/vol) l-glutamine (Invitrogen), and 1% sodium pyruvate (Vero cells only). The origin and preparation of LACV have been described already (26).
Preparation of primary rat neuronal cultures.
As previously described (8, 55), primary rat neuronal cultures were prepared from embryonic day 17 Sprague Dawley rat pups. Cells were plated on 24-well plates precoated with poly-l-lysine (Sigma), with or without coverslips, at a density of 2 × 105 cells per well in neurobasal medium supplemented with B27 (Invitrogen), and they were maintained at 37°C and 5% CO2. These cultures were infected at 7 days in vitro.
Quantitative PCR (qPCR).
Total RNA was isolated from BHK-21 cells and primary rat neuronal cultures with the RNeasy extraction kit (Qiagen). The quantification of RNA levels for the LACV M segment was performed by reverse transcription-PCR (RT-PCR) on the 7900HT real-time PCR system using the TaqMan assay (Applied Biosystems, Carlsbad, CA). Levels of LACV M RNAs were normalized to the expression of the housekeeping 18S RNA. The primers and probe used for LACV (32) were the following: LACV 935, 5′-6-carboxyfluorescein (FAM)-TATAAAAGCCTAAGAGCTGCCAGAGT-black hole quencher (BHQ)-3′; LACV 1018c, 5′-FAM-GACCAGTACTGCAGTAATTATAGACAAT-BHQ-3′; and LACV 963 probe, 5′-FAM-TGTGCAAGTCGAAAGGGCCTGCA-BHQ-3′.
Reagents.
Stock solutions were prepared as follows: 20 mM dynasore (DYN; Tocris Bioscience, MO), 5 mg/ml nystatin (EMD Chemicals, NJ), 50 mM 5-ethylisopropyl amiloride (EIPA; Sigma), 1 mM latrunculin A (EMD Millipore), 1 mM jasplakinolide (EMD Millipore), and 1 mg/ml cytochalasin D (EMD Millipore) were prepared in dimethyl sulfoxide (DMSO), while 0.01 g/ml chlorpromazine (CPZ; Sigma) was prepared in water. Cell death did not exceed 5% after incubation of cell cultures with different inhibitors at the indicated working concentrations, as determined by the trypan blue exclusion method. Short interfering RNA (siRNA) were from Ambion (Austin, TX), and transfections were performed using Lipofectamine 2000 reagent (Invitrogen, CA) according to the manufacturer's instructions. siRNA samples were used at 33 nM in each transfection (HeLa cells were transfected twice; the second transfection was performed 24 h after the first). Assays were performed 24 h after the second transfection.
Western blot analysis.
Cells were washed with cold phosphate-buffered saline (PBS) before and after centrifugation and then lysed with radio immunoprecipitation assay (RIPA) buffer containing miniprotease inhibitor cocktail (Roche Applied Science), and supernatants were clarified by centrifugation. For Western blot analysis, 15 to 20 μg of protein was separated by 4 to 15% Tris-HCl Ready Gel or Mini-Protean TGX precast gel (Bio-Rad) and transferred onto nitrocellulose membranes. These membranes were incubated with mouse-anti-clathrin heavy chain (1:600; BD Biosciences), mouse-anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:7,000; Advanced ImmunoChemical Inc.), rabbit-anti-caveolin-1 (1:1,000; Abcam), or rabbit-anti-ß-tubulin (1:1,000; Cell Signaling) followed by goat-anti-mouse IgG fluorescein isothiocyanate (FITC) (1:1,000; Sigma) and Alexa Fluor 633 goat anti-rabbit IgG (1:2,000; Invitrogen). Proteins were visualized using a Typhoon 9200 variable-mode imager with ImageQuant 5.2 and FluorSep 2.2 software (GE Healthcare).
Plasmid constructs.
Plasmids expressing green fluorescent protein (GFP)-tagged dominant-negative Eps15 (Eps15Δ95/295-GFP) and control Eps15 DIIIΔ2 were generous gifts from Alexandre Benmerah (Inserm, Paris, France). The Eps15Δ95/295 mutant disrupts clathrin-mediated endocytosis, while the control construct does not affect endocytosis (3, 4). The pcDNA3-EGFP plasmid was a gift of Doug Golenbock (plasmid 13031; Addgene), and the Rab5A-S34N-EGFP plasmid was a gift of Qing Zhong (plasmid 28045; Addgene). The wild-type DsRed-Rab5 (plasmid 13050; Addgene), dominant-negative DsRed-Rab5 (plasmid 13051; Addgene), wild-type Rab7-GFP (plasmid 12605; Addgene), and dominant-negative Rab7-GFP (plasmid 12660; Addgene) plasmids were gifts of Richard Pagano. Transient expression was performed using Lipofectamine 2000 reagent (Invitrogen, CA) according to the manufacturer's instructions. Briefly, HeLa cells were plated at 4 × 104 cells per well, incubated overnight, and transfected with 600 to 800 ng of plasmid with 1.2 to 2 μl of transfection reagent. Assays were performed 24 h after transfection.
Immunofluorescence and microscopy.
Cells were grown on poly-l-lysine (Sigma)-precoated glass coverslips in 24-well plates and then fixed for 20 min with PBS-4% paraformaldehyde and 4% sucrose, followed by permeabilization in 100% methanol (10 min) and PBS-0.2% Triton X-100 (10 min). Nonspecific binding was then blocked with PBS-10% goat serum for 1 h at room temperature. Working dilutions of the primary antibodies used were 1:200 rabbit-anti-clathrin heavy chain (Abcam, MA), 1:1,000 rabbit-anti-caveolin-1 (Abcam, MA), 1:20,000 chicken-anti-MAP2 (Abcam, MA), and 1:400 to 1:500 mouse-anti-LACV Gc (807.31 and 807.27) in PBS-10% goat serum. Secondary antibodies were goat-anti-mouse-IgG FITC (1:100; Sigma), goat-anti-chicken DyLight-549 (1:100; Jackson ImmunoResearch Laboratories), sheep-anti-mouse IgG R-phycoerythrin (1:100; Sigma), goat-anti-rabbit-tetramethyl rhodamine isocyanate (TRITC; 1:100; Sigma), or goat-anti-rabbit IgG R-phycoerythrin (1:100; Sigma). Hoecht's stain was used at 1:100. Cells were imaged using a Leica DMI6000 B inverted microscope and Leica Application Suite Advanced Fluorescence Software (Leica Microsystems).
Image processing.
The images were processed and merged with Adobe Photoshop CS4 on an iMac (Apple, Inc.). Image processing was kept minimal (only adjustments in contrast and brightness) and was applied equally to all parts of the image. Additionally, the processing was applied to each set of experiments, including controls. For the colocalization studies, four z sections were taken of the same field, and then each z section's brightness was equally dimmed and subsequently overlaid with the other z sections.
Transferrin, high-molecular-weight dextran, and cholera toxin uptake assays.
BHK-21 cells were grown on poly-l-lysine-precoated glass coverslips (Sigma) in 24-well plates at a density of 8 × 104 cells per well. Cells were pretreated with the appropriate inhibitor for 30 min at 37°C in infection medium prior to incubation with 10 μg/ml Alexa Fluor-labeled human transferrin (TF-594; Molecular Probes) in infection medium for 45 min at 37°C and 5% CO2. Noninternalized TF-594 was removed by acid washing at 4°C with 0.1 M glycine, 0.1 M NaCl, pH 3.0.
Similarly, high-molecular-weight dextran uptake (10,000-MW dextran conjugated to Alexa Fluor 594; DX-594; Molecular Probes) was assayed by treating BHK-21 cells with DMSO or EIPA for 30 min at 37°C and 5% CO2, followed by the addition of 0.5 mg of DX-594 for an additional 30 min. Cells were then washed with ice-cold PBS and acid washed with ice-cold 0.1 M sodium acetate, 0.05 NaCl, pH 5.5, solution for 10 min to remove noninternalized ligand.
For the Alexa Fluor 594-conjugated cholera toxin subunit B (CTX-594; Molecular Probes) assay, BHK-21 cells were pretreated with nystatin (NYS) for 30 min on ice, followed by the addition of CTX-594 (5 μg) for 30 min on ice. Subsequently, cells were moved to 37°C and 5% CO2 for 30 min and then fixed and stained with Hoecht's stain at 1:100.
Drug treatments and fusion from within (FFWI).
BHK-21 cells were plated at a concentration of 2 × 104 cells per well in poly-l-lysine-precoated 96-well plates (Sigma). Subconfluent monolayers of cells were pretreated with DMSO, DYN, CPZ, sucrose, NYS, or EIPA in infection medium (2% FBS) at 37°C, followed by virus infection at an multiplicity of infection (MOI) of 0.5 for 1 h in the presence of the inhibitors. The monolayers then were washed with PBS and incubated in infection medium at 37°C. For virus infection rescue experiments, virus was adsorbed to cells in the presence of inhibitors at 4°C for 1 h, washed with PBS at 4°C, exposed to pH-adjusted (pH 5.5 or 7.0) medium at 37°C, and incubated for an hour in the presence of inhibitors at 37°C. All inhibitors were removed after incubation with the virus unless otherwise noted. Eighteen h postinfection, cells were washed with PBS, exposed to pH-adjusted (pH 5.5 or 7.0) medium for 30 to 60 s at 37 or 4°C, and subsequently incubated at 37 or 4°C for 30 min in infection medium with 10 mM 2-(N-morpholino)ethanesulfonic acid (MES). Cells were then fixed, stained with Giemsa (Difco Laboratories), and counted. The fusion index (FI) was determined as FI = 1 − (C/N), where C and N are the number of cells and nuclei, respectively.
RESULTS
Entry and early infection events of LACV are dynamin and clathrin dependent in HeLa cells and primary rat neurons.
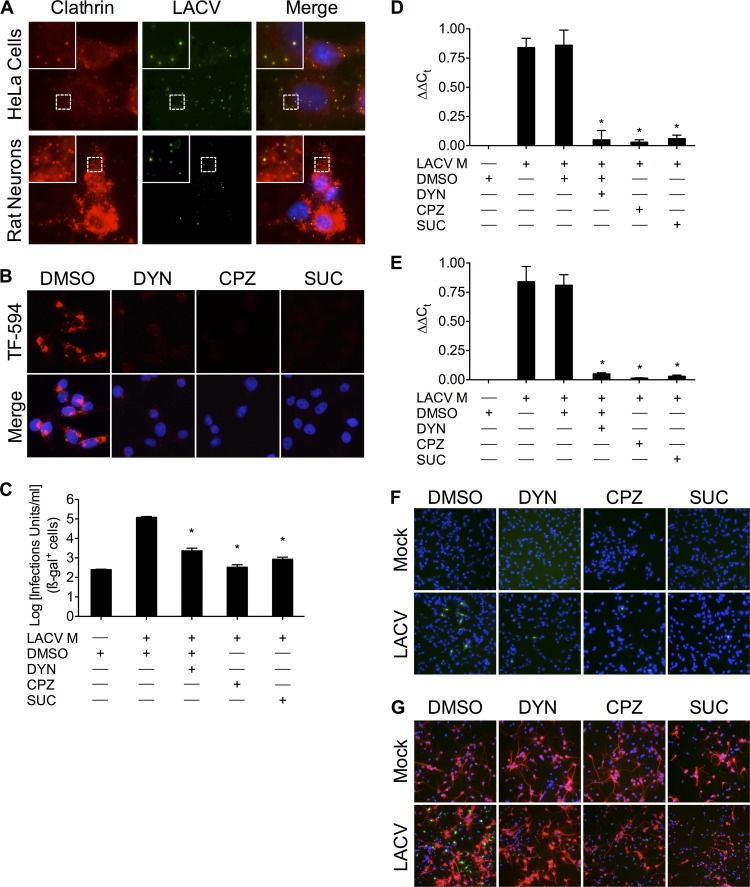
Previous experiments demonstrating the sensitivity of LACV to lysosomotropic agents (46) suggested that LACV enters host cells by receptor-mediated endocytosis (5, 13, 17, 53). However, the precise mechanism, and particularly any relevance to neuronal cells, has not been examined. To determine if LACV enters cells in CCVs, HeLa cells and primary rat neuronal cultures were incubated with LACV (MOI, >10) on ice for 90 min and subsequently moved to a 37°C incubator, allowing the medium to slowly warm. Following virus adsorption on ice, cells were fixed at regular intervals (up to 20 min) as the medium warmed and were stained with specific antibodies against LACV Gc and clathrin heavy chain. Primary rat neuronal cultures were also stained with anti-MAP2 antibody, followed by anti-chicken Cy5 antibody (not shown). In both HeLa cells and primary rat neuronal cultures, LACV colocalized with clathrin shortly after the onset of infection (Fig. 1A; 20-min time point shown), suggesting LACV enters cells in CCVs.
Fig 1.
LACV requires dynamin- and clathrin-mediated endocytosis during early infection in HeLa cells and primary neurons. (A) Colocalization of LACV with clathrin shortly after LACV internalization. LACV (MOI, >10) was incubated with either HeLa cells or primary rat neuronal cultures on ice for 90 min. Cells were then slowly shifted to 37°C to allow LACV uptake and fixed at 0 to 20 min after adsorption/during warming (images shown were taken at 20 min after the onset of warming). Cells were then permeabilized and stained with anti-LACV Gc and anti-clathrin antibodies, followed by secondary antibodies conjugated to FITC and R-phycoerythrin, respectively. Images were taken of four z-sections of the same field and then overlaid. Nuclei (blue) were stained with Hoecht's stain. Green, LACV Gc; red, endogenous clathrin. (B) Dynasore (DYN), chlorpromazine (CPZ), and hypertonic medium blocks transferrin uptake. BHK-21 cells (untreated or pretreated with DMSO [0.4%, vol/vol], DYN [40 or 80 μM], CPZ [5 or 10 μg/ml], or sucrose [SUC; 0.3 or 0.45 M]) were incubated with Alexa Fluor-594-conjugated transferrin (TF-594; red). After 45 min, cells were fixed and analyzed by fluorescence microscopy for the uptake of transferrin. (C) MLV pseudotypes of LACV transduce CHME-5 cells less efficiently in the presence of DYN, CPZ, or hypertonic medium treatment (*, P < 0.01 by Tukey test). (D to G) DYN, CPZ, and hypertonic medium inhibit LACV entry in BHK-21 cells and primary rat neurons. Following 30 min of pretreatment with DYN, CPZ, or hypertonic medium, BHK-21 cells (D and F) or primary rat neuronal cultures (E and G) were mock infected or infected with LACV (MOI, 0.5 [C to E] or 0.2 [F and G]) in the presence of inhibitors of clathrin-mediated endocytosis for 1 h. RNA from cell lysates 6 h postinfection was isolated and subjected to qPCR using LACV M segment-specific primers. DYN, CPZ, and hypertonic medium decreased LACV M segment RNA (relative to 18S RNA) compared to LACV-infected, control, or DMSO-treated cells (*, P < 0.001 by Student Newman-Keuls test). Ct, threshold cycle. The results are means ± standard deviations (SD) from 3 independent experiments. BHK-21 cells (F) and primary rat neuronal cultures (G) were fixed 8 h after infection, permeabilized, and stained with anti-LACV Gc (green) and anti-MAP2 (G; red) antibodies. Only higher concentrations of each treatment are shown in panels B to G.
Nevertheless, colocalization does not automatically indicate a functional role for CME in LACV entry. Therefore, the effects of well-described inhibitors of dynamin and CME, dynasore (DYN), chlorpromazine (CPZ), and hypertonicity, were tested in this system. DYN is a specific inhibitor of the GTPase activity of dynamins (34, 41), thus inhibiting CME. CPZ inhibits CME by interfering with clathrin disassembly and receptor recycling to the plasma membrane (22, 59), while hypertonic medium blocks and removes membrane-associated clathrin lattices (19, 20). The internalization of Alexa Fluor 594-conjugated transferrin (TF-594), which is mediated by CME, was used as a positive control. DYN, CPZ, and hypertonicity indeed reduced the uptake of TF-594 (Fig. 1B). Murine leukemia virus (MLV) pseudotype particles incorporating LACV M segment constructs [MLV(LACV)], prepared using a previously described three-plasmid system (44, 45, 56), were then used in a previously described entry assay (44, 45). CHME-5 cells were transduced with MLV(LACV) pseudotypes in the presence of DYN, CPZ, or hypertonic medium and then stained for ß-galactosidase to quantify the expression of the indicator enzyme (44, 45). MLV(LACV) pseudotypes transduced CHME-5 cells significantly less well (Fig. 1C) in the presence of DYN, CPZ, or hypertonicity than the control DMSO treatment. These experiments showed that inhibitors of CME have a marked effect on entry mediated by the LACV glycoproteins.
To further demonstrate the dependence of LACV entry on dynamin- and clathrin-mediated endocytosis and extend our findings to neuronal cells, we assessed the presence of LACV RNA following infection with LACV. BHK-21 cells or primary rat neuronal cultures were infected with LACV in the presence of DYN, CPZ, or hypertonic medium for 1 h. RNA from cell lysates obtained 6 h postinfection was purified and quantified by qPCR using LACV M segment-specific primers (modified from reference 54) for CCHFV. Relative LACV RNA levels decreased significantly (Fig. 1D; *, P < 0.001 by Student Newman-Keuls) after any of these treatments in BHK-21 cells and, importantly, also in primary rat neuronal cultures (Fig. 1E; *, P < 0.001 by Student Newman-Keuls). Additionally, 8 h after LACV infection, BHK-21 and primary rat neuronal cultures showed fewer LACV-infected cells by immunofluorescence of viral antigen (Fig. 1F and G). Taken together, these data demonstrate that entry and early infection events of LACV are dependent on dynamin- and clathrin-mediated endocytosis in mammalian cells and neuronal cells.
Productive infection of LACV and orthobunyaviruses is dependent on dynamin- and clathrin-mediated endocytosis.
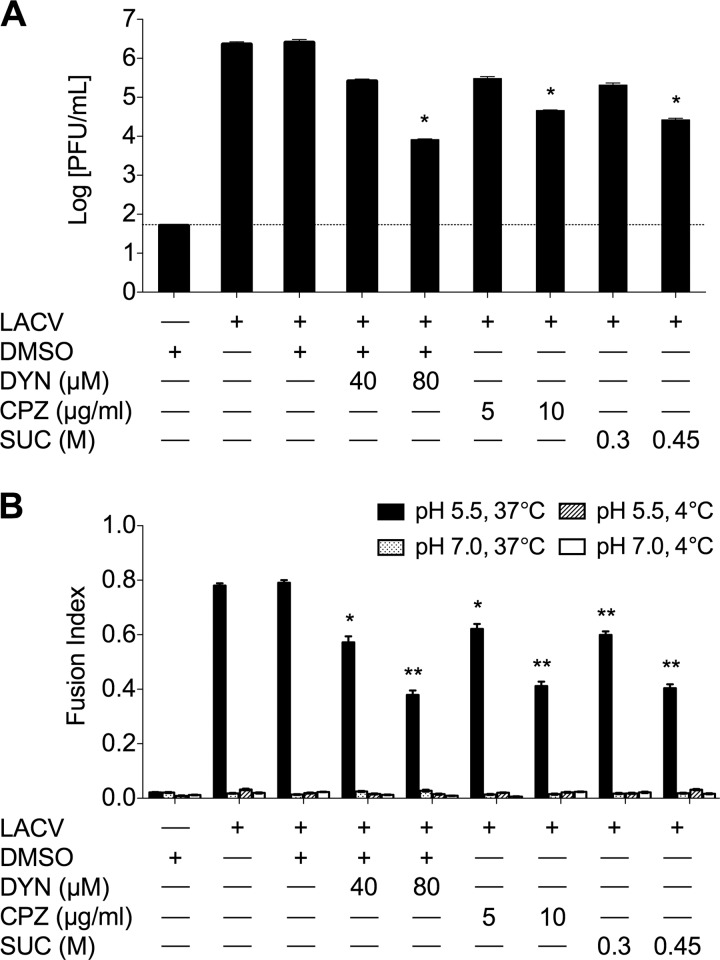
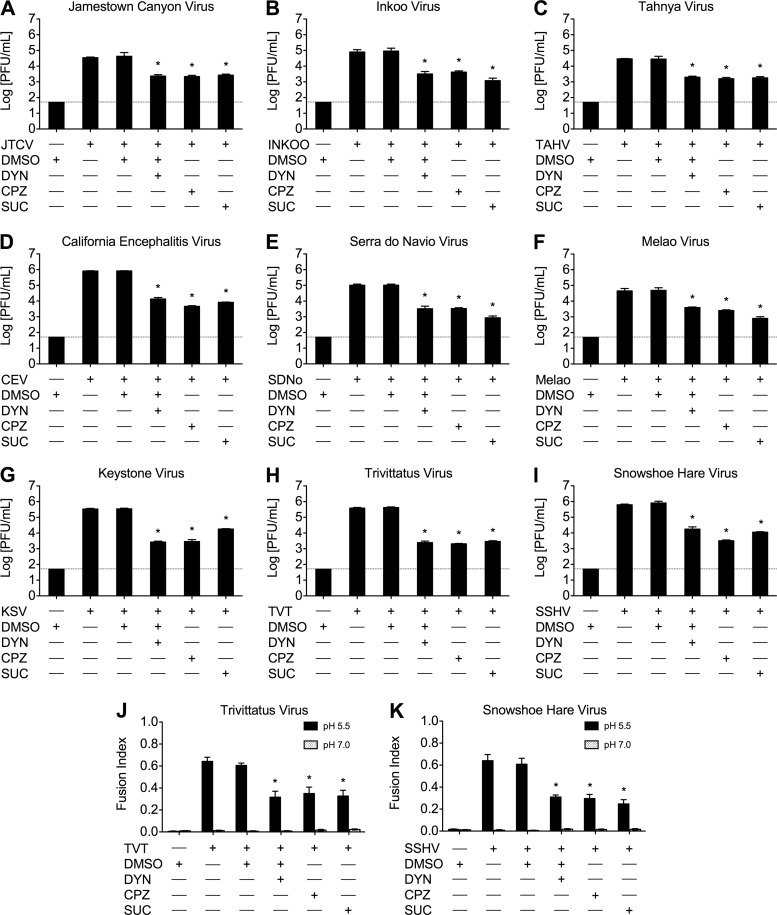
FFWI allows for the study of more orthobunyaviruses without large-scale preparation and purification of viruses and their proteins, and it is a result of productive orthobunyavirus infection. We used FFWI assay and standard plaque assays to determine the effects of the same interventions on the productive infection of LACV and other orthobunyaviruses. BHK-21 cells pretreated with DYN, CPZ, or hypertonic medium were infected with LACV at a low MOI in the presence of the treatments for 1 h, and supernatants were harvested 24 h after infection. Viral titers were reduced at both concentrations tried (Fig. 2A), with each of the treatments having clear dose dependence and up to 100-fold reduction at the higher concentrations (P < 0.01 by Tukey test). Additionally, the extent of virus-mediated cell-to-cell fusion was determined by FFWI assay as a surrogate for productive infection to assess the effects on later events in LACV infection due to the presence of these inhibitors. Briefly, BHK-21 cells were pretreated with DYN, CPZ, or hypertonic medium and infected with LACV in the presence of the treatments for 1 h. Cells were exposed to low-pH (5.5) medium, which induces virus-mediated cell-to-cell fusion, or neutral (pH 7) medium for 30 to 60 s. Fusion indices (calculated as 1 − C/N, where C is the number of cells and N is the number of nuclei) were determined where a higher fusion index represents more cell-to-cell fusion and thus a more robust LACV infection. As controls, pH-adjusted media (4°C) were also used, since fusion does not occur at 4°C. The inhibition of CME by DYN, CPZ, and hypertonic medium resulted in significant decreases in fusion indices of LACV-infected cultures in a dose-dependent manner (Fig. 2B) compared to control or DMSO treatments (P < 0.01 and P < 0.001 by Student's t test). These results, taken together with the inhibition of LACV replication in the first 6 h of infection, indicate that LACV internalization into host cells is dependent on dynamin- and clathrin-dependent endocytosis in both nonneuronal and neuronal cells. Furthermore, the inhibitory effects of DYN, CPZ, and hypertonicity on other orthobunyaviruses were also assessed by viral titers and FFWI assays to determine if CME is a common route of entry for the orthobunyavirus genus. As with LACV, viral titers at 24 h (Fig. 3A to I) and FFWI (Fig. 3J and K) were significantly reduced by DYN, CPZ, and hypertonic medium (P < 0.001 by Tukey test) in the other orthobunyaviruses tested, including members of the California, Melao, and Trivitattus serogroups, suggesting that CME is common to this genus.
Fig 2.
Productive infection of LACV is dependent on dynamin- and clathrin-mediated endocytosis. BHK-21 cells (untreated or pretreated with DMSO, DYN, CPZ, or sucrose) were mock infected or infected with LACV in the presence of each treatment for 1 h. (A) To assess the effect of DYN, CPZ, and sucrose on LACV replication, cells were infected with LACV (MOI, 0.005) and supernatants were harvested 24 h postinfection. Viral titers were determined by standard plaque assays on VERO cells. The results are means ± SD of 3 independent experiments (*, P < 0.01 by Tukey test). (B) A fusion-from-within (FFWI) assay was used to measure the extent of LACV infection 18 h postinfection (MOI, 0.5). FFWI in LACV-infected cells was significantly decreased by inhibitors of dynamin- and clathrin-mediated endocytosis. As expected, cell-to-cell fusion was not observed in mock-infected control cells (data not shown) and LACV-infected cells fused at 4°C. Error bars represent standard errors of the means from three independent experiments performed in duplicate (*, P ≤ 0.01; **, P ≤ 0.001; both by Tukey test). The dashed line represents the limit of detection.
Fig 3.
Orthobunyavirus growth and virus-mediated cell-to-cell fusion is reduced by chemical inhibitors of dynamin- and clathrin-mediated endocytosis. BHK-21 cells (untreated or pretreated with DMSO [0.4%, vol/vol], DYN [80 μM], CPZ [10 μg/ml], or sucrose [0.45 M]) were mock infected or infected with an orthobunyavirus (MOI, 0.01) in the presence of each treatment for 1 h, and supernatants were harvested 24 h postinfection. Orthobunyaviruses examined were Jamestown Canyon virus (JTCV) (A), Inkoo virus (B), Tahnya virus (TAHV) (C), California encephalitis virus (CEV) (D), Serra do Navio virus (SDNo) (E), Melao virus (F), Keystone virus (KSV) (G), Trivittatus virus (TVT) (H), and snowshoe hare virus (SSHV) (I). Viral titers were determined by standard plaque assays on Vero cells. The results are means ± SD from 3 independent experiments (*, P < 0.001 by Tukey test). The dashed line represents the limit of detection. (J and K) An FFWI assay was used to measure the extent of TVT (MOI, 0.5) (J) and SSHV (MOI, 5) (K) infection 18 h postinfection. Inhibitors of dynamin- and clathrin-mediated endocytosis significantly decreased FFWI in TVT- and SSHV-infected cells. As expected, cell-to-cell fusion was not observed in mock-infected control cells (data not shown). Error bars represent standard errors of the means from three independent experiments performed in duplicate (*, P < 0.01 by Student's t test).
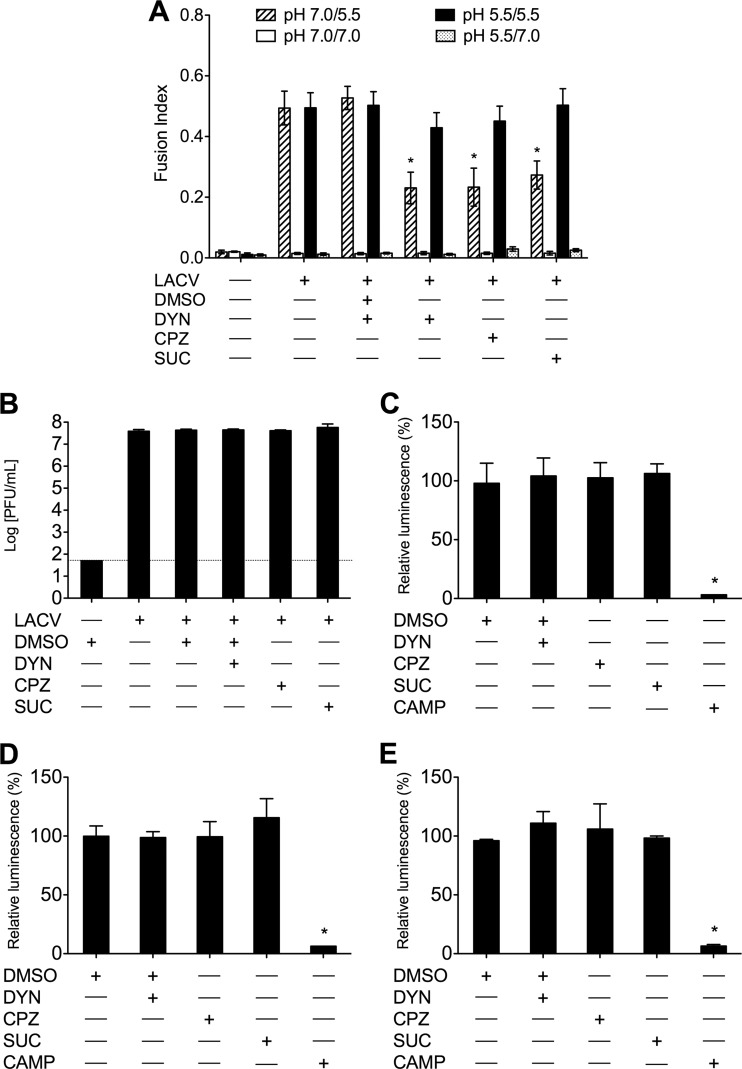
Importantly, LACV infection can be rescued in the presence of these inhibitors of CME. LACV adsorbed at 4°C in the presence of DYN, CPZ, or hypertonic medium followed by brief exposure to pH 5.5 medium at 37°C allows the virus to fuse with the plasma membrane and bypass the endocytic route in the presence of these inhibitors, which were present for 1 h following low-pH treatment. Following the initial exposure to pH-adjusted medium, FFWI assay 18 h later demonstrated an increase of fusion indices of LACV that fused at the plasma membrane (pH 5.5/5.5) in the presence of DYN, CPZ, or hypertonic medium compared to those of the same treatment exposed to neutral pH following adsorption (pH 7.0/5.5) (Fig. 4A). Furthermore, when cells were exposed to neutral pH medium following virus adsorption, there was still a significant decrease (P < 0.01) in fusion indices due to treatment with DYN, CPZ, or hypertonic medium (Fig. 4A). Thus, the inhibitory effects of DYN, CPZ, and hypertonicity were specific for the inhibition of infection during the entry step/endocytic uptake of LACV, and bypassing this route of entry allows for a productive infection to occur. To demonstrate that the antiviral effects of these inhibitors were not the result of direct inactivation of the virus or nonspecific effects on viral replication, DYN, CPZ, or hypertonic medium was added for 1 h, after which cultures were incubated with LACV for 1 h. LACV growth was not significantly affected by the addition of DYN, CPZ, or hypertonic medium following infection (Fig. 4B). Furthermore, to ensure that these inhibitors were not significantly affecting cell viability, BHK-21 cells, HeLa cells, and primary rat neuronal cultures were treated with these inhibitors, and cell viability was determined by the CellTiter-Glo luminescent cell viability assay. Figure 4C to E demonstrates that the viability of the cells was not significantly affected by treatment with DYN, CPZ, or hypertonic medium. Taken together, these data confirm that the antiviral effects of these inhibitors are due to inhibitions on LACV entry.
Fig 4.
Inhibition of LACV entry observed in cells treated with inhibitors of dynamin- and clathrin-mediated endocytosis is not the result of drug-mediated toxicity or nonspecific effects. (A) BHK-21 cells were untreated or were treated with DMSO (0.4%, vol/vol), DYN (80 μM), CPZ (10 μg/ml), or sucrose (0.45 M) and incubated with LACV (MOI, 0.5) or mock infected at 4°C for 1 h to adsorb virus. Cells were briefly exposed (30 to 60 s) to prewarmed, 37°C medium of pH 7.0 or 5.5 (mediates membrane fusion). Fresh medium with inhibitors was added, and cells were incubated for an additional 1 h at 37°C. FFWI assays were performed 18 h postinfection. FFWI in LACV-infected cells exposed to pH 5.5 medium following virus adsorption was rescued (pH 5.5/5.5), while infected cells exposed to medium at pH 7.0 following adsorption demonstrated decreases in fusion indices (pH 7.0/5.5). As expected, cell-to-cell fusion was not observed in mock-infected cells or cells exposed to pH 7.0 during the FFWI assay. Error bars represent standard errors of the means from three independent experiments performed in duplicate (*, P < 0.01 by Student's t test). (B) BHK-21 cells were treated with DMSO (0.4%, vol/vol), DYN (80 μM), CPZ (10 μg/ml), or sucrose (0.45 M) for 1 h following 1 h of incubation with LACV (MOI, 0.005). Supernatants were harvested 24 h postinfection, and viral titers were determined by standard plaque assays. HeLa cells (C), BHK-21 cells (D), and primary rat neuronal cultures (E) were treated with DMSO (0.4%, vol/vol), DYN (80 μM), CPZ (10 μg/ml), or sucrose (0.45 M), or they were treated with camptothecin (CAMP; 20 mM) as a positive control for cell death and damage, for 1 h. Cell viability was determined 18 h following treatment with the CellTiter-Glo luminescent cell viability assay. Cell viability was not altered by the addition of DYN, CPZ, or sucrose for any of these cell lines. Cell viability was significantly decreased in the CAMP-treated cells (*, P ≤ 0.001 by Student's t test).
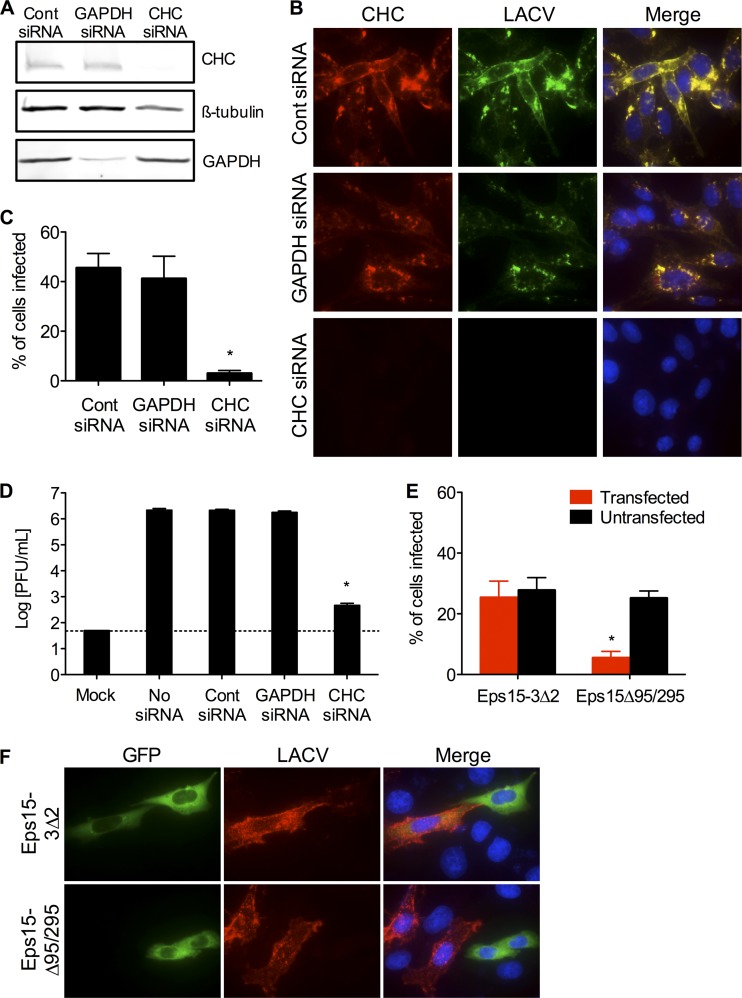
To more specifically and potently inhibit CME and confirm its role in LACV entry into mammalian cells, siRNA designed to target the clathrin heavy chain (CHC) was synthesized. Control siRNA and GAPDH-targeting siRNA were used as controls to ensure any effects of siRNA knockdown of CHC on LACV infection were specific. Knockdown of CHC by the specific siRNA was confirmed by Western blotting (Fig. 5A) and immunofluorescence (Fig. 5B). When CHC siRNA-transfected cells were infected with LACV and examined by immunofluorescence 24 h postinfection, a significant reduction (P < 0.01 by Student's t test) in the number of LACV-infected cells was observed compared to control siRNA or GAPDH siRNA (Fig. 5B and C). Under these experimental conditions, 45.5% ± 9.37% of control siRNA-transfected cells and 41.2% ± 9.39% of GAPDH siRNA-transfected cells were infected by LACV, whereas transfection of siRNA specific for CHC reduced LACV infectivity to only 3% ± 2.75% of cells (Fig. 5C). Furthermore, most of the cells infected by LACV were those where CHC knockdown was least efficient by immunofluorescent observation (data not shown). Concomitantly, viral titers at 24 h were significantly lower (P < 0.001 by Tukey test) in cells transfected with CHC siRNA compared to control or GAPDH siRNA-transfected cells (Fig. 5D).
Fig 5.
Clathrin heavy chain and Eps15 are required for LACV infection and replication. (A) Knockdown of clathrin heavy chain by siRNA. HeLa cells were serially transfected with siRNA (control [cont], GAPDH, or clathrin heavy chain [CHC]). Cell lysates were subjected to Western blotting for CHC, GAPDH, and ß-tubulin to confirm knockdown. (B) Knockdown of clathrin heavy chain decreases LACV infection. Twenty-four h after LACV infection (MOI, 0.5), HeLa cells were fixed and stained with anti-LACV Gc (green) and anti-clathrin (red) antibodies, followed by anti-mouse IgG and anti-rabbit IgG conjugated to FITC and R-phycoerythrin, respectively. Nuclei (blue) were stained with Hoecht's stain. (C) The number of LACV-infected cells was counted, and the percentage of cells infected with LACV was determined for each condition. The percentage of CHC siRNA-transfected cells was significantly decreased compared to levels of control or GAPDH siRNA-transfected cells (*, P < 0.01 by Student's t test). (D) Knockdown of CHC decreases LACV titers. HeLa cells were infected with LACV (MOI, 0.01) 24 h following the second siRNA transfection. Supernatants were harvested 24 h postinfection. Viral titers were determined by standard plaque assays. LACV titers were significantly reduced in CHC siRNA-transfected cells compared to control or GAPDH siRNA-transfected cells (*, P < 0.001 by Tukey test). (E) Overexpression of dominant-negative Eps15 (Eps15Δ95/295) inhibits LACV infection. Twenty-four h after transfection with control Eps15 (Eps15-3Δ2-GFP) or dominant-negative Eps15 (Eps15Δ95/295-GFP), HeLa cells were infected with LACV. Twenty-four h later, cells were fixed and stained with anti-LACV Gc (red) followed by anti-mouse IgG conjugated to R-phycoerythrin. The numbers of LACV-positive transfected and untransfected cells were counted, and the percentage of cells (transfected [GFP+] or untransfected [GFP−]) infected with LACV was determined for each condition (*, P < 0.01 by Student's t test). (F) Representative images of the immunofluorescence assay. The results are means ± SD of 3 independent experiments. The dashed line represents the limit of detection.
As an alternative approach to siRNA, we used a previously described dominant-negative form of the cellular epidermal growth factor receptor substrate 15 (Eps15) protein which lacks its second and third EH domains (Eps15Δ95/295), resulting in potent inhibition of CME (3) without affecting clathrin-independent pathways (43). HeLa cells overexpressing a green fluorescent protein (GFP)-tagged Eps15Δ95/295 (Eps15Δ95/295-GFP) or control Eps15 lacking all interacting domains (Eps15-3Δ2-GFP) were infected with LACV. Expression of dominant-negative Eps15Δ95/295-GFP significantly decreased (P < 0.01 by Student's t test) the percentage of GFP-expressing cells that were infected with LACV compared to untransfected HeLa cells of the same culture, as well as compared to control Eps15-3Δ2-GFP-expressing cells (Fig. 5E and F). These data clearly show an effect of Eps15Δ95/295-GFP on LACV infection, further demonstrating the functional requirement of CME for LACV infection.
Macropinocytosis and caveolar endocytosis are not involved in LACV entry.
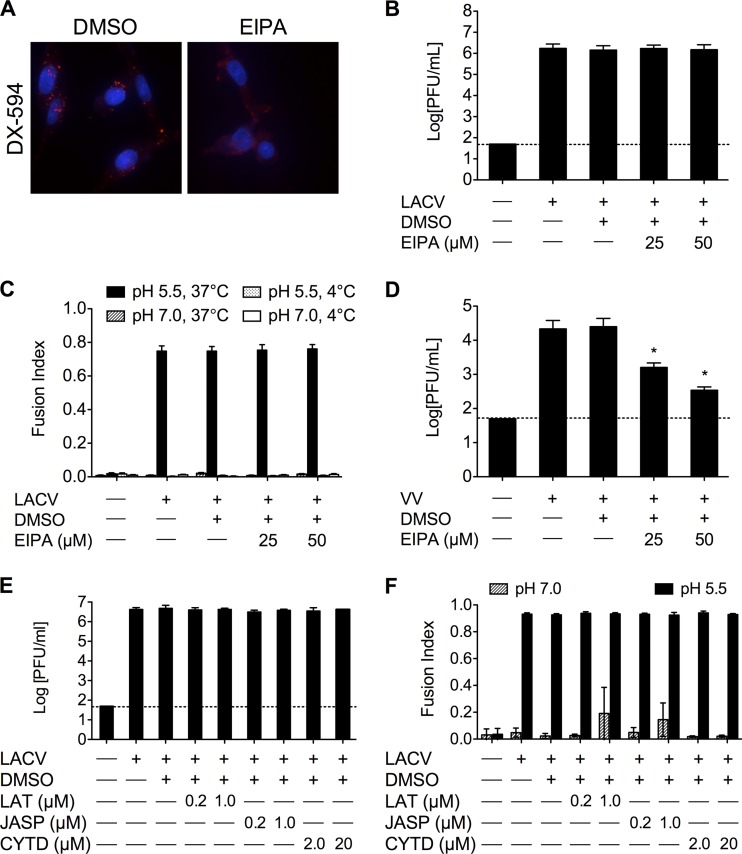
Even though LACV enters cells via CME, other viruses (e.g., influenza virus and Ebola virus [2, 47]) have been shown to use multiple routes of entry. Additionally, recent studies have demonstrated that some viruses can enter cells via macropinocytosis and be trafficked through the classical endosomal pathway for a productive infection. The requirement for LACV infection on macropinocytosis was determined using 5-ethylisopropyl amiloride (EIPA) as an inhibitor of macropinocytosis. EIPA is a potent and specific inhibitor of the Na+/H+ exchanger activity that is critical for macropinosome formation. To test the efficacy of EIPA, the uptake of Alexa Fluor 594-labeled high-molecular-weight dextran (DX-594) was examined. Consistent with its inhibitory activity, EIPA decreased the amount of DX-594 taken up in BHK-21 cells (Fig. 6A). When BHK-21 cells were pretreated with 25 or 50 μM EIPA and infected with LACV for 1 h in the presence of EIPA, no significant differences were seen in the LACV titer at 24 h (Fig. 6B) or in FFWI (Fig. 6C). As a positive control, the effect of EIPA treatment on viral replication was determined in BHK-21 cells using vaccinia virus, which is known to enter cells by macropinocytosis. As expected, EIPA significantly decreased vaccinia virus titers at 24 h (Fig. 6D; *, P < 0.001 by Tukey test). Additionally, other inhibitors of macropinocytosis were tested on LACV replication and FFWI. LACV replication (Fig. 6E) and FFWI (Fig. 6F) were not significantly affected by treatment with latrunculin A, jasplakinolide, or cytochalasin D. Taken together, these results demonstrated that LACV entry into mammalian cells is not dependent on macropinocytosis.
Fig 6.
LACV infection is not dependent on macropinocytosis. (A) EIPA blocks high-molecular-weight dextran uptake. To confirm the activity of EIPA, BHK-21 cells (untreated or pretreated with DMSO [0.1%] or EIPA [25 or 50 μM]) were incubated with Alexa-Fluor594-labeled high-molecular-weight dextran (DX-594). After 45 min, cells were fixed and analyzed by fluorescence microscopy for the uptake of DX-594. Nuclei (blue) were stained with Hoecht's stain. Only the higher concentrations of EIPA are shown. (B) LACV replication is not affected by EIPA treatment. BHK-21 cells were infected with LACV (MOI, 0.005) in the presence of DMSO or EIPA (25 or 50 μM) for 1 h. Supernatants were harvested 24 h postinfection. (C) LACV FFWI is not affected by inhibiting macropinocytosis. BHK-21 cells were infected with LACV (MOI, 0.5) in the presence of each treatment for 1 h. (D) EIPA decreases vaccinia virus (VV) replication. BHK-21 cells were infected with VV (MOI, 0.01) in the presence of DMSO or EIPA for 1 h. Supernatants were harvested 24 h postinfection (*, P < 0.001 by Tukey test). (E) To assess the effect of latrunculin A (LAT), jasplakinolide (JASP), and cytochalasin D (CYTD) on LACV replication, cells were infected with LACV (MOI, 0.005) and supernatants were harvested 24 h postinfection. Viral titers were determined by standard plaque assays on Vero cells. The results are means ± SD from 3 independent experiments. (F) An FFWI assay was used to measure the extent of LACV infection 18 h postinfection (MOI, 0.5). The dashed line represents the limit of detection.
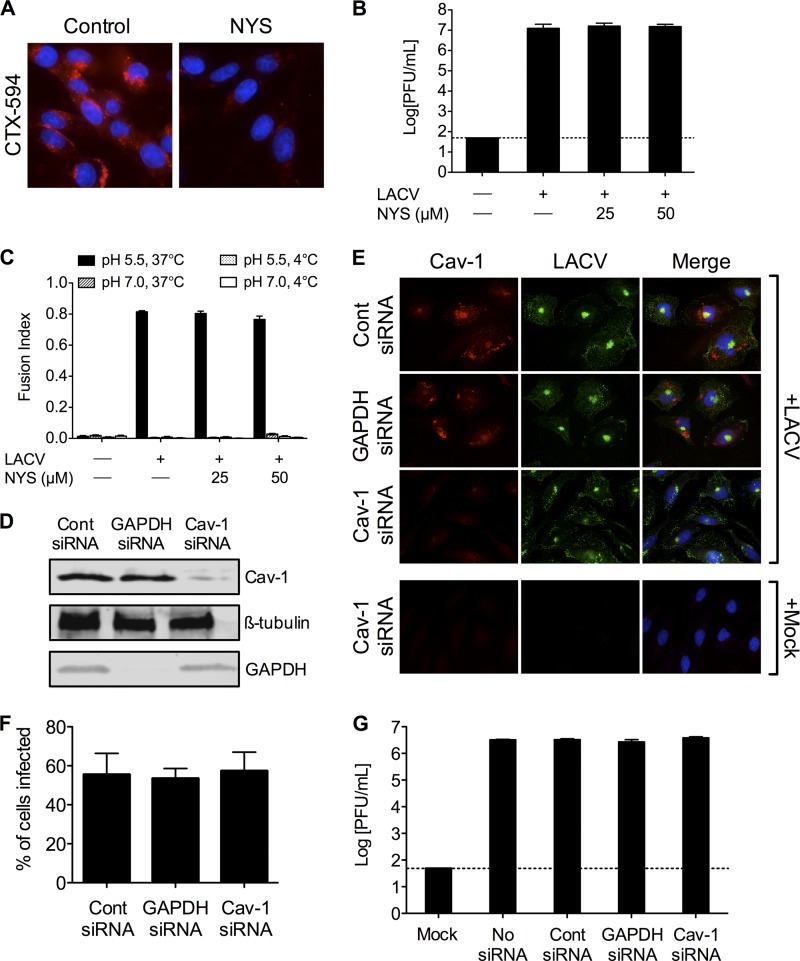
As with CME, caveolar endocytosis requires dynamin for scission of the vesicle from the plasma membrane, thus entry through caveolar endocytosis was determined as well. Nystatin, a cholesterol-sequestering drug that disrupts membrane lipid rafts and consequently prevents caveola formation, was used to inhibit this endocytic pathway. Cholera toxin is generally used as a marker for caveolar endocytosis, so the cellular uptake of Alexa Fluor 594-conjugated cholera toxin subunit B (CTX-594) was used to confirm nystatin's inhibition of caveolar endocytosis. Treatment with nystatin in BHK-21 cells decreased the internalization of CTX-594 (Fig. 7A), demonstrating the effectiveness of the inhibitor.
Fig 7.
LACV infection is not dependent on caveolar endocytosis. (A) Nystatin blocks uptake of cholera toxin subunit B. To confirm the activity of nystatin (NYS), BHK-21 cells (untreated or pretreated with NYS [25 or 50 μM]) were incubated with Alexa Fluor 594-conjugated cholera toxin subunit B (CTX-594). After 45 min, cells were fixed and analyzed by fluorescence microscopy for the uptake of CTX-594. Nuclei (blue) were stained with Hoecht's stain. Only the higher concentration of NYS is shown. (B) LACV replication is not affected by NYS treatment. BHK-21 cells were infected with LACV (MOI, 0.005) in the presence of NYS for 1 h. Supernatants were harvested 24 h postinfection. (C) LACV FFWI is unaffected by inhibiting caveolar endocytosis. BHK-21 cells were mock infected or infected with LACV (MOI, 0.5) in the presence of NYS for 1 h. The dashed line represents the limit of detection. (D) HeLa cells were serially transfected with siRNA (control [cont], GAPDH, or caveolin-1 [Cav-1]). Cell lysates were subjected to Western blotting for Cav-1, GAPDH, and ß-tubulin to confirm knockdown. (E) Knockdown of Cav-1 does not decrease LACV infection. Twenty-four h after LACV infection (MOI, 0.5), HeLa cells were fixed and stained with anti-LACV Gc (green) and anti-Cav-1 (red) antibodies, followed by anti-mouse IgG and anti-rabbit IgG conjugated to FITC and TRITC, respectively. (F) The number of LACV-infected cells was counted, and the percentage of cells infected with LACV was determined for each condition. (G) Knockdown of Cav-1 does not decrease LACV titers. HeLa cells were infected with LACV (MOI, 0.01) 24 h following the second siRNA transfection. Supernatants were harvested 24 h postinfection. Viral titers were determined by standard plaque assays.
BHK-21 cells pretreated with 25 or 50 μM nystatin for 30 min and infected with LACV for 1 h in the presence of nystatin showed no significant differences in the LACV titer at 24 h (Fig. 7B) or FFWI (Fig. 7C). To more specifically demonstrate that LACV entry is not dependent on caveolar endocytosis, siRNA knockdown of caveolin-1 (confirmed by Western blotting; Fig. 7D) did not significantly affect LACV infection (Fig. 7E and F) or replication (Fig. 7G). These results demonstrate that caveolar endocytosis is not a major entry route for LACV, and that membrane lipid rafts are not a critical component in LACV's ability to enter cells.
Trafficking to early endosomes is required for productive LACV infection.
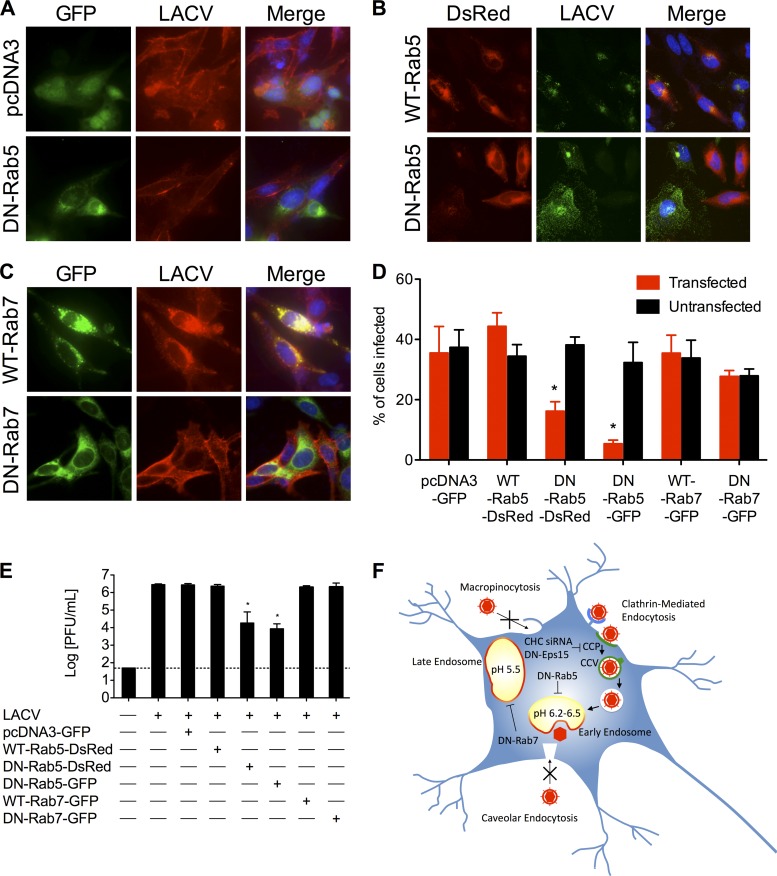
The requirement of low endosomal pH for the entry of LACV has previously been shown using lysosomotropic agents, which raise the pH of cellular vacuolar compartments (46). Following uptake of virus, endocytic vesicles are trafficked through various pathways to a site where virus-cell membrane fusion can occur. The GTPases Rab5 and Rab7 are the key regulators of transport of these vesicles to early and late endosomes, respectively (29, 52, 61). Many enveloped viruses have evolved to utilize a low-pH-dependent conformational change to mediate fusion, penetration, and/or uncoating, and concomitantly the sites of virus-cell membrane fusion are the early (pH ∼6.2 to 6.5) and/or late (pH ∼5.5) endosomes. To assess the requirement of transport to early and late endosomes in the entry of LACV, dominant-negative mutants of Rab5 and Rab7 were used. Dominant-negative Rab5-GFP/DsRed expression reduced LACV infection, as determined by immunofluorescence (Fig. 8A, B, and D; *, P < 0.01 by Student's t test compared to either untransfected cells in the same culture or GFP/wild-type Rab5-DsRed-transfected cells), and decreased viral titers at 24 h compared to GFP/wild-type Rab5-DsRed-transfected cultures (Fig. 8E; *, P < 0.0001 by Tukey test). In contrast, expression of dominant-negative Rab7-GFP did not reduce the percentage of cells infected with LACV significantly. Therefore, following CME, the LACV-containing vesicle is trafficked into early endosomes, where most virus-cell membrane fusion occurs due to the drop in pH, and then into late endosomes, where the remaining viruses mediate membrane fusion.
Fig 8.
Trafficking to early, but not late, endosomes is required for productive LACV infection. (A to E) Overexpression of dominant-negative Rab5, but not dominant-negative Rab7, decreases LACV infection. HeLa cells were infected with LACV (MOI, 0.5) 24 h after transfection with (A) pcDNA3-GFP or dominant-negative (DN)-Rab5-GFP; (B) wild-type (WT)-Rab5-DsRed or DN-Rab5-DsRed; and (C) WT-Rab7-GFP or DN-Rab7-GFP. After 24 h, cells were fixed and stained with anti-LACV Gc (A and C, red; B, green) followed by anti-mouse IgG conjugated to R-phycoerythrin (A and C) or FITC (B). Nuclei (blue) were stained with Hoecht's stain. (D) The numbers of LACV-positive transfected and untransfected cells were counted, and the percentage of cells (transfected [GFP/DsRed+] or untransfected [GFP/DsRed−]) infected with LACV was determined for each condition (*, P < 0.01 by Student's t test). (C) To assess the effect of overexpression of pcDNA3-GFP, WT-Rab5, DN-Rab5, WT-Rab7, or DN-Rab7 on LACV replication, HeLa cells were infected with LACV (0.005) 24 h following the second siRNA transfection. Supernatants were harvested 24 h postinfection. Viral titers were determined by standard plaque assays on Vero cells (*, P < 0.0001 by Tukey test). (D) Model of LACV entry via dynamin- and clathrin-mediated endocytosis with subsequent trafficking to early endosomes. LACV binds to its receptor (unidentified), inducing a clathrin-coated pit to form, which invaginates, and the scission of the clathrin-coated vesicle from the plasma membrane mediated by dynamin. The LACV-containing vesicle is trafficked in a Rab5-dependent process to early endosomes where LACV membrane fusion occurs. The results are means ± SD from 3 independent experiments. The dashed line represents the limit of detection.
DISCUSSION
Endocytic uptake of viruses provides efficient transport past the plasma membrane and into the cytoplasm while protecting the virions from immune surveillance. LACV is sensitive to lysosomotropic agents, indicating that it enters cells by receptor-mediated endocytosis and not by direct fusion at the plasma membrane (46), but its precise route has not been described. Bunyaviruses from the genera Nairovirus (CCHFV) and Hantavirus (Hantaan virus) are susceptible to CPZ and hypertonic treatment (27, 54), and the role of clathrin in CCHFV entry has been further supported by siRNA knockdown of CHC (54). In contrast to nairoviruses and hantaviruses, bunyaviruses of the genus Phlebovirus do not depend on CME; these viruses use noncoated vesicles to enter the classical endosomal system for infection (33).
Previous studies demonstrating the colocation of Oropouche virus (genus Orthobunyavirus) with clathrin during early events in infection and the inhibition of Oropouche virus replication by CPZ suggested that CME is required for orthobunyavirus entry (51). However, although there is ample evidence that CPZ disrupts CCPs, its specificity has been questioned, because CPZ can also interfere with the biogenesis of large intracellular vesicles, including phagosomes and macropinosomes (23). Accordingly, CPZ is a useful tool, but the requirement of CME for virus entry cannot be determined based solely on its use. In this study, we employed chemical and molecular inhibitors of CME as well as chemicals known to inhibit alternative pathways of virus entry to demonstrate that CME is indeed the principal route of entry for LACV and other orthobunyaviruses in both nonneuronal and neuronal cells.
Three separate chemical treatments, including CPZ, DYN (a potent inhibitor of dynamin), and hypertonicity, were used to inhibit CME. A previously described MLV(LACV) pseudotype entry assay demonstrated that the inhibitory effects of DYN, CPZ, or hypertonic medium on LACV result from the inhibition of entry rather than a later event in infection. In addition, we demonstrated that both early replication (6 h postinfection) as determined by qPCR (Fig. 1D and E) and later replication/infection (Fig. 2A and B) were all significantly decreased by these inhibitors of CME compared to control or DMSO treatments. Furthermore, we modified our FFWI assay for LACV (46, 55) to rescue LACV infection in the presence of these inhibitors of CME by inducing virus-to-cell membrane fusion at the plasma membrane (Fig. 4A). These data confirm that the inhibition of LACV infection observed during treatment with DYN, CPZ, and hypertonic medium are the result of inhibiting virus entry and not a later stage in replication or as a result of direct inactivation of the virus or nonspecific cell damage. CPZ, DYN, and hypertonicity also decreased replication of the other orthobunyaviruses examined (Fig. 3). Importantly, we extended these findings to primary rat neuronal cells, supporting the functional requirement of CME in LACV entry to cells of the CNS (Fig. 1E).
In addition to chemical inhibition of CME, two additional molecular techniques were used to more specifically and potently inhibit CME: siRNA against CHC (Fig. 5A and B) and overexpression of dominant-negative Eps15 (Eps15Δ95/295; Fig. 5F). Clathrin is recruited to the CCP by adaptor protein 2 (AP-2) (7), which has two large subunits involved in the recruitment of accessory proteins (57). One such protein is Eps15, which is required for CCP assembly and invagination during CME (10, 11). Therefore, we were able to inhibit CME by two independent techniques targeting different proteins. Both of these independent methods significantly decreased the infectivity of LACV as determined by reductions in the percentage of cells infected with LACV due to siRNA knockdown of CHC (Fig. 5B and C) or overexpression of Eps15Δ95/295 (Fig. 5E and F) during immunofluorescence examination. These studies were critical in determining the functional requirement of CME for LACV infection, because pharmacological inhibitors often affect multiple cellular functions.
Some viruses, including influenza virus and Ebola virus (2, 35, 49), use multiple routes of entry. Previously, the relationship between orthobunyavirus entry and macropinocytosis had not been examined; the acidification of macropinosomes when they intersect the classical endosomal pathway makes macropinocytosis an attractive alternative route for LACV entry (38). Therefore, it was of interest to assess the potential role in macropinocytosis and caveolar/raft-dependent endocytosis on LACV infection using inhibitors of macropinocytosis (Fig. 6) and nystatin (Fig. 7A to C), inhibitors of macropinocytosis and caveolar/raft-dependent endocytosis, respectively. Neither EIPA nor nystatin affected LACV FFWI or replication. Previous reports showed that nystatin affected the production of Oropouche virus in the supernatants even when added as late as 4 h postinfection, but it led to the significant accumulation of infectious intracellular virus, suggesting that nystatin inhibits other raft-dependent processes, like constitutive exocytosis (50, 51). Therefore, the effects seen were most likely due to inhibition of later steps of orthobunyavirus replication. In contrast, the nystatin treatment we used was only 30 min prior to and during the 1 h addition of virus, and the paucity of an observed effect suggests that cholesterol-rich lipid raft domains, including caveolae, are not required for productive entry of LACV (Fig. 7A to C). To specifically inhibit caveolar endocytosis, we use siRNA knockdown of caveolin-1 (Fig. 7D and E). Knockdown of caveolin-1 did not significantly affect LACV infection (Fig. 7F) or replication (Fig. 7G). Taken together, our results demonstrate that LACV enters cells, including neuronal cells, in a dynamin- and clathrin-mediated endocytic mechanism, and that it does not use caveolar endocytosis or macropinocytosis as major routes of entry into host cells.
There is ample evidence that orthobunyaviruses as well as hantaviruses, phleboviruses, and nairoviruses require endosomal acidification; however, these studies have also relied on lysosomotropic agents (27, 46, 51, 54). To build upon these observations, we utilized dominant-negative mutants of Rab5 and Rab7. The results demonstrated that Rab5, a key regulator of endocytic transport to early endosomes, is required for LACV infection. Similar to our findings, Uukeniemi virus (genus Phlebovirus) requires trafficking through early endosomes for infection, but unlike LACV, membrane fusion occurs in late endosomal compartments, probably because of the lower pH threshold for viral membrane fusion in Uukeniemi virus (33). Furthermore, colocalization studies demonstrate that Hantaan virus is similarly trafficked through early endosomes to late endosomes, where membrane fusion is thought to occur (27), although functional requirements of Rab5 and/or Rab7 have not been examined. Importantly, virus trafficking through early and late endosomes allows many enveloped viruses to utilize a low-pH-dependent mechanism to mediate virus-cell membrane fusion. In addition to bunyaviruses, flaviviruses and alphaviruses rely on low-pH-dependent membrane fusion proteins to mediate virus-cell membrane fusion (15, 44–46, 55). Thus, many arboviral encephalitides share common routes of entry and mechanisms of virus-cell membrane fusion, which could aid in the development of broad-spectrum antivirals for neuronal infections of viruses using mechanisms of entry and fusion that are similar to those of LACV.
Preventing binding to a receptor or inhibiting entry into the host cell once virions are bound are potential strategies for interfering with virus infection. Successful examples of such antiviral therapies include the fusion inhibitor T-20 for HIV and the receptor/attachment inhibitors PRO 140, PRO 542, and anti-CD81 for HIV (24, 25, 30, 58) and hepatitis C virus (39), respectively. Unfortunately, the identification of viral receptors as antiviral targets can be problematic, and many viruses do not share common receptors. However, many viruses use similar postbinding entry mechanisms, making these ideal targets for the development of antivirals with a broad spectrum. Here, we have identified a number of cellular factors, including accessory proteins involved in CME (e.g., Eps15) and those involved in the trafficking and maturation of early endosomes (e.g., Rab5), that play a role in LACV entry into host cells, including neurons. Importantly, these cellular factors are potential targets for the development of therapeutics and/or antivirals.
This study demonstrates that LACV uses an entry mechanism that is similar to that of other viral encephalitides, such as West Nile virus (12) and Japanese encephalitis virus (42), which use CME as a route of entry and cause CNS infection. Importantly, this study is the first to identify CME as a route of virus entry in primary neurons, identifying an important step of LACV infection in the cells, whose death and/or dysfunction leads to severe neurological consequences in vivo. Thus, LACV shares common mechanisms of entry and fusion with other important arboviral encephalitides, including several members of the Bunyaviridae, Flaviviridae, and Togaviridae, indicating that these and future findings can be extended to disparate viruses (15, 40). Moreover, this study supports LACV infection of primary neuronal cultures as a useful model to study entry into cells of the CNS and to assess antivirals targeting common entry pathways for numerous arboviral encephalitides.
ACKNOWLEDGMENTS
This work was supported by PHS grants NS-074626, NS-074626-02S1 (J.W.F.), and T32-NS-07180 (B.S.H.). In addition, we are grateful to Peter Crino for the use of the Leica DMI6000 B inverted microscope, which was purchased with the support of PHS grant NS-045021.
We thank Marc Dichter and Margaret Maronski for providing rat embryonic brain tissue and Denise R. Cook for critical review of the manuscript.
We declare that there are no conflicts of interest.
Footnotes
Published ahead of print 23 May 2012
REFERENCES
- 1. Acosta EG, Castilla V, Damonte EB. 2008. Functional entry of dengue virus into Aedes albopictus mosquito cells is dependent on clathrin-mediated endocytosis. J. Gen. Virol. 89:474–484 [DOI] [PubMed] [Google Scholar]
- 2. Aleksandrowicz P, et al. 2011. Ebola virus enters host cells by macropinocytosis and clathrin-mediated endocytosis. J. Infect. Dis. 204(Suppl. 3):S957–S967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benmerah A, Bayrou M, Cerf-Bensussan N, Dautry-Varsat A. 1999. Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J. Cell Sci. 112:1303–1311 [DOI] [PubMed] [Google Scholar]
- 4. Benmerah A, et al. 1998. AP-2/Eps15 interaction is required for receptor-mediated endocytosis. J. Cell Biol. 140:1055–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bishop D. 1996. Biology and molecular biology of bunyaviruses, p 19–53 In Elliot R. (ed), The Bunyaviridae. Plenum Press, New York, NY [Google Scholar]
- 6. Borucki MK, Kempf BJ, Blitvich BJ, Blair CD, Beaty BJ. 2002. La Crosse virus: replication in vertebrate and invertebrate hosts. Microbes Infect. 4:341–350 [DOI] [PubMed] [Google Scholar]
- 7. Boucrot E, Saffarian S, Zhang R, Kirchhausen T. 2010. Roles of AP-2 in clathrin-mediated endocytosis. PLoS One 5:e10597 doi:10.1371/journal.pone.0010597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brewer GJ. 1995. Serum-free B27/neurobasal medium supports differentiated growth of neurons from the striatum, substantia nigra, septum, cerebral cortex, cerebellum, and dentate gyrus. J. Neurosci. Res. 42:674–683 [DOI] [PubMed] [Google Scholar]
- 9. Calisher CH. 1996. History, classification, and taxonomy of viruses in the family Bunyaviridae, p 1–17 In Elliot R. (ed), The Bunyaviridae. Plenum Press, New York, NY [Google Scholar]
- 10. Carbone R, et al. 1997. eps15 and eps15R are essential components of the endocytic pathway. Cancer Res. 57:5498–5504 [PubMed] [Google Scholar]
- 11. Chen H, et al. 1998. Epsin is an EH-domain-binding protein implicated in clathrin-mediated endocytosis. Nature 394:793–797 [DOI] [PubMed] [Google Scholar]
- 12. Chu JJ, Ng ML. 2004. Infectious entry of West Nile virus occurs through a clathrin-mediated endocytic pathway. J. Virol. 78:10543–10555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Elliott RM. 1990. Molecular biology of the Bunyaviridae. J. Gen. Virol. 71:501–522 [DOI] [PubMed] [Google Scholar]
- 14. Gaidarov I, Santini F, Warren RA, Keen JH. 1999. Spatial control of coated-pit dynamics in living cells. Nat. Cell Biol. 1:1–7 [DOI] [PubMed] [Google Scholar]
- 15. Garry CE, Garry RF. 2004. Proteomics computational analyses suggest that the carboxyl terminal glycoproteins of bunyaviruses are class II viral fusion protein (beta-penetrenes). Theor. Biol. Med. Model. 1:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gerhardt RR, et al. 2001. First isolation of La Crosse virus from naturally infected Aedes albopictus. Emerg. Infect. Dis. 7:807–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gonzalez-Scarano F, Nathanson N. 1996. Bunyaviridae, p 1473–1505 In Howley PM, et al. (ed), Virology. Lippincott-Raven, New York, NY [Google Scholar]
- 18. Haddow AD, Odoi A. 2009. The incidence risk, clustering, and clinical presentation of La Crosse virus infections in the eastern United States, 2003–2007. PLoS One 4:e6145 doi:10.1371/journal.pone.0006145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hansen SH, Sandvig K, van Deurs B. 1993. Clathrin and HA2 adaptors: effects of potassium depletion, hypertonic medium, and cytosol acidification. J. Cell Biol. 121:61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heuser JE, Anderson RG. 1989. Hypertonic media inhibit receptor-mediated endocytosis by blocking clathrin-coated pit formation. J. Cell Biol. 108:389–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hollidge BS, Gonzalez-Scarano F, Soldan SS. 2010. Arboviral encephalitides: transmission, emergence, and pathogenesis. J. Neuroimmune Pharmacol. 5:428–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hunt RC, Marshall-Carlson L. 1986. Internalization and recycling of transferrin and its receptor. Effect of trifluoperazine on recycling in human erythroleukemic cells. J. Biol. Chem. 261:3681–3686 [PubMed] [Google Scholar]
- 23. Ivanov AI. 2008. Pharmacological inhibition of endocytic pathways: is it specific enough to be useful? Methods Mol. Biol. 440:15–33 [DOI] [PubMed] [Google Scholar]
- 24. Jacobson JM, et al. 2000. Single-dose safety, pharmacology, and antiviral activity of the human immunodeficiency virus (HIV) type 1 entry inhibitor PRO 542 in HIV-infected adults. J. Infect. Dis. 182:326–329 [DOI] [PubMed] [Google Scholar]
- 25. Jacobson JM, et al. 2008. Antiviral activity of single-dose PRO 140, a CCR5 monoclonal antibody, in HIV-infected adults. J. Infect. Dis. 198:1345–1352 [DOI] [PubMed] [Google Scholar]
- 26. Janssen R, Gonzalez-Scarano F, Nathanson N. 1984. Mechanisms of bunyavirus virulence. Comparative pathogenesis of a virulent strain of La Crosse and an avirulent strain of Tahyna virus. Lab. Investig. 50:447–455 [PubMed] [Google Scholar]
- 27. Jin M, et al. 2002. Hantaan virus enters cells by clathrin-dependent receptor-mediated endocytosis. Virology 294:60–69 [DOI] [PubMed] [Google Scholar]
- 28. Jones KE, et al. 2008. Global trends in emerging infectious diseases. Nature 451:990–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jordens I, Marsman M, Kuijl C, Neefjes J. 2005. Rab proteins, connecting transport and vesicle fusion. Traffic 6:1070–1077 [DOI] [PubMed] [Google Scholar]
- 30. Kilby JM, et al. 1998. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat. Med. 4:1302–1307 [DOI] [PubMed] [Google Scholar]
- 31. Kirkham M, Parton RG. 2005. Clathrin-independent endocytosis: new insights into caveolae and non-caveolar lipid raft carriers. Biochim. Biophys. Acta 1745:273–286 [DOI] [PubMed] [Google Scholar]
- 32. Lambert AJ, et al. 2005. Nucleic acid amplification assays for detection of La Crosse virus RNA. J. Clin. Microbiol. 43:1885–1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lozach PY, et al. 2010. Entry of bunyaviruses into mammalian cells. Cell Host Microbe 7:488–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Macia E, et al. 2006. Dynasore, a cell-permeable inhibitor of dynamin. Dev. Cell 10:839–850 [DOI] [PubMed] [Google Scholar]
- 35. Marsh M, Helenius A. 1980. Adsorptive endocytosis of Semliki Forest virus. J. Mol. Biol. 142:439–454 [DOI] [PubMed] [Google Scholar]
- 36. Mayor S, Pagano RE. 2007. Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol. 8:603–612 [DOI] [PubMed] [Google Scholar]
- 37. McJunkin JE, et al. 2001. La Crosse encephalitis in children. N. Engl. J. Med. 344:801–807 [DOI] [PubMed] [Google Scholar]
- 38. Mercer J, Helenius A. 2009. Virus entry by macropinocytosis. Nat. Cell Biol. 11:510–520 [DOI] [PubMed] [Google Scholar]
- 39. Meuleman P, et al. 2008. Anti-CD81 antibodies can prevent a hepatitis C virus infection in vivo. Hepatology 48:1761–1768 [DOI] [PubMed] [Google Scholar]
- 40. Nalca A, Fellows PF, Whitehouse CA. 2003. Vaccines and animal models for arboviral encephalitides. Antiviral Res. 60:153–174 [DOI] [PubMed] [Google Scholar]
- 41. Nankoe SR, Sever S. 2006. Dynasore puts a new spin on dynamin: a surprising dual role during vesicle formation. Trends Cell Biol. 16:607–609 [DOI] [PubMed] [Google Scholar]
- 42. Nawa M, Takasaki T, Yamada K, Kurane I, Akatsuka T. 2003. Interference in Japanese encephalitis virus infection of Vero cells by a cationic amphiphilic drug, chlorpromazine. J. Gen. Virol. 84:1737–1741 [DOI] [PubMed] [Google Scholar]
- 43. Nichols BJ, et al. 2001. Rapid cycling of lipid raft markers between the cell surface and Golgi complex. J. Cell Biol. 153:529–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Plassmeyer ML, Soldan SS, Stachelek KM, Martin-Garcia J, Gonzalez-Scarano F. 2005. California serogroup Gc (G1) glycoprotein is the principal determinant of pH-dependent cell fusion and entry. Virology 338:121–132 [DOI] [PubMed] [Google Scholar]
- 45. Plassmeyer ML, et al. 2007. Mutagenesis of the La Crosse virus glycoprotein supports a role for Gc (1066-1087) as the fusion peptide. Virology 358:273–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pobjecky N, Smith J, Gonzalez-Scarano F. 1986. Biological studies of the fusion function of California serogroup bunyaviruses. Microb. Pathog. 1:491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rust MJ, Lakadamyali M, Zhang F, Zhuang X. 2004. Assembly of endocytic machinery around individual influenza viruses during viral entry. Nat. Struct. Mol. Biol. 11:567–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rust RS, Thompson WH, Matthews CG, Beaty BJ, Chun RW. 1999. La Crosse and other forms of California encephalitis. J. Child. Neurol. 14:1–14 [DOI] [PubMed] [Google Scholar]
- 49. Saeed MF, Kolokoltsov AA, Albrecht T, Davey RA. 2010. Cellular entry of ebola virus involves uptake by a macropinocytosis-like mechanism and subsequent trafficking through early and late endosomes. PLoS Pathog. 6:e1001110 doi:10.1371/journal.ppat.1001110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Salaun C, James DJ, Chamberlain LH. 2004. Lipid rafts and the regulation of exocytosis. Traffic 5:255–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Santos RI, et al. 2008. Oropouche virus entry into HeLa cells involves clathrin and requires endosomal acidification. Virus Res. 138:139–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schimmoller F, Simon I, Pfeffer SR. 1998. Rab GTPases, directors of vesicle docking. J. Biol. Chem. 273:22161–22164 [DOI] [PubMed] [Google Scholar]
- 53. Schmaljohn C. 1996. Bunyaviridae: the viruses and their replication, p 1447–1472 In Howley PM, et al. (ed), Virology. Lippincott-Raven, New York, NY [Google Scholar]
- 54. Simon M, Johansson C, Mirazimi A. 2009. Crimean-Congo hemorrhagic fever virus entry and replication is clathrin-, pH- and cholesterol-dependent. J. Gen. Virol. 90:210–215 [DOI] [PubMed] [Google Scholar]
- 55. Soldan SS, Hollidge BS, Wagner V, Weber F, Gonzalez-Scarano F. 2010. La Crosse virus (LACV) Gc fusion peptide mutants have impaired growth and fusion phenotypes, but remain neurotoxic. Virology 404:139–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Soneoka Y, et al. 1995. A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res. 23:628–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Traub LM, Downs MA, Westrich JL, Fremont DH. 1999. Crystal structure of the alpha appendage of AP-2 reveals a recruitment platform for clathrin-coat assembly. Proc. Natl. Acad. Sci. U. S. A. 96:8907–8912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Trkola A, et al. 2001. Potent, broad-spectrum inhibition of human immunodeficiency virus type 1 by the CCR5 monoclonal antibody PRO 140. J. Virol. 75:579–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wang LH, Rothberg KG, Anderson RG. 1993. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 123:1107–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Weaver SC, Reisen WK. 2010. Present and future arboviral threats. Antiviral Res. 85:328–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zerial M, McBride H. 2001. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2:107–117 [DOI] [PubMed] [Google Scholar]