Abstract
The E6 proteins from high-risk alpha human papillomavirus (HPV) types (e.g., HPV16) are characterized by the presence of a PDZ-binding motif through which they interact with a number of cellular PDZ domain-containing substrates and cooperate in their degradation. The ability of these E6 proteins to bind to PDZ domain proteins correlates with the oncogenic potential of the virus. The E6 proteins of oncogenic HPV from the genus Betapapillomavirus (betaPV, e.g., HPV8) do not encode a PDZ-binding motif. We found that the PDZ domain protein syntenin-2 is transcriptionally downregulated in primary human epidermal keratinocytes (PHEK) by HPV8 E6. The mRNA levels of the known HPV16 E6 PDZ protein targets Dlg, Scribble, Magi-1, Magi-3, PSD95, and Mupp1 were not changed by HPV8 E6. Decreased protein levels of syntenin-2 were observed in cell extracts from PHEK expressing HPV5, -8, -16, -20, and -38 E6 but not in HPV1 and -4 E6-positive keratinocytes. Surprisingly, HPV16 E6 also repressed transcription of syntenin-2 but with a much lower efficiency than HPV8 E6. In healthy human skin, syntenin-2 expression is localized in suprabasal epidermal layers. In organotypic skin cultures, the differentiation-dependent expression of syntenin-2 was absent in HPV8 E6- and E6E7-expressing cells. In basal cell carcinomas of the skin, syntenin-2 was not detectable, whereas in squamous cell carcinomas, expression was located in differentiated areas. Short hairpin RNA-mediated knockdown of syntenin-2 led to an inhibition of differentiation and an increase in the proliferation capacity in PHEK. These results identified syntenin-2 as the first PDZ domain protein controlled by HPV8 and HPV16 at the mRNA level.
INTRODUCTION
Human papillomaviruses (HPV) are associated with epithelial cancers of the genital tract, and high-risk HPV of the genus Alphapapillomavirus (alphaPV) have been proven to be a necessary causative factor in the development of cervical cancer (42). HPV types of the genus Betapapillomavirus (betaPV) have been implicated in the development of cutaneous tumors, especially squamous cell carcinomas (SCC) and actinic keratoses (AK) as their precursor lesions (13). The first evidence implicating cutaneous HPV types in the pathogenesis of cutaneous SCC was based on the detection of betaPV types, predominantly HPV5 or HPV8 and rarely HPV type 14, 17, 19, 20, 24, or 47, in SCC of epidermodysplasia verruciformis (EV) patients (11, 15, 27, 29). Also, in non-EV patients, betaPV are frequently detected in cutaneous SCC and AK. Interestingly, the high prevalence and viral load of betaPV types in actinic keratoses (30, 38) and the decrease of viral load during skin carcinogenesis may point to a particular involvement of HPV in the early stages of skin cancer development. UV radiation is recognized as the main risk factor in SCC development (28), and HPV is discussed as a cofactor in concert with UV radiation (7, 16, 18, 21, 33, 35, 37). However, the role of betaPV and their cell-transforming mechanisms are still less clear than those of high-risk alphaPV (4).
The tumorigenic potential of alphaPV (e.g., HPV16 and HPV18) E6 and E7 oncoproteins depends in part on their capacity to form complexes with and inactivate the tumor suppressor proteins pRb and p53 (25). Recent studies have also revealed p53-independent functions of high-risk alphaPV E6 that are important for immortalization of human cells. HPV16 E6 mutants have been identified that are unable to degrade p53 but still retain the ability to immortalize epithelial cells. These data suggest that interactions with other cellular proteins may be essential for immortalization of cells. One such important interaction of E6 appears to be with proteins of the PDZ family (reviewed in reference 22). PDZ proteins contain a conserved domain that associates with the PSD95, Dlg, and ZO-1 proteins (hence the name PDZ). To date, PDZ domain-containing proteins are known to be targeted exclusively by high-risk alphaPV E6 through a PDZ-binding motif at their extreme C termini (ETQL in HPV16 E6 and ETQV in HPV18 E6), which results in the proteasomal degradation of the PDZ protein (34). A number of PDZ domain-containing proteins have been identified as E6 targets, including Dlg, Scribble, Mupp1, Magi, PATJ, PSD95, and NHERF-1 (1, 40). The PDZ domain is often found in proteins localized in areas of cell-cell contact, such as tight junctions in epithelial cells. PDZ proteins play a major role in maintenance of cell polarity, formation of cell-cell adherence junctions, and organization of multiprotein signaling complexes as scaffolding proteins (26). Their targeting by E6 may cause loss of cell polarity or morphological conversion, associated with epithelial-mesenchymal transition, and may be involved in transformation and carcinogenesis (40). Since E6 proteins of oncogenic betaPV types 5 and 8 do not encode a C-terminal PDZ-binding motif (36), no cellular PDZ protein is described to be targeted for degradation by these viruses and no data exist on the effects of betaPV types on the levels of PDZ proteins.
Further PDZ domain proteins are syntenin-1 and syntenin-2. Syntenin-1 was originally identified as a protein that binds directly to the cytoplasmic domain of the syndecan family of heparan sulfate proteoglycans (17), which are implicated in cell adhesion and several growth factor signaling pathways (10, 41). Syntenin-2 shows a sequence identity of about 58%, which increases up to 69% identity if considering only the tandem PDZ domains. When testing for syntenin-1 and -2 in HPV8 E6-expressing keratinocytes, it was surprising to see a reduced protein level of syntenin-2 in these cells. Here, we show that E6 of HPV8 and HPV16 is able to efficiently inhibit the transcription of the PDZ domain protein syntenin-2.
MATERIALS AND METHODS
Plasmid constructs.
The construction of pLXSN-8-E7 and pLXSN-8-E6E7 has been described previously in reference 5, and the generation of pLXSN-based E6 expression vectors for HPV types 1, 4, 5, 8, 16, 20, and 38 is described in reference 3. pLXSN-8E6Δ132-136 was generated by in vitro mutagenesis of pLXSN-8-E6 with appropriate primers to delete amino acids 132 to 136. The construction of pcDNA-NAP-HA is described in reference 31, and that of pCDNA-p53-Flag, pCDNA-16E6wt-Flag, and pCDNA-16E6ΔETQL-Flag in reference 36. pCDNA-16E6P59V-Flag was obtained by in vitro mutagenesis. To generate an expression vector for syntenin-2, the open reading frame (ORF) fragment was first cut out from pOTB7-syntenin-2 (ImaGenes, Berlin, Germany) by EcoRI and XhoI digestion and Klenow fragment treatment and subcloned into the pCDNA3.1-Flag vector, which was also treated with EcoRI and XhoI and later with Klenow fragment. Syntenin-2 could thus be cloned in frame with the Flag tag.
To obtain a retroviral syntenin-2 knockdown vector, the two oligonucleotide primers GATCCCCGCAACGGGCTCCTCACCAATTTTCAAGAGAAATTGGTGAGGAGCCCGTTGCTTTTTA and GGGCGTTGCCCGAGGAGTGGTTAAAAGTTCTCTTTAACCACTCCTCGGGCAACGAAAAATTCGA, containing BglII and HindIII restriction sites, were annealed and then introduced into the corresponding sites of pSuperior.retro.puro (OligoEngine, Seattle, WA) to produce pSuperior.retro-shSyn-2.
Cell lines and treatments.
Cells of the retroviral packaging cell line PT67 and the HPV-negative cervical carcinoma-derived cell line C33a were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS). Primary human epidermal keratinocytes (PHEK) were purchased from Cambrex and cultivated in KGM-Gold medium (Lonza, Cologne, Germany). The production of recombinant pLXSN- and pSuperior.retro-based retroviruses, their subsequent transduction into PHEK, and the selection of stable, 100% transduced clones were performed as described previously (5). UVB irradiation of PHEK was done as described in reference 3. C33a cells were transfected with FuGene (Roche, Mannheim, Germany) transfection reagent according to the manufacturer's recommendations.
Organotypic skin cultures.
Retrovirally transduced N/TERT keratinocytes (PHEK immortalized by overexpression of the human telomerase catalytic subunit [12]) with pLZRS vector containing the E6E7, E6, or E7 gene of HPV8 (33) were grown in raft cultures as described previously (9, 19). In brief, collagen-based dermal equivalents were generated with type IA rat collagen (2.2 mg/ml; BD Biosciences) containing 2 × 105 3T3-J2 fibroblasts and supplemented with 1× Ham's F12, 1× reconstitution buffer (0.22% NaHCO3, 0.005 N NaOH, 20 mM HEPES buffer), and 5% FCS. Upon establishing the collagen gel in a 1.9-cm2 well, 1.5 × 105 keratinocytes were seeded on top of the collagen. After 1 day of culturing, the collagen gel matrix with the attached keratinocytes was lifted to the air-liquid interphase and maintained in culture for 10 days. Skin cultures with primary keratinocytes expressing empty retroviral vector pSuperior or pSuperior/shSyntenin-2 (for short hairpin RNA-mediated knockdown of syntenin-2) were based on de-epidermalized human dermis as the matrix and prepared as described previously (5, 39).
Patient material and ethics statement.
Archival paraffin blocks of healthy human skin and of 9 cutaneous basal cell carcinoma (BCC) and 16 SCC (6 betaPV negative and 10 betaPV positive) samples were used for immunohistochemistry. The ethical approval for this study was obtained from the ethics committee of the University of Cologne. Written informed consent was obtained from all participants in this study.
Quantitative reverse transcription-PCR (qRT-PCR).
Total RNA was isolated from cells using the miRNeasy kit, and DNase digestion was performed on a column using RNase-free DNase according to the manufacturer's instructions (Qiagen, Hilden, Germany). One microgram of total RNA was reverse transcribed using the Omniscript RT kit (Qiagen, Hilden, Germany) with 10 μM random nonamers (TIB MOLBIOL, Berlin, Germany) and 1 μM oligo(dT23) primer (Sigma, Deisenhofen, Germany), as well as 10 units of RNase inhibitor (Fermentas, St. Leon-Rot, Germany). Quantitative PCR (qPCR) was performed using the Light-Cycler system (Roche, Mannheim, Germany). The total copy numbers of the target gene were normalized to the total copy number of the housekeeping gene encoding hypoxanthine phosphoribosyltransferase 1 (HPRT1). One PCR mixture contained 2 μl of 1:10-diluted cDNA in a total volume of 20 μl, 1.25 units Platinum Taq polymerase and the buffer provided in the kit (Invitrogen, Karlsruhe, Germany), 4 mM MgCl2, 1.6 μl of a 1:1,000 dilution of SYBGreen (Sigma, Deisenhofen, Germany), 5% dimethyl sulfoxide (DMSO), 0.5 μM each forward and backward primer, 500 ng/μl nonacetylated bovine serum albumin (Fermentas, St. Leon-Rot, Germany), and 0.2 mM each deoxynucleotide triphosphate. Amplified PCR fragments of the syntenin-2, syntenin-1, and HPRT1 genes were cloned into pJET1.2 (Qiagen, Hilden, Deutschland) to generate absolute standards with primers also used for subsequent qPCR analysis. Samples were analyzed in duplicate together with a 10-fold dilution series of standard plasmid. The cycling protocol conditions were 60 s at 95°C, followed by 40 cycles of 1 s at 95°C (20°C/s), 5 s at annealing temperature (Tan; melting temperature of primer − 5°C) (20°C/s), and 15 s at 72°C (20°C/s). The primers used in this study had the following 5′-to-3′ sequences: Syntenin-1-fw, TCCAGCAATTTTGTCAGAAGC; Syntenin-1-bw, GCTCTGGATACAGTCTGGGATAG; Syntenin-2-fw, GTGGACGGGCAGAATGTTAT; Syntenin-2-bw, ATGGAGATTCTGGCCACG; HPRT1-fw, CCTAAGATGAGCGCAAGTTGAA; HPRT1-bw, CCACAGGACTAGAACACCTGCTAA; Dlg1-fw, GCCATGAAACTGGAACAGG; Dlg1-bw, TGATCTGTTTCACTTGGTTGTAAAT; Scribble-fw, AATCTCCCTGCTCCCCTAGT; Scribble-bw, GTAGGCCTGCTTCACATTGG; Mupp1-fw, TGCCGCTTTGCTAAAGGT; Mupp1-bw, TGGATCCAGAGAGTGGAAAAG; PSD95-fw, GCCACCTCTATGGGACCAG; PSD95-bw, ATTGGCCGAGACATCGAG; Magi1-fw, CATCGACAGCTGCAAGGAG; Magi1-bw, AATGTCGCAGGTCCTTGTTC; Magi3-fw, TCATTCAGGCTGGTGGAAAT; Magi3-bw, ATCACATTTGAAGACGAAGGATT; 8E6-fw, CCGCAACGTTTGAATTTAATG; 8E6-bw, ATTGAACGTCCTGTAGCTAATTCA; 16E6-fw, TGTTTCAGGACCCACAGGA; and 16E6-bw, TTGTTTGCAGCTCTGTGCAT.
Western blotting.
Western blot analysis was performed as described previously (2). The blots were probed with antibodies raised against syntenin-2 (10407-1-AP, diluted 1:1,000; Proteintech, Chicago, IL), syntenin-1 (ab19903, diluted 1:1,000; Abcam, Cambridge, United Kingdom), involucrin (clone SY5, diluted 1:1,000; Sigma, St. Louis, MO), p53 (DO-1, diluted 1:1,000; Santa Cruz, Heidelberg, Germany), tubulin (YL1/2, diluted 1:5,000; Abcam), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (ab9484, diluted 1:1,000; Abcam), which was used as loading control. Immunoreactive proteins were visualized using peroxidase-coupled secondary antibodies (Dako, Hamburg, Germany) and the chemiluminescence system ECL Plus Western blotting detection system (GE Healthcare, Buckinghamshire, United Kingdom). Coimmunoprecipitations were performed as described in reference 24. In order to investigate in vivo degradation of syntenin-2 or p53, C33a cells were transiently cotransfected into 6-cm culture dishes with expression vectors for Flag-p53 or Flag–syntenin-2 and HPV16 E6-Flag expression vectors, and p53 or syntenin-2 levels were detected by Western blotting.
Immunohistochemistry.
Four-micrometer tissue sections of paraffin-embedded tissues were deparaffinized with xylene and ethanol incubations and treated with 3% H2O2 in methanol to inactivate endogenous peroxidases. Nonspecific binding sites were blocked by incubating the sections for 1 h with 50% heat-inactivated horse serum (diluted in phosphate-buffered saline [PBS]). Sections were incubated overnight in the absence (control) or in the presence of the primary antibody to syntenin-2 at 4°C (diluted 1:250 in 2% horse serum diluted in PBS). After extensive washing in PBS, sections were incubated at room temperature for 30 min with a biotinylated secondary antibody and processed with a streptavidin-biotin-peroxidase detection system (Vectastain ABC kit; Vector Labs) as recommended by the manufacturer. Sections were developed using DAB (3,3-diaminobenzidine) and counterstained with hematoxylin.
Flow cytometric analysis.
To analyze 5-bromodeoxyuridine (BrdU) incorporation and total DNA content, flow cytometric analyses using the MACSQuant Analyzer (MACS Miltenyi Biotec, Bergisch Gladbach, Germany) were performed as described previously (6). Briefly, cells were incubated with 10 μM of BrdU (Sigma, Steinheim, Germany) for 30 min and then harvested by trypsinization and centrifugation. After fixation in 70% ethanol, the samples were pelleted, washed twice in PBS, and treated with 2 M HCl for 30 min. They were then washed twice in PBST (PBS containing 0.1% BSA and 0.2% Tween 20). Cells were then incubated with fluorescein isothiocyanate (FITC)-labeled anti-BrdU antibody (Becton Dickinson, Oxford, United Kingdom) for 30 min in the dark. Cells were then washed twice in PBST and treated with RNase A (20 μg/ml; Fermentas, St. Leon-Rot, Germany) and propidium iodide (1 μg/ml; MACS Miltenyi Biotec). Samples were incubated at room temperature for 30 min prior to flow cytometric analysis. The flow cytometric data were analyzed using FlowJo software (Tree Star, Inc., Ashland, OR).
RESULTS
Syntenin-2 protein levels are downregulated in PHEK expressing alphaPV and betaPV E6.
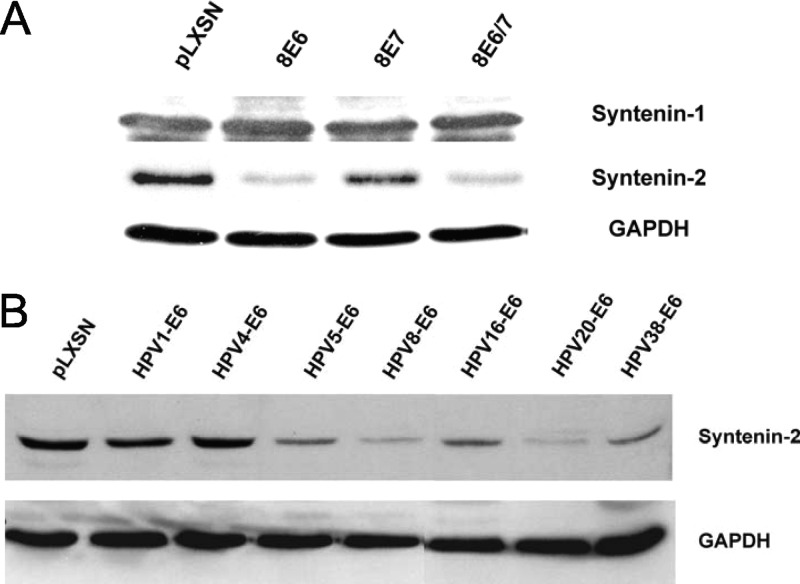
To test whether HPV8 is able to modulate the expression of syntenins, we analyzed the protein levels of the two highly homologous proteins syntenin-1 and -2 in PHEK expressing either HPV8 E6 or E7 or E6E7 together. A clear reduction in syntenin-2 protein levels was observed in E6- and E6E7-positive cells, whereas E7 alone had no effect on syntenin-2 expression. In contrast to the effect on syntenin-2, HPV8 oncoproteins had no effect on syntenin-1 protein levels (Fig. 1A).
Fig 1.
Downregulation of syntenin-2 protein expression in keratinocytes expressing HPV E6. (A) Western blot analysis of syntenin-1 and -2 in keratinocytes infected with retrovirus coding for HPV8 E6, E6E7, and E7. (B) Western blot analysis of syntenin-2 in keratinocytes infected with retrovirus coding for E6 of HPV type 1, 4, 5, 8, 16, 20, or 38 or with empty retrovirus pLXSN. GAPDH was used as loading control.
This initial data indicated that the expression of HPV8 E6 is responsible for modulating syntenin-2 protein expression in keratinocytes. To investigate whether syntenin-2 protein levels are also modulated by E6 of other HPV types, immunoblot analysis of total cell extracts from PHEK expressing E6 of HPV1 (muPV), HPV4 (gammaPV), HPV types 5, 8, 20, and 38 (betaPV), and HPV16 (alphaPV) were performed. The results showed strong reductions of syntenin-2 levels by E6 from HPV types 5, 8, 16, 20, and 38. HPV1 E6 and HPV4 E6 had no effect on syntenin-2 protein expression (Fig. 1B). These data identified the cellular PDZ domain protein syntenin-2 as a target for high-risk alphaPV type 16 and betaPV types.
HPV8 E6 targets the PDZ domain protein syntenin-2 at the transcriptional level.
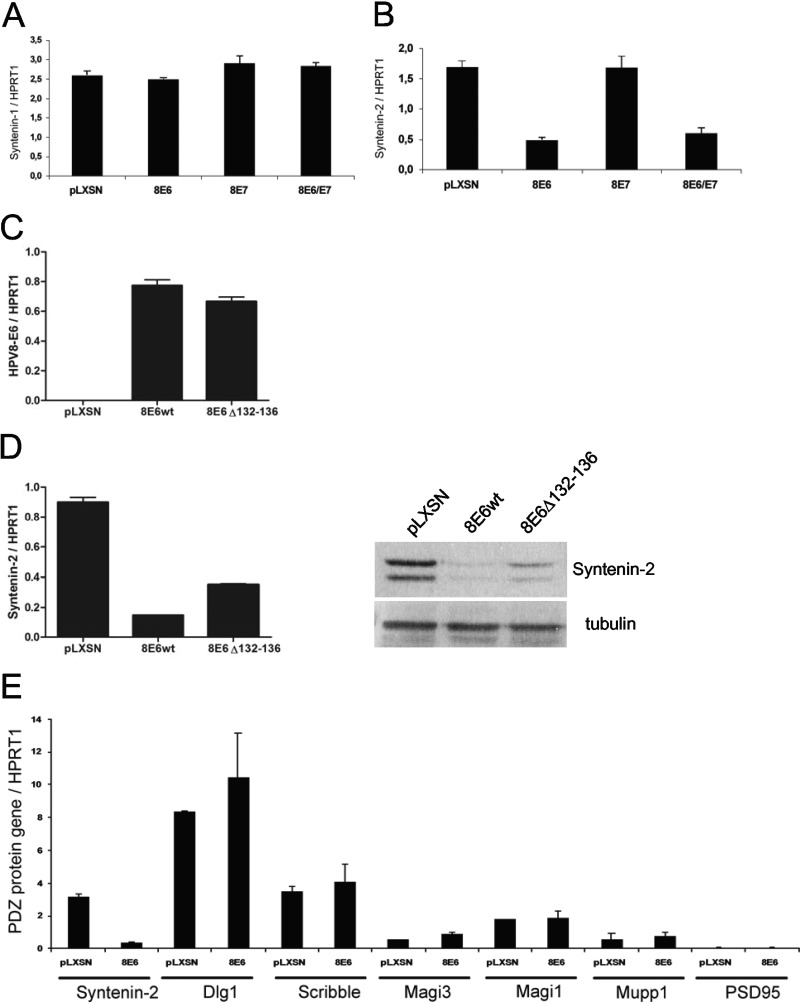
Since the HPV8 E6 oncoprotein does not contain a PDZ-binding motif through which, e.g., HPV16 E6 mediates proteasomal degradation of cellular PDZ proteins, we next checked whether syntenin-2 downregulation by HPV8 E6 is due to transcriptional repression. We therefore analyzed the syntenin-2 mRNA level by qRT-PCR in PHEK expressing HPV8 E6 either alone or together with E7. In line with the observations at the protein level, HPV8 E6, E7, and E6E7 had no effect on syntenin-1 mRNA expression (Fig. 2A). A strong reduction in syntenin-2 mRNA expression was observed in HPV8 E6 and E6E7 cells, whereas E7 alone had no effect on syntenin-2 mRNA expression (Fig. 2B). In order to map sequences on HPV8 E6 that are necessary for transcriptional downregulation of syntenin-2, we used a mutant with a deletion of amino acids 132 to 136 (8E6Δ132-136), which abolishes the interaction with the cellular transcriptional regulator p300 (24). After confirming equal expression levels of HPV8 E6 wild type (wt) and HPV8 E6Δ132-136 (Fig. 2C), it could be demonstrated that the grade of HPV8 E6-mediated repression of transcription depends in part on the presence of the p300 binding domain, since the mutant did not repress syntenin-2 transcription and protein expression as strongly as HPV8 E6 wt (Fig. 2D). HPV8 E6 was not able to repress the mRNA expression of the known HPV16 E6 PDZ protein targets Dlg, Scribble, Mupp1, Magi-1, Magi-3, and PSD95, which underlines a potential specificity of the interference with syntenin-2 transcription (Fig. 2E). No basal mRNA expression of Magi-2 and PATJ was detectable in cultured keratinocytes (data not shown).
Fig 2.
Syntenin-2 is downregulated at the transcriptional level in HPV8 E6-expressing cells. (A) Syntenin-1 mRNA expression in keratinocytes retrovirally infected with the empty construct pLXSN or constructs coding for HPV8 E6, E7, or E6E7. (B) Syntenin-2 mRNA expression was quantified in keratinocytes positive for pLXSN, HPV8 E6, E7, or E6E7. (C and D) PHEK expressing HPV8 E6 wt or HPV8 E6Δ132-136 were studied for E6 (C) and for syntenin-2 (D) mRNA and protein expression. (E) MRNA levels of syntenin-2, Dlg1, Scribble, Magi-1 and -3, Mupp1, and PSD95 in pLXSN- or HPV8 E6-positive primary keratinocytes. MRNA levels of gene targets were normalized to HPRT1 mRNA levels. Error bars show standard deviations.
HPV16 E6 also targets syntenin-2 at the transcriptional level.
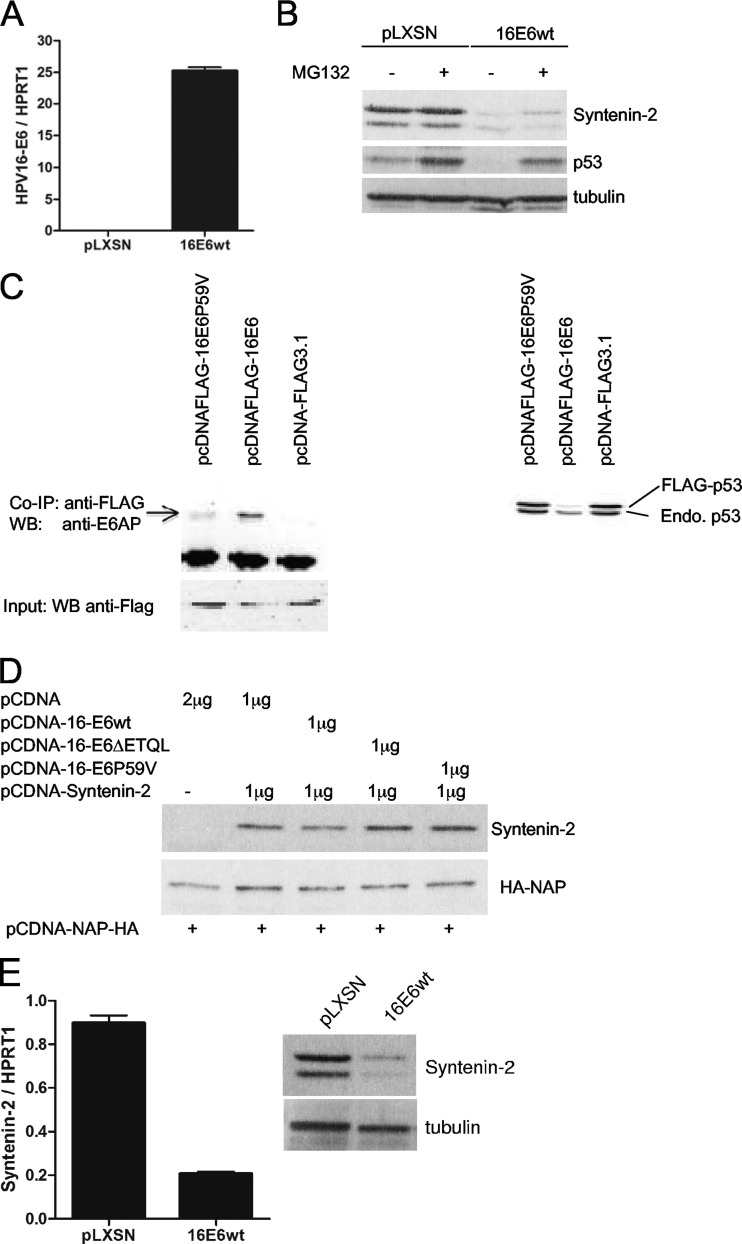
To investigate the mechanism by which HPV16 E6 causes decreased expression of syntenin-2, we first determined the contribution of proteasome-mediated degradation, since HPV16 E6 contains a PDZ protein-binding motif through which it can induce proteasomal degradation of cellular PDZ proteins. PHEK were transduced with the empty retroviral vector pLXSN or HPV16 E6 wt-expressing retrovirus. HPV16 E6 gene expression was monitored by qRT-PCR (Fig. 3A), and syntenin-2 protein levels were determined by Western blotting before and after treatment with the proteasome inhibitor MG132 (Fig. 3B). Treatment of HPV16 E6 cells with MG132 restored the levels of p53 protein, which was used as a surrogate marker for the activity of HPV16 E6. In control cells, the syntenin-2 protein levels were high and unchanged after MG132 treatment. The syntenin-2 protein levels were significantly lower in HPV16 E6 wt-transduced cells, but the addition of MG132 to the culture medium, surprisingly, did not lead to the accumulation of syntenin-2. Without evidence for proteasomal degradation, we then speculated that reduced levels of syntenin-2 protein in HPV16 E6 wt cells are due to transcriptional repression, as observed for HPV8 E6. To confirm this hypothesis, we transfected syntenin-2, under the control of a heterologous promoter (pCDNA-Syntenin-2), into the syntenin-2 null cervical cancer cell line C33a. The cells were cotransfected with expression vector pCDNA-16-E6wt, pCDNA-16-E6ΔETQL (coding for HPV16 E6 lacking the PDZ-binding domain), or pCDNA-16-E6P59V (with a mutation of the P in position 59 to V). The HPV16 E6 P59V mutant is no longer able to bind to endogenous E6AP (Fig. 3C, left) or to mediate E6AP-dependent degradation of cotransfected Flag-p53 (Fig. 3C, right). As indicated by the results in Fig. 3D, syntenin-2 protein levels were not affected by the three HPV16 E6 expression vectors, supporting the notion that HPV16 E6 does not regulate syntenin-2 at the protein level. However, when analyzing syntenin-2 mRNA levels by qRT-PCR, HPV16 E6 could be shown to inhibit the transcription of syntenin-2 in PHEK (Fig. 3E). It should be noted that a level of inhibition comparable to that by HPV8 E6 was achieved by an approximately 30-fold-higher level of HPV16 E6 mRNA (compare Fig. 3A and 2C).
Fig 3.
Syntenin-2 is targeted by HPV16 E6 at the transcriptional level. (A) PHEK expressing HPV16 E6 wt were studied for E6 mRNA expression by qRT-PCR. (B) HPV16 E6-positive PHEK were incubated in the presence of either MG132 or solvent before harvesting and then analyzed by Western blotting. (C) Coimmunoprecipitation (Co-IP) showed that the HPV16 E6P59V mutant does not bind to E6AP (left). Extracts from C33a cells, transiently transfected into 10-cm culture dishes with empty vectors or vectors for HPV16 E6-Flag or HPV16 E6P59V-Flag (15 μg each) were incubated with anti-Flag–M2 affinity beads. Bound E6AP was detected in the subsequent Western blot analysis by using a monoclonal E6AP antibody. To verify the presence of Flag-tagged protein, an aliquot of the extract was used as input control in a Western blot developed with the M2 anti-Flag antibody. In vivo degradation assay showed that the HPV16 E6P59V mutant does not degrade p53 (right). C33a cells were transiently cotransfected into 6-cm culture dishes with expression vectors for Flag-p53 and vectors coding for HPV16 E6-Flag or HPV16 E6P59V-Flag (2.5 μg each), and then p53 levels were detected by Western blotting using the Do-1 anti-p53 antibody. (D) Transient transfection of C33a with HPV16 E6 expression vectors was performed as indicated. Additionally, transfected HA-NAP was included as an internal transfection control. (E) Syntenin-2 mRNA expression was quantified in pLXSN- and HPV16 E6-positive PHEK by qRT-PCR, and protein levels detected by Western blotting. MRNA levels of gene targets were normalized to HPRT1 mRNA levels. Error bars show standard deviations.
UVB-induced syntenin-2 expression is blocked by HPV8 E6.
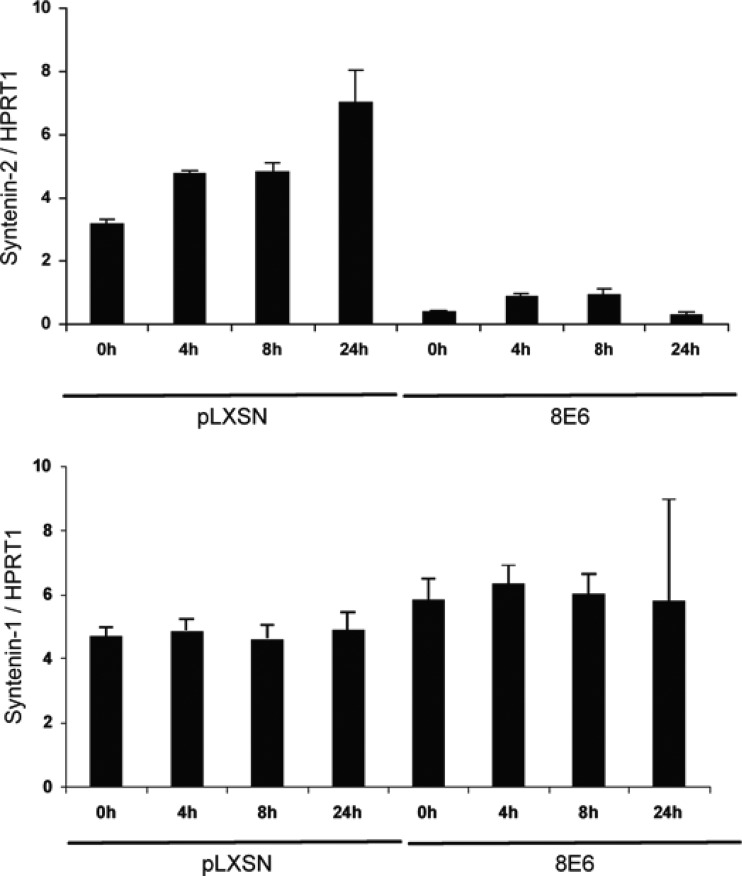
To test whether UVB irradiation may affect syntenin-2 mRNA expression in HPV8 E6-expressing PHEK, total RNA was extracted from HPV8 E6-positive and control cells at different time points after irradiation with a sublethal dose of UVB and analyzed by qRT-PCR. As shown in Fig. 4, UVB exposure of control cells led to induction of syntenin-2 mRNA expression at 4 h, 8 h, and 24 h after treatment. As expected, the baseline level of syntenin-2 in HPV8 E6-expressing cells was much lower than the baseline level in control cells and no significant stimulation of syntenin-2 mRNA production after UVB irradiation could be detected in these cells. In contrast, syntenin-1 mRNA levels were not changed either in control or in HPV8 E6-positive keratinocytes after UVB treatment.
Fig 4.
Syntenin-1 and -2 mRNA levels in control or HPV8 E6-expressing primary keratinocytes after UVB irradiation. Syntenin-1 and -2 mRNA levels were normalized to HPRT1 mRNA levels. Error bars show standard deviations.
Expression of syntenin-2 in normal human skin and basal and squamous cell carcinomas.
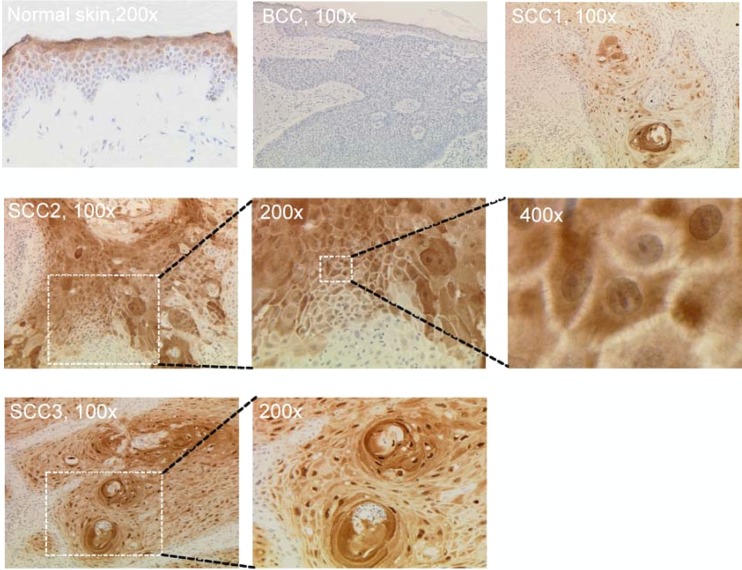
In order to demonstrate the presence of syntenin-2 in human skin, the target tissue of cutaneous betaPV, we analyzed the syntenin-2 expression pattern in nonlesional skin, as well as BCC and SCC. Immunohistochemical analysis of healthy skin revealed no syntenin-2 expression in basal and immediate suprabasal cell layers, whereas expression was detected in progressively differentiated keratinocyte layers (Fig. 5). In 9 BCC specimens, syntenin-2 was not detectable in any part of the tissues analyzed. In contrast, in 10 betaPV-positive and 6 betaPV-negative SCC specimens, syntenin-2 was expressed in keratinocytes of the variably differentiated squamous epithelium but was absent in basal cells. When analyzing syntenin-2 expression in SCC variants with different grades of keratinization, poorly differentiated SCC (SCC1) presented a weak cytoplasmic staining with few cells displaying nuclear expression. In well-differentiated SCC, syntenin-2 was strongly expressed in the cytoplasm alone (SCC2) or in both the cytoplasm and nuclei (SCC3) (Fig. 5). Although syntenin-2 expression was generally not detected as membranous staining, intercellular bridges were also slightly stained (×400 magnification). Thus, lack of syntenin-2 expression in basal cells but detection in differentiated keratinocytes of normal or malignant epithelium would suggest an involvement of syntenin-2 in cell differentiation. We therefore also concluded that the reinitiated expression of syntenin-2 in monolayer cultures of PHEK was most probably due to the cell culture conditions.
Fig 5.
Syntenin-2 expression patterns in healthy human skin and skin malignancies. Immunohistochemical analysis of syntenin-2 expression in healthy human skin and BCC and SCC specimens. Sections of paraffin-embedded tissue were stained with anti-syntenin-2 antibody (brown staining) and counterstained with hematoxylin. The SCC1 specimen is representative of poorly differentiated SCC, and the SCC2 and SCC3 specimens are representative for highly differentiated SCCs.
Downregulation of syntenin-2 in organotypic skin cultures of HPV8 E6-positive keratinocytes.
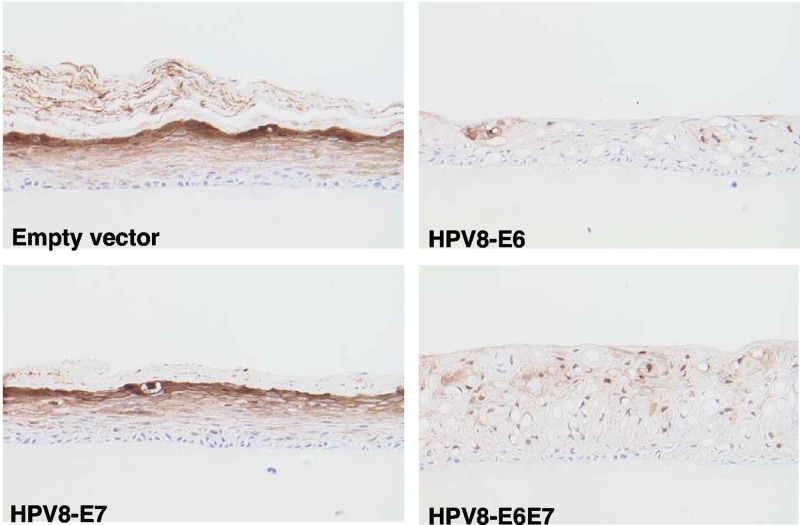
To test whether HPV8 oncoproteins are able to downregulate syntenin-2 expression and thereby modulate keratinocyte differentiation, N/TERT keratinocytes were retrovirally transduced with retrovirus containing HPV8 E6, E7, or E6E7 and used for organotypic skin cultures on a collagen-based matrix. At 10 days after air exposure, the N/TERT control and HPV8 E7 organotypic cultures regenerated a normal epithelium with all epidermal layers present, while the HPV8 E6 and E6E7 cultures showed altered keratinocyte differentiation, demonstrated by loss of normal stratification and the absence of stratum corneum development. Additional features observed in both the E6 and E6E7 cultures were the increase in cellular vacuolization in the suprabasal keratinocyte layers and the presence of a thicker epidermis in the E6E7 culture. Immunohistochemical staining showed syntenin-2 expression in the suprabasal cells in control and HPV8 E7-positive organotypic cultures, with increasing staining in the uppermost layers as found for healthy human skin. Cultures with HPV8 E6- and E6E7-positive keratinocytes showed an almost complete loss of syntenin-2 expression (Fig. 6).
Fig 6.
Expression of syntenin-2 in organotypic skin cultures of N/TERT keratinocytes expressing HPV8 E6, E7, or E6E7. Sections of paraffin-embedded cultures were stained with anti-syntenin-2 antibody (brown staining) and counterstained with hematoxylin. Magnification, ×200.
Knockdown of syntenin-2 alters the proliferation and differentiation capacities of primary keratinocytes.
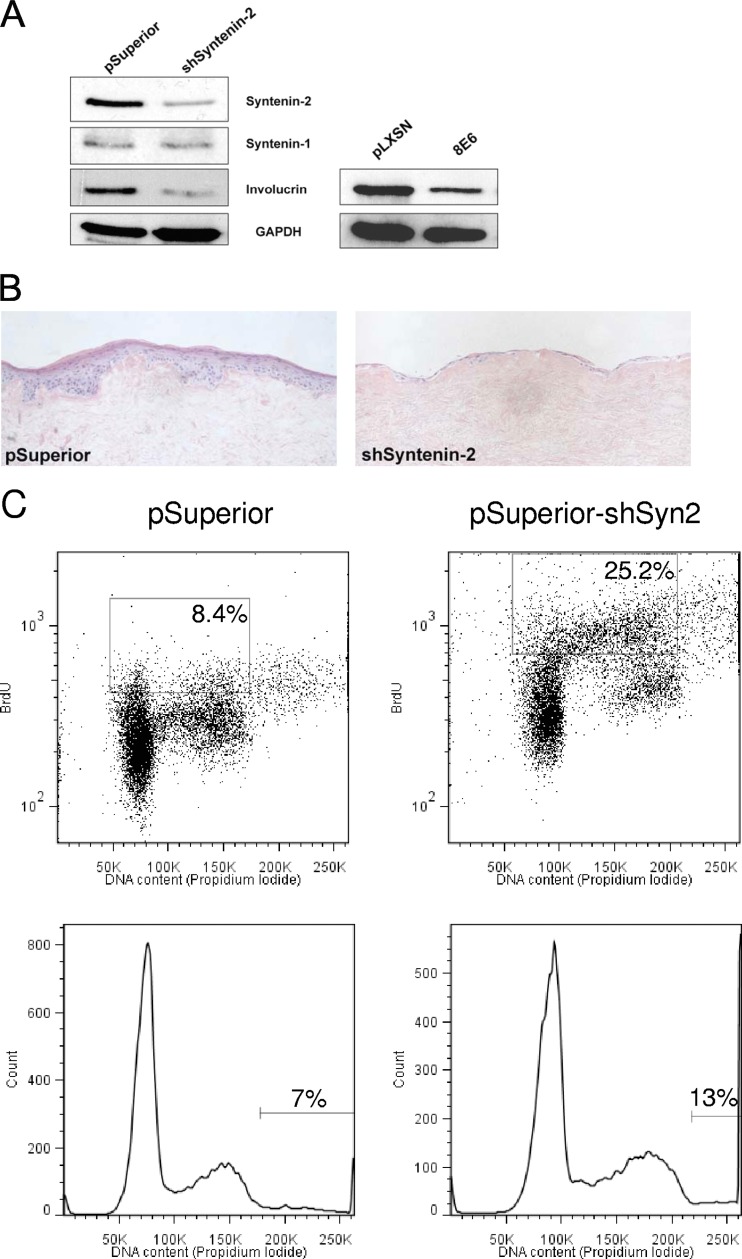
In order to assess the role of syntenin-2 for keratinocyte growth, we specifically silenced syntenin-2 expression by using short hairpin RNA (shRNA). In monolayer cultures of PHEK, knockdown of syntenin-2 led to reduced expression levels of the differentiation marker involucrin (Fig. 7A). Interestingly, overexpression of HPV8 E6 in the PHEK from the same donor also led to a reduction in involucrin expression (Fig. 7A). In organotypic cultures, PHEK expressing the empty retroviral vector pSuperior generated a normal epithelium with distinct strata of keratinocyte differentiation. In line with the modulation of differentiation markers in monolayer growth, shSyntenin-2-expressing cells were not able to develop a differentiated epithelium as in the control organotypic keratinocyte cultures (Fig. 7B). In normal keratinocytes, proliferation and differentiation are strictly coupled. The differences observed in the differentiation status of shSyntenin-2-expressing cells suggested that they might also have an altered proliferation capacity. To examine the role of syntenin-2 in the control of cell proliferation, shSyntenin-2-expressing cells were incubated in the presence of BrdU and stained with antibodies to BrdU and with propidium iodide to measure relative DNA content. The results of a representative experiment are shown in Fig. 7C. Flow cytometric analysis revealed that shSyntenin-2-expressing cells showed both a significant increase in the percentage of BrdU-positive cells (25.2%) compared with the percentage in the pSuperior control culture (8.4%) and a significant increase in the number of polyploid cells (13% versus 7%). These data suggested that syntenin-2 is involved in the control of normal keratinocyte differentiation and proliferation.
Fig 7.
Specific knockdown of syntenin-2 led to changes in keratinocyte differentiation and proliferation. (A) Western blot analysis of syntenin-2, syntenin-1, and involucrin in keratinocytes infected with the empty retroviral construct pSuperior or retroviral constructs coding for shSyntenin-2. Involucrin levels were also analyzed in keratinocytes expressing pLXSN or HPV8 E6. GAPDH was used as the loading control. (B) Organotypic skin cultures of PHEK expressing the empty retroviral construct pSuperior or the construct coding for shSyntenin-2. These were repeated three times, and sections of representative cultures are shown which were stained with hematoxylin and eosin and analyzed for histological changes. Magnification, ×200. (C) Flow cytometric analysis of pSuperior- and shSyntenin-2-expressing cells. Dot plots (top; gated on cells in the S-phase of the cell cycle), and histogram illustrations (bottom; gated on polyploid cells) of BrdU- and propidium iodide-treated cells.
DISCUSSION
We observed for the first time that the levels of a cellular PDZ protein, namely, syntenin-2, are reduced not only by genital high-risk alphaPV like HPV16 but also by various betaPV. In contrast to the well-established degradation of PDZ proteins via the proteasome-dependent pathway induced by HPV16 E6, our results demonstrated that HPV8 E6 repressed syntenin-2 by blocking mRNA expression.
Using the HPV8 E6Δ132-136 mutant, which is devoid of binding to the transcriptional coactivator p300, we could show that repression by HPV8 E6 is in part dependent on interaction with p300. The characterization of the minimal syntenin-2 promoter in the future will help to clarify in more detail to what degree E6-mediated syntenin-2 repression is through modulation of the expression of cellular transcription factors regulating syntenin-2 expression or through other mechanisms. It was surprising to see that reduced levels of syntenin-2 in HPV16 E6-expressing cells were not due to proteasomal degradation as expected for a PDZ protein but were also due to transcriptional repression. However, our results presented here suggest that higher levels of HPV16 E6 are needed to achieve the same degree of syntenin-2 repression as HPV8 E6.
Syntenin-2 is the closest homologue of the well-studied protein syntenin-1 but does not bind to the known peptide partners of syntenin-1. So far, only a few syntenin-2 binding partners have been proposed (8, 20, 32). Despite differences in protein binding, syntenin-2 shares with syntenin-1 the ability to interact with phosphatidylinositol 4-5-bisphosphate (PIP2). In monolayer cultures of the breast cancer cell line MCF-7 and the osteosarcoma cell line U2OS, syntenin-2 was found to be concentrated at three PIP2-rich regions, i.e., at the plasma membrane, in nucleoli, and in nuclear speckles (14, 23). Mortier and colleagues demonstrated that RNA interference-mediated specific knockdown of syntenin-2 in these cells resulted in dispersed smaller speckles, which had a dramatic consequence for the nuclear PIP2 pattern and impeded cell survival and cell division (23). In line with these functions in osteosarcoma cells, specific silencing of syntenin-2 in PHEK was shown here to lead to a complete block of normal differentiation, an increase in cell proliferation, and an increase in the number of polyploid cells, which might contribute to genomic instability. This suggests an essential role for syntenin-2 during keratinocyte growth processes.
In normal human skin and betaPV-negative and -positive SCC, we could demonstrate differentiation-dependent expression of syntenin-2 with a predominantly cytoplasmic and nuclear expression pattern. No differences were observed between betaPV-negative and -positive SCC. In contrast to mucosal cancers, in which alphaPV types persist in all tumor cells, betaPV loads in cutaneous SCC are generally very low. Because of the high prevalence and viral load of betaPV types in actinic keratoses and the decrease of viral load during skin carcinogenesis, it is generally assumed that betaPV may be preferentially involved as a cofactor to UV irradiation in the early stages of skin cancer development (30, 38). The deregulation of syntenin-2 by betaPV during early stages may lead to the establishment of a disturbed growth capacity of keratinocytes, which may contribute to skin cancer initiation. The syntenin-2 gene is a UVB-responsive gene which is upregulated in PHEK upon UVB irradiation. The fact that this upregulation is efficiently blocked by HPV8 E6 may also contribute to the generation of transformed skin cells. At later tumor stages, syntenin-2 is expressed according to the tumor-specific differentiation pattern, independent of HPV status. Elucidation of cellular pathways downstream from syntenin-2 will provide further aspects in our understanding of HPV pathogenesis in the future.
ACKNOWLEDGMENTS
We thank Pascale Zimmermann (Department of Human Genetics, K.U. Leuven, Belgium) for kindly providing the shRNA sequence for syntenin-2 and James G. Rheinwald (Deptment of Dermatology, Harvard Medical School, Boston MA) for the kind gift of the N/TERT cell line.
The human tissue samples were provided through the Z2 project of the SFB 829 through the Deutsche Forschungsgemeinschaft at the University of Cologne. This study was supported by the Deutsche Krebshilfe (grant number 109524) and by the Köln Fortune Program, Faculty of Medicine, University of Cologne (grant number 87/2011).
Footnotes
Published ahead of print 23 May 2012
REFERENCES
- 1. Accardi R, et al. 2011. E6 and E7 from human papillomavirus type 16 cooperate to target the PDZ protein Na/H exchange regulatory factor 1. J. Virol. 85:8208–8216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Akgül B, et al. 2011. Upregulation of lipocalin-2 in human papillomavirus-positive keratinocytes and cutaneous squamous cell carcinomas. J. Gen. Virol. 92:395–401 [DOI] [PubMed] [Google Scholar]
- 3. Akgül B, et al. 2010. Human papillomavirus 5 and 8 E6 downregulate interleukin-8 secretion in primary human keratinocytes. J. Gen. Virol. 91:888–892 [DOI] [PubMed] [Google Scholar]
- 4. Akgül B, Cooke JC, Storey A. 2006. HPV-associated skin disease. J. Pathol. 208:165–175 [DOI] [PubMed] [Google Scholar]
- 5. Akgül B, et al. 2005. The E7 protein of cutaneous human papillomavirus type 8 causes invasion of human keratinocytes into the dermis in organotypic cultures of skin. Cancer Res. 65:2216–2223 [DOI] [PubMed] [Google Scholar]
- 6. Akgül B, et al. 2007. HPV8 early genes modulate differentiation and cell cycle of primary human adult keratinocytes. Exp. Dermatol. 16:590–599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Akgül B, Lemme W, Garcia-Escudero R, Storey A, Pfister HJ. 2005. UV-B irradiation stimulates the promoter activity of the high-risk, cutaneous human papillomavirus 5 and 8 in primary keratinocytes. Arch. Virol. 150:145–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Borrell-Pages M, et al. 2000. The carboxy-terminal cysteine of the tetraspanin L6 antigen is required for its interaction with SITAC, a novel PDZ protein. Mol. Biol. Cell 11:4217–4225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boxman IL, et al. 2001. Transduction of the E6 and E7 genes of epidermodysplasia-verruciformis-associated human papillomaviruses alters human keratinocyte growth and differentiation in organotypic cultures. J. Invest. Dermatol. 117:1397–1404 [DOI] [PubMed] [Google Scholar]
- 10. Couchman JR. 2003. Syndecans: proteoglycan regulators of cell-surface microdomains? Nat. Rev. Mol. Cell Biol. 4:926–937 [DOI] [PubMed] [Google Scholar]
- 11. Dell'Oste V, et al. 2009. High beta-HPV DNA loads and strong seroreactivity are present in epidermodysplasia verruciformis. J. Invest. Dermatol. 129:1026–1034 [DOI] [PubMed] [Google Scholar]
- 12. Dickson MA, et al. 2000. Human keratinocytes that express hTERT and also bypass a p16(INK4a)-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol. Cell. Biol. 20:1436–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Feltkamp MC, de Koning MN, Bavinck JN, Ter Schegget J. 2008. Betapapillomaviruses: innocent bystanders or causes of skin cancer. J. Clin. Virol. 43:353–360 [DOI] [PubMed] [Google Scholar]
- 14. Gallardo R, Ivarsson Y, Schymkowitz J, Rousseau F, Zimmermann P. 2010. Structural diversity of PDZ-lipid interactions. Chembiochem 11:456–467 [DOI] [PubMed] [Google Scholar]
- 15. Gewirtzman A, Bartlett B, Tyring S. 2008. Epidermodysplasia verruciformis and human papilloma virus. Curr. Opin. Infect. Dis. 21:141–146 [DOI] [PubMed] [Google Scholar]
- 16. Giampieri S, Storey A. 2004. Repair of UV-induced thymine dimers is compromised in cells expressing the E6 protein from human papillomaviruses types 5 and 18. Br. J. Cancer 90:2203–2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grootjans JJ, et al. 1997. Syntenin, a PDZ protein that binds syndecan cytoplasmic domains. Proc. Natl. Acad. Sci. U. S. A. 94:13683–13688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jackson S, Harwood C, Thomas M, Banks L, Storey A. 2000. Role of Bak in UV-induced apoptosis in skin cancer and abrogation by HPV E6 proteins. Genes Dev. 14:3065–3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kazem S, van der Meijden E, Struijk L, de Gruijl FR, Feltkamp MC. 2012. Human papillomavirus 8 E6 disrupts terminal skin differentiation and prevents pro-Caspase-14 cleavage. Virus Res. 163:609–616 [DOI] [PubMed] [Google Scholar]
- 20. Koroll M, Rathjen FG, Volkmer H. 2001. The neural cell recognition molecule neurofascin interacts with syntenin-1 but not with syntenin-2, both of which reveal self-associating activity. J. Biol. Chem. 276:10646–10654 [DOI] [PubMed] [Google Scholar]
- 21. Leverrier S, et al. 2007. Role of HPV E6 proteins in preventing UVB-induced release of pro-apoptotic factors from the mitochondria. Apoptosis 12:549–560 [DOI] [PubMed] [Google Scholar]
- 22. Mantovani F, Banks L. 2001. The human papillomavirus E6 protein and its contribution to malignant progression. Oncogene 20:7874–7887 [DOI] [PubMed] [Google Scholar]
- 23. Mortier E, et al. 2005. Nuclear speckles and nucleoli targeting by PIP2-PDZ domain interactions. EMBO J. 24:2556–2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Muller-Schiffmann A, Beckmann J, Steger G. 2006. The E6 protein of the cutaneous human papillomavirus type 8 can stimulate the viral early and late promoters by distinct mechanisms. J. Virol. 80:8718–8728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Münger K, Howley PM. 2002. Human papillomavirus immortalization and transformation functions. Virus Res. 89:213–228 [DOI] [PubMed] [Google Scholar]
- 26. Niessen CM. 2007. Tight junctions/adherens junctions: basic structure and function. J. Invest. Dermatol. 127:2525–2532 [DOI] [PubMed] [Google Scholar]
- 27. Orth G. 2006. Genetics of epidermodysplasia verruciformis: insights into host defense against papillomaviruses. Semin. Immunol. 18:362–374 [DOI] [PubMed] [Google Scholar]
- 28. Pfeifer GP, Besaratinia A. 2012. UV wavelength-dependent DNA damage and human non-melanoma and melanoma skin cancer. Photochem. Photobiol. Sci. 11:90–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pfister H. 2003. Chapter 8: Human papillomavirus and skin cancer. J. Natl. Cancer Inst. Monogr. 2003(31):52–56 [DOI] [PubMed] [Google Scholar]
- 30. Pfister H, et al. 2003. High prevalence of epidermodysplasia verruciformis-associated human papillomavirus DNA in actinic keratoses of the immunocompetent population. Arch. Dermatol. Res. 295:273–279 [DOI] [PubMed] [Google Scholar]
- 31. Rehtanz M, Schmidt HM, Warthorst U, Steger G. 2004. Direct interaction between nucleosome assembly protein 1 and the papillomavirus E2 proteins involved in activation of transcription. Mol. Cell. Biol. 24:2153–2168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rual JF, et al. 2005. Towards a proteome-scale map of the human protein-protein interaction network. Nature 437:1173–1178 [DOI] [PubMed] [Google Scholar]
- 33. Struijk L, et al. 2008. Specific betapapillomaviruses associated with squamous cell carcinoma of the skin inhibit UVB-induced apoptosis of primary human keratinocytes. J. Gen. Virol. 89:2303–2314 [DOI] [PubMed] [Google Scholar]
- 34. Thomas M, Dasgupta J, Zhang Y, Chen X, Banks L. 2008. Analysis of specificity determinants in the interactions of different HPV E6 proteins with their PDZ domain-containing substrates. Virology 376:371–378 [DOI] [PubMed] [Google Scholar]
- 35. Tomlins C, Storey A. 2010. Cutaneous HPV5 E6 causes increased expression of Osteoprotegerin and Interleukin 6 which contribute to evasion of UV-induced apoptosis. Carcinogenesis 31:2155–2164 [DOI] [PubMed] [Google Scholar]
- 36. Töpffer S, Muller-Schiffmann A, Matentzoglu K, Scheffner M, Steger G. 2007. Protein tyrosine phosphatase H1 is a target of the E6 oncoprotein of high-risk genital human papillomaviruses. J. Gen. Virol. 88:2956–2965 [DOI] [PubMed] [Google Scholar]
- 37. Underbrink MP, Howie HL, Bedard KM, Koop JI, Galloway DA. 2008. E6 proteins from multiple human betapapillomavirus types degrade Bak and protect keratinocytes from apoptosis after UVB irradiation. J. Virol. 82:10408–10417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weissenborn SJ, et al. 2005. Human papillomavirus-DNA loads in actinic keratoses exceed those in non-melanoma skin cancers. J. Invest. Dermatol. 125:93–97 [DOI] [PubMed] [Google Scholar]
- 39. Westphal K, Akgül B, Storey A, Nindl I. 2009. Cutaneous human papillomavirus E7 type-specific effects on differentiation and proliferation of organotypic skin cultures. Cell Oncol. 31:213–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yugawa T, Kiyono T. 2009. Molecular mechanisms of cervical carcinogenesis by high-risk human papillomaviruses: novel functions of E6 and E7 oncoproteins. Rev. Med. Virol. 19:97–113 [DOI] [PubMed] [Google Scholar]
- 41. Zimmermann P, David G. 1999. The syndecans, tuners of transmembrane signaling. FASEB J. 13(Suppl):S91–S100 [DOI] [PubMed] [Google Scholar]
- 42. zur Hausen H. 2002. Papillomaviruses and cancer: from basic studies to clinical application. Nat. Rev. Cancer. 2:342–350 [DOI] [PubMed] [Google Scholar]