Abstract
T cell exhaustion and loss of memory potential occur during many chronic viral infections and cancer. We investigated when during chronic viral infection virus-specific CD8 T cells lose the potential to form memory. Virus-specific CD8 T cells from established chronic infection were unable to become memory CD8 T cells if removed from infection. However, at earlier stages of chronic infection, these virus-specific CD8 T cells retained the potential to partially or fully revert to a memory differentiation program after transfer to infection-free mice. Conversely, effector CD8 T cells primed during acute infection were not protected from exhaustion if transferred to a chronic infection. We also tested whether memory and exhausted CD8 T cells arose from different subpopulations of effector CD8 T cells and found that only the KLRG1lo memory precursor subset gave rise to exhausted CD8 T cells. Together, these studies demonstrate that CD8 T cell exhaustion is a progressive developmental process. Early during chronic infection, the fate of virus-specific CD8 T cells remains plastic, while later, exhausted CD8 T cells become fixed in their differentiation state. Moreover, exhausted CD8 T cells arise from the memory precursor and not the terminally differentiated subset of effector CD8 T cells. These studies have implications for our understanding of senescence versus exhaustion and for therapeutic interventions during chronic infection.
INTRODUCTION
During acute infections, antigen-specific CD8 T cells are activated through stimulation of the T cell receptor (TCR) and costimulatory receptors, as well as inflammatory signals (10, 25, 42). Upon activation, CD8 T cells proliferate extensively and differentiate, acquiring new properties, such as the ability to produce cytokines and chemokines, to kill virally infected cells, and to develop new migratory potential (14, 21, 42). While most of the effector CD8 T cell population dies (14, 21, 42), a subset further differentiates into long-lived memory T cells capable of undergoing antigen-independent maintenance and self-renewal (18, 20). Upon reinfection, these memory CD8 T cells rapidly expand and redifferentiate into secondary effector T cells (14, 21, 42). Considerable evidence now supports a model of gradual development of CD8 T cell memory in vivo. For example, while CD8 T cells committed to the memory fate can be identified early in the effector response (15, 18–20, 23), these cells require further differentiation after clearance of infection to acquire canonical memory T cell properties such as self-renewal and robust recall responses.
In contrast to acute infections, during chronic viral infections, CD8 T cells can develop functional defects collectively referred to as exhaustion (38). Exhausted CD8 T cells undergo a hierarchical loss of function and progressively lose the ability to produce interleukin-2 (IL-2), to kill efficiently ex vivo, and to produce tumor necrosis factor (TNF) or gamma interferon (IFN-γ) (13, 40, 43). Exhausted CD8 T cells also fail to acquire memory T cell properties, such as antigen-independent self-renewal and the ability to mount robust recall responses (38). A central question about CD8 T cell exhaustion, however, is whether the loss of effector functions and failed memory CD8 T cell differentiation develop because of improper/altered priming or because of events that occur later during persisting infection. Other types of T cell dysfunction, such anergy, arise because of changes in initial T cell activation, and these events commit T cells to a state of hyporesponsiveness and/or deletion (30), though in vivo tolerance might be reversible under some conditions (29). While there is some evidence that CD8 T cells can acquire and maintain the ability to produce cytokines if removed from a chronic infection after 5 days of priming (9), the flexibility of the CD8 T cell exhaustion developmental program and impact on memory T cell properties remain poorly understood.
In this study, we examined whether events during the priming phase of infection can predetermine the fate of CD8 T cells during either chronic or acute infections. While virus-specific CD8 T cells from established chronic infection failed to form memory when removed from infection, memory T cell potential gradually increased in virus-specific CD8 T cells isolated from earlier time points postinfection (p.i.). Additionally, virus-specific CD8 T cells primed during acute infection were not protected from exhaustion by presumably optimal priming conditions, since these effector CD8 T cells became exhausted if adoptively transferred to chronically infected mice. Finally, we defined the lineage origin of exhausted CD8 T cells on the basis of the effector subsets described during acute infection. While the population of KLRG1hi effector CD8 T cells found during the first week of acute or chronic infection rapidly disappeared from chronically infected mice, the KLRG1lo subset of effector CD8 T cells that persisted during chronic infection gave rise to exhausted CD8 T cells. Together, these studies demonstrate that CD8 T cell exhaustion arises from a progressive differentiation process with gradual loss of developmental plasticity and increased commitment to exhaustion. Furthermore, the identification of the KLRG1lo subset of effector CD8 T cells as precursors to exhausted CD8 T cells suggests that terminally differentiated effector and exhausted CD8 T cells can arise via distinct pathways. These studies have implications for the reversibility of CD8 T cell dysfunction during chronic infection and our understanding of senescence versus exhaustion.
MATERIALS AND METHODS
Mice, virus, and infections.
Five-week-old C57BL/6 and B6LY5.2CR (B6) mice (expressing Ly5.1 [Ly5.1+]) were purchased from NCI (Frederick, MD). Lymphocytic choriomeningitis virus (LCMV) strains were propagated, titers were determined, and the strains were used as previously described (40). B6 mice were infected with LCMV Armstrong (Arm; 2 × 105 PFU) intraperitoneally or with LCMV clone 13 (2 × 106 PFU) intravenously. All mice were maintained at the University of Pennsylvania under protocols approved by the Institutional Animal Care and Use Committee.
Adoptive transfers.
CD8 T cells were purified using negative-selection magnetic beads (magnetically activated cell sorting beads; Miltenyi Biotec). The KLRG1 sorts were performed on an Aria II (BD Immunocytometry Systems, San Jose, CA). In each individual experiment, identical numbers of DbGP33+ CD8 T cells were adoptively transferred to each separate recipient mouse. Donor populations in the peripheral blood were monitored by retro-orbital blood collection as described previously (40).
Flow cytometry and ICS.
Lymphocytes were isolated from the spleen and peripheral blood as previously described (7, 40). Major histocompatibility complex class I peptide tetramers were made and used as described previously (39, 40). Antibodies were purchased from eBioscience (San Diego, CA), Biolegend (San Diego, CA), Invitrogen (Carlsbad, CA), Abcam (Cambridge, MA), R&D Systems (Minneapolis, MN), or BD Biosciences (San Diego, CA). Lymphocytes were stained and analyzed, and intracellular cytokine staining (ICS) was performed as previously described (7, 40). Cells were analyzed on an LSR II flow cytometer (BD Immunocytometry Systems, San Jose, CA). Data analysis was performed using FlowJo (version 9.3.1) software (TreeStar, San Carlos, CA). Dead cells were removed by gating on a LIVE/DEAD Aqua kit (Invitrogen) versus forward scatter (FSC-A).
RESULTS
During chronic viral infections, CD8 T cells become exhausted rather than develop into functional memory CD8 T cells. It is unclear, however, when during chronic infection virus-specific CD8 T cells lose the potential to become memory CD8 T cells. To address this question, we used two well-described strains of LCMV, LCMV Arm and LCMV clone 13. Infection with LCMV Arm results in an acute infection, with viral clearance by day 8 p.i. LCMV clone 13, on the other hand, causes a chronic infection with viremia for 2 to 3 months and long-term viral replication in tissues such as the kidneys and brain (2, 40, 43). Using this system and a series of adoptive transfer experiments, we tested the loss of CD8 T cell memory potential and commitment to exhaustion at different stages of chronic viral infection.
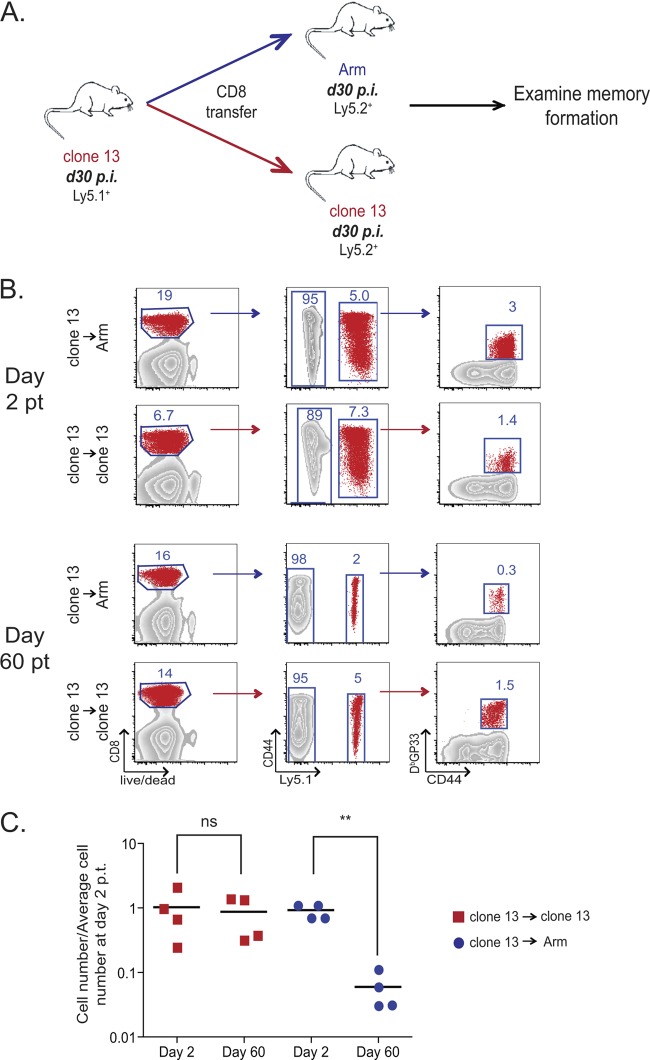
We first examined the ability of exhausted CD8 T cells from established chronic infection (day 30 p.i. clone 13) to convert to functional memory CD8 T cells when adoptively transferred to infection-free mice. To achieve this goal, exhausted CD8 T cells were purified from clone 13-infected Ly5.1+ C57BL/6 mice and adoptively transferred to Ly5.2+ LCMV Arm-immune mice (Fig. 1A). The use of LCMV Arm-immune mice as recipients in these experiments allowed the control of any residual virus transferred with the donor CD8 T cells by the robust cellular and humoral immunity of the recipient mice. These exhausted CD8 T cells were also adoptively transferred to Ly5.2+ clone 13 infection-matched (day 30 p.i.) recipient mice as a control. On day 2 posttransfer (p.t.), donor (Ly5.1+) LCMV DbGP33-specific CD8 T cells were easily detected in both LCMV Arm-immune and clone 13-infected recipient mice (Fig. 1B and C). However, while donor-derived virus-specific CD8 T cells persisted at constant levels in the chronically infected recipients, these donor cells failed to persist in the infection-free Arm-immune recipients (Fig. 1B and C). These observations are consistent with those from previous studies (35, 39) demonstrating that after prolonged (i.e., 3-plus months) chronic infection, exhausted virus-specific CD8 T cells are maintained in an antigen-dependent rather than cytokine (IL-7/IL-15)-dependent manner and fail to persist in antigen-free mice. The current data suggest that this antigen dependence is already established by day 30 p.i. Furthermore, these data indicate that by day 30 of chronic LCMV infection, virus-specific CD8 T cells have lost the potential to form antigen-independent memory CD8 T cells if removed from chronic infection.
Fig 1.
Virus-specific CD8 T cells at day 30 after LCMV clone 13 infection do not persist if adoptively transferred to infection-free recipients. (A) Experimental design. Ly5.1+ CD8 T cells were purified from day 30 LCMV clone 13-infected mice and adoptively transferred to day 30 LCMV Arm-immune or day 30 LCMV clone 13-infected recipient mice. Approximately 2 × 105 DbGP33+ CD8 T cells were adoptively transferred to each recipient mouse. Donor virus-specific CD8 T cells were examined on days 2 and 60 p.t. (B) Gating strategy used to identify donor CD8 T cell population. Representative examples of the day 30 clone 13 → Arm and day 30 clone 13 → clone 13 recipient mice on both day 2 p.t. and day 60 p.t. (C) Absolute numbers of DbGP33+ CD8 T cells in the spleens of recipient mice on days 2 and 60 p.t. normalized to the number of cells on day 2 p.t. Each symbol represents one mouse, with red squares signifying antigen-specific CD8 T cells transferred to clone 13-infected recipient mice and blue circles representing cells transferred to day 30 Arm-infected recipient mice. ns, not significant; **, P < 0.01.
Partial loss of memory CD8 T cell potential prior to day 30 of chronic infection.
To begin to determine when during chronic infection virus-specific CD8 T cells lose the potential to become memory T cells, Ly5.2+ virus-specific CD8 T cells were purified at day 15 of clone 13 infection and adoptively transferred into Ly5.1+ recipient mice that had also been infected 15 days previously with either Arm or clone 13. Persistence and differentiation of the Ly5.2+ virus-specific donor CD8 T cell population were examined over time (Fig. 2A).
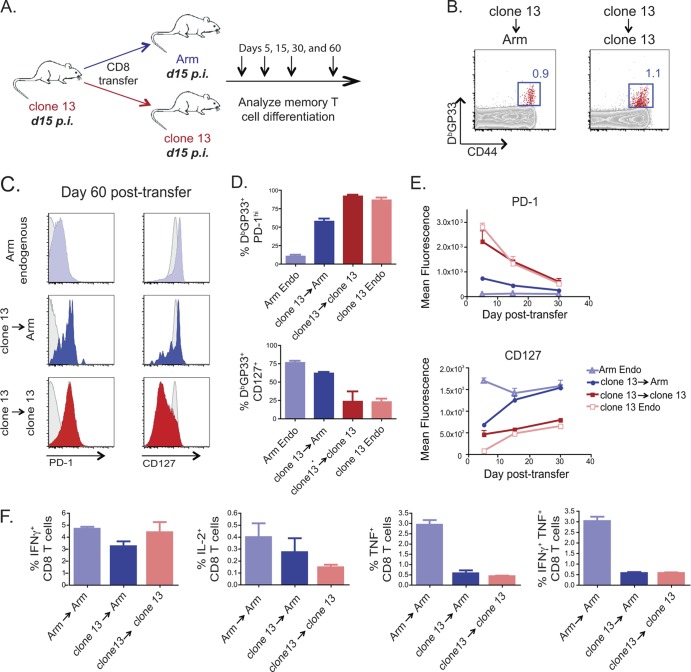
Fig 2.
CD8 T cells from day 15 clone 13-infected mice persisted but formed memory poorly in infection-free recipients. (A) Experimental design. Ly5.2+ CD8 T cells were purified from day 15 LCMV clone 13-infected mice and adoptively transferred to day 15 Arm- or day 15 clone 13-infected recipient mice. Approximately 1 × 105 or 1.5 × 105 donor DbGP33+ CD8 T cells were adoptively transferred to each recipient mouse, with equal numbers of adoptively transferred cells transferred to all donor mice for each individual experiment. Donor virus-specific CD8 T cells were examined on days 5, 15, 30, and 60 p.t. in recipient mouse spleens. (B) Representative fluorescence-activated cell sorter plots of CD44hi DbGP33+ day 15 clone 13 → Arm-infected and day 15 clone 13 → clone 13-infected recipient mice on day 60 p.t. The cells are gated on live CD8+ Ly5.2+ T cells. (C) Mean fluorescence of CD127 and PD-1. The gray histogram in each plot is gated on CD8+ CD44lo naïve T cells. (D) Frequency of PD-1hi and CD127+ CD8+ DbGP33+ T cells on day 60 p.t. (E) Mean fluorescence of CD127 and PD-1 on days 5, 15, and 30 p.t. (F) Percentage of IFN-γ, IL-2, TNF, and IFN-γ plus TNF produced by CD8 T cells following restimulation ex vivo with the GP33 peptide on day 30 p.t. Light blue, endogenous (Endo) Arm response; dark blue, clone 13 → Arm transfer; red, clone 13 → clone 13 transfer; pink, endogenous clone 13 response.
Unlike the exhausted CD8 T cells found at day 30 of chronic infection, the DbGP33-specific CD8 T cell population isolated from clone 13-infected mice at day 15 p.i. persisted when adoptively transferred to virus-free mice (Fig. 2B). However, this persistence was not associated with a complete conversion to a normal memory CD8 T cell phenotype. While virus-specific CD8 T cells from mice with clone 13 infection at day 15 transferred into Arm-infected recipients at day 15 of infection expressed high levels of CD127, similar to memory CD8 T cells, the same cells also had elevated expression of the inhibitory receptor PD-1 (Fig. 2C to E). These virus-specific CD8 T cells removed from chronic infection on day 15 also produced cytokines at a level intermediate between that observed for virus-specific memory CD8 T cells that develop after an acute infection (Arm endogenous) and that observed for the clone 13 → clone 13 control transfer (Fig. 2F). Therefore, at day 15 of chronic infection, while virus-specific CD8 T cells had the capacity to persist and develop some phenotypic characteristics of memory CD8 T cells upon adoptive transfer to infection-free mice, these cells retained some phenotypic scars of chronic infection.
Plasticity and memory T cell potentials exist early during clone 13 viral infection.
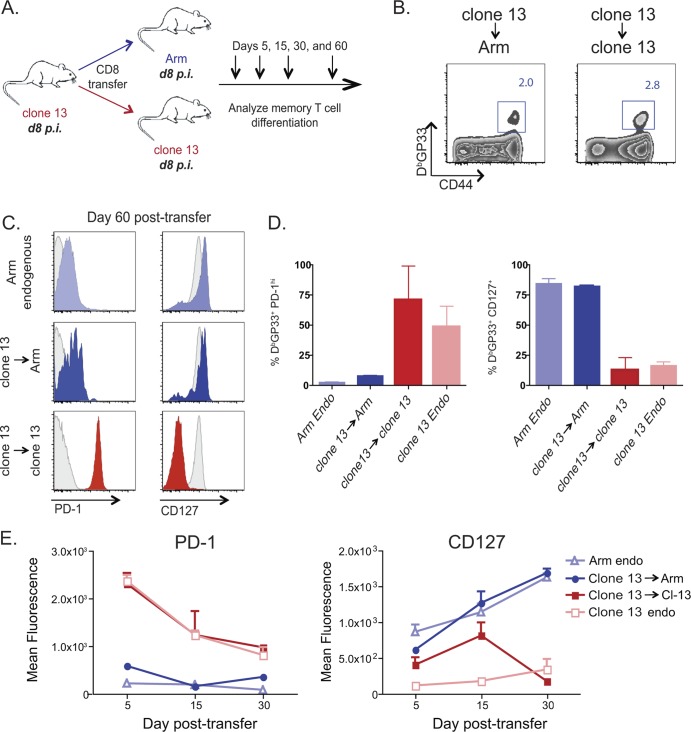
We next investigated whether virus-specific CD8 T cells present earlier in chronic infection retained greater memory potential than those observed at day 15 or day 30 p.i. To address this question, at day 8 after clone 13 infection, virus-specific CD8 T cells were adoptively transferred to congenic day 8 clone 13-infected mice or day 8 Arm-infected recipient mice (Fig. 3A). The DbGP33 donor CD8 T cell population was monitored for ∼2 months to examine memory CD8 T cell differentiation (Fig. 3A). Like the day 15 donor cells, these day 8 donor cells persisted following adoptive transfer to infection-free mice (Fig. 3B). In contrast to what was observed at day 15 p.i., however, DbGP33 CD8 T cells removed from chronic infection at day 8 p.i. reverted to low PD-1 expression in infection-free recipient mice (Fig. 3C to E). The CD8 T cells transferred from clone 13-infected to Arm-infected mice on day 8 p.i. also expressed high levels of CD127, consistent with normal memory CD8 T cell differentiation (Fig. 3C to E). PD-1 expression remained high and CD127 expression remained low if the same donor CD8 T cells were instead adoptively transferred back into clone 13-infected recipient mice (Fig. 3B to E). While donor cell recovery did not permit rigorous assessment of cytokine production, the expression of CD127, PD-1, CD62L, and KLRG1 (Fig. 3C to E and data not shown), as well as cellular recovery, is consistent with efficient development of memory CD8 T cells from cells primed for 8 days during LCMV clone 13 infection. Thus, early during chronic infection, virus-specific CD8 T cells possess memory potential and can form robust memory CD8 T cells if removed from chronic infection.
Fig 3.
Virus-specific CD8 T cells isolated from LCMV clone 13-infected donor mice on day 8 p.i. can form memory in infection-free recipient mice. (A) Experimental design. Ly5.2+ CD8 T cells were purified from day 8 clone 13-infected mice and adoptively transferred to day 8 Arm- or day 8 clone 13-infected recipient mice. In each experiment, the same number of CD8 T cells was transferred to each recipient mouse, with between 2 × 105 and 3 × 105 donor DbGP33+ CD8 T cells transferred to each recipient per experiment. Donor virus-specific CD8 T cells were examined on days 5, 15, 30, and 60 p.t. in recipient mouse spleens. (B) Representative fluorescence-activated cell sorter plots of CD44hi DbGP33+ day 8 clone 13 → Arm-infected and day 8 clone 13 → clone 13-infected recipient mice on day 30 p.t. The cells are gated on live CD8+ Ly5.2+ T cells. (C) Mean fluorescence of CD127 and PD-1. The gray histogram in each plot is gated on CD8+ CD44lo naïve T cells. (D) Frequency of PD-1hi and CD127+ CD8+ DbGP33+ T cells on day 60 p.t. (E) Mean fluorescence of CD127 and PD-1 on days 5, 15, and 30 p.t. Light blue, endogenous Arm response; dark blue, clone 13 → Arm transfer; red, clone 13 → clone 13 transfer; pink, endogenous clone 13 response.
LCMV Arm-primed effector CD8 T cells become exhausted in clone 13-infected mice.
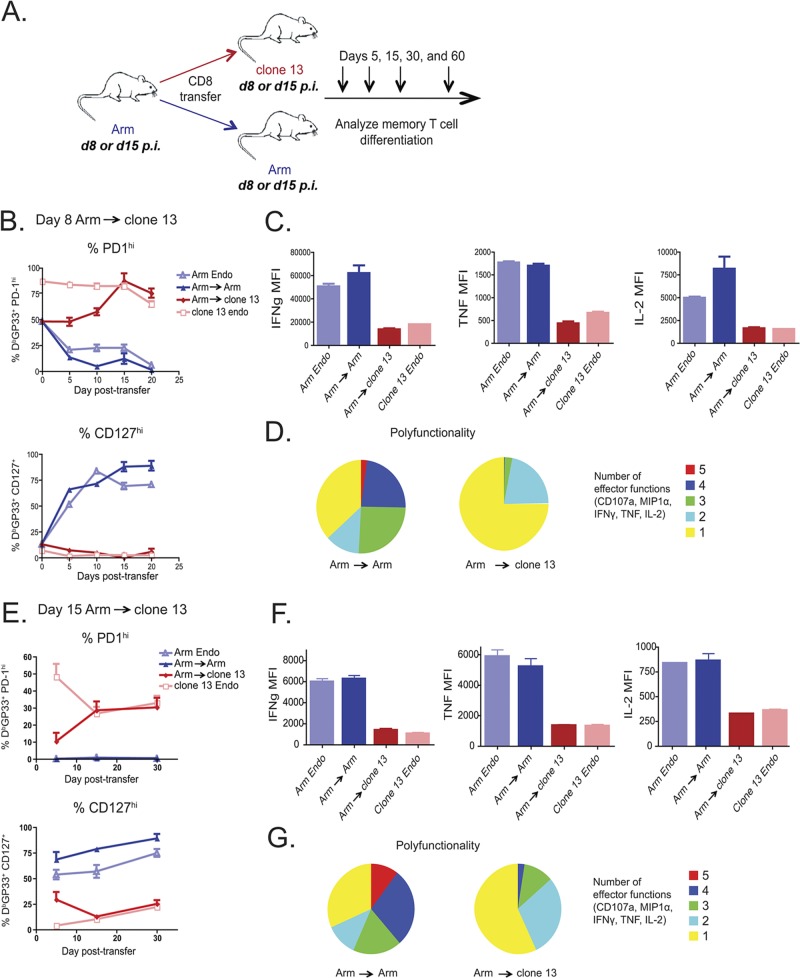
The results described above suggest considerable developmental plasticity in virus-specific CD8 T cells early during chronic infection. Fully developed memory CD8 T cells generated following acute infection can either completely control chronic infection (1, 17, 19, 27) or undergo physical deletion (37), depending on the number of memory CD8 T cells present. It is unclear, however, whether priming during acute infection prevents the development of exhaustion. To investigate this issue, CD8 T cells were purified at either day 8 or 15 p.i. with Arm and transferred into time point-matched clone 13- or Arm-infected congenic recipient mice (i.e., day 8 → day 8 and day 15 → day 15) to track the differentiation of the donor virus-specific CD8 T cells over time (Fig. 4A). LCMV Arm-primed CD8 T cells adoptively transferred to clone 13-infected recipient mice at day 8 p.i. upregulated PD-1 and remained CD127lo, while the same cells became PD1lo and CD127hi if transferred to Arm-infected recipient mice (Fig. 4B). This reprogramming occurred quickly, as day 8 Arm → clone 13 CD8 T cells expressed levels of PD-1 and CD127 similar to those of the endogenous clone 13 response by day 15 p.t. (Fig. 4B). At 2 months p.t., the day 8 Arm → clone 13 donor cells produced significantly lower levels of IFN-γ, TNF, and IL-2 (Fig. 4C) than the day 8 Arm → Arm donor cells. The ability of a cell to produce multiple cytokines is also correlated with T cell quality. As shown in Fig. 4D, the day 8 Arm → clone 13 donor cells not only produced lower levels of individual cytokines, but their polyfunctionality was also reduced. This decrease in cytokine production in the day 8 Arm → clone 13 donor CD8 T cells suggests that optimal priming alone does not prevent the development of exhaustion.
Fig 4.
Optimal priming does not prevent exhaustion of effector CD8 T cells transferred to a chronic infection. (A) Experimental design. Ly5.1+ CD8 T cells were purified from either day 8 or day 15 Arm-infected mice and adoptively transferred (day 8, ∼5 × 105 DbGP33+ CD8 T cells/recipient; day 15, ∼1 × 105 DbGP33+ CD8 T cells/recipient) to infection-matched clone 13- or Arm-infected recipient mice. Donor virus-specific CD8 T cells were examined on days 5, 15, 30, and 60 p.t. in recipient spleens. (B to D) Day 8 Arm → clone 13. (B) Percentage of Ly5.1+ DbGP33+ CD8 T cells expressing CD127 or high levels of PD-1 in the blood; (C) mean fluorescence of IFN-γ, IL-2, and TNF expressed by CD8 T cells restimulated ex vivo with the GP33 peptide on day 60 p.t.; (D) polyfunctionality of day 8 Arm → Arm and day 8 Arm → clone 13. Colors represent the number of cytokines (1 to 5) coproduced per cell. (E to G) Day 15 Arm → clone 13. (E) Percentage of Ly5.1+ DbGP33+ CD8 T cells expressing CD127 or high levels of PD-1 in the blood; (F) mean fluorescence of IFN-γ, IL-2, and TNF expressed by CD8 T cells restimulated ex vivo with the GP33 peptide on day 60 p.t.; (G) polyfunctionality of day 15 Arm → Arm and day 15 Arm → clone 13 displayed as in panel D. MFI, mean fluorescence intensity; MIP1α, macrophage inflammatory protein-1α.
Since fully formed memory CD8 T cells might not be susceptible to exhaustion (37), we next tested whether CD8 T cells at early stages of memory CD8 T cell differentiation following acute infection could become exhausted. Thus, CD8 T cells from day 15 Arm-infected donors were also adoptively transferred to clone 13-infected recipients at day 15 p.i. Again, by day 15 p.t., these donor cells already expressed levels of PD-1 and CD127 similar to those expressed by the endogenous exhausted virus-specific CD8 T cells (Fig. 4E). By day 60 p.t., the day 15 Arm → clone 13 donor CD8 T cells produced substantially lower levels of cytokine and were much less polyfunctional than the donor CD8 T cells transferred into Arm-infected recipient mice (Fig. 4F and G). Interestingly, 15 days of effector and memory cell differentiation in Arm-infected mice offered little protection from exhaustion for CD8 T cells transferred into clone 13-infected recipients. This observation contrasts with what we observed in the day 15 clone 13 → Arm transfer, where the clone 13 donor CD8 T cells could not become functional memory cells but were still able to survive and gain some memory-like characteristics (i.e., CD127 expression). In other words, these experiments suggest that there is greater plasticity at day 15 in CD8 T cells generated following acute infection, since these cells can become memory or exhausted, while the cells at day 15 of chronic infection cannot fully progress to a normal memory state.
Exhausted CD8 T cells arise from memory precursors.
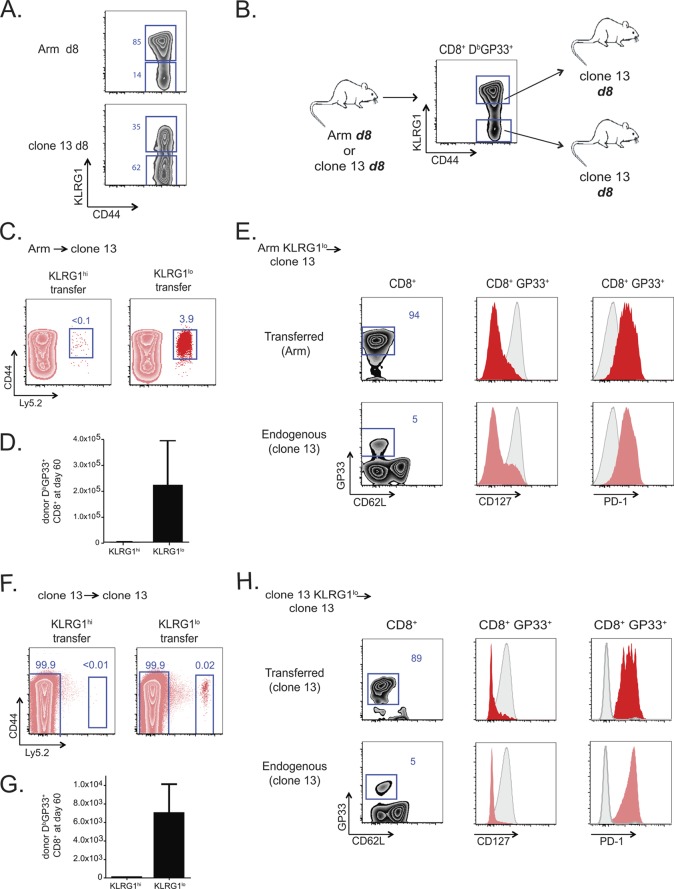
During acute infection, effector CD8 T cells that express CD127 but lack KLRG1 are precursors of long-lived memory CD8 T cells, while the CD127lo KLRG1hi subset of effector CD8 T cells has poor memory potential (18). The phenotypic heterogeneity for CD127 and KLRG1 found during acute infection is also observed during the first week of chronic infection. As shown in Fig. 5A, subsets defined by KLRG1 expression exist for virus-specific CD8 T cell populations at day 8 of either Arm or clone 13 infection. Using these defined effector subsets, we next investigated the lineage origin of exhausted CD8 T cells. Day 8 effector CD8 T cells from Arm- or clone 13-infected mice were sorted into KLRG1hi and KLRG1lo subsets, and the fate of each of the four subpopulations (Arm KLRG1hi, Arm KLRG1lo, clone 13 KLRG1hi, clone 13 KLRG1lo) was determined after adoptive transfer to mice infected 8 days earlier with clone 13 (Fig. 5B).
Fig 5.
Exhausted virus-specific CD8 T cells arise from the KLRG1lo memory precursor population of effector cells. (A) KLRG1 expression on day 8 p.i. with Arm and clone 13. (B) Experimental design. Ly5.2+ CD44hi DbGP33+ CD8 T cells from either day 8 Arm- or day 8 clone 13-infected donor mice were sorted into KLRG1hi and KLRG1lo populations. Equal numbers of virus-specific KLRG1hi- or KLRG1lo-sorted populations were adoptively transferred into day 8 clone 13-infected recipient mice and analyzed at 1 to 2 months p.t. Approximately 2.9 × 105 KLRG1hi or KLRG1lo DbGP33+ CD8 T cells were transferred to each recipient in the Arm → clone 13 experiment, and 1.4 × 105 cells were transferred in the clone 13 → clone 13 control experiment. (C to E) KLRG1hi and KLRG1lo CD8+ DbGP33+ T cells from day 8 Arm-infected mice adoptively transferred into day 8 clone 13-infected recipients. (C) Representative plots of the endogenous (Ly5.2-negative [Ly5.2−]) and KLRG1-sorted (Ly5.2+) donor populations at 2 months p.t.; (D) absolute number of each transferred population on day 60 p.t.; (E) representative plots from KLRG1lo recipient mice on day 60 p.t. The mean fluorescence of CD127 and PD-1 is shown in red or pink, with CD8+ CD44lo naïve T cells shown in the gray histograms. (F and G) KLRG1hi or KLRG1lo CD8+ DbGP33+ T cells from day 8 clone 13-infected mice adoptively transferred into day 8 clone 13-infected recipients. (F) Representative plots of the endogenous (Ly5.2−) and KLRG1-sorted (Ly5.2+) donor populations at 2 months p.t.; (G) absolute number of each transferred population on day 60 p.t. (H) Representative plots from KLRG1lo recipient mice on day 60 p.t. The mean fluorescence of CD127 and PD-1 is shown in red or pink, with CD8+ CD44lo naïve T cells shown in the gray histograms.
Equal numbers of KLRG1hi or KLRG1lo DbGP33-specific CD8 T cells were adoptively transferred into congenic day 8 clone 13-infected recipient mice. At 2 months p.t., few if any of the Arm-primed KLRG1hi donor cells were found in the clone 13-infected recipient mice, while the KLRG1lo donor cells were readily detected (Fig. 5C and D). The DbGP33 tetramer-positive donor cells derived from the KLRG1lo effector subset had by that time developed characteristic features of T cell exhaustion, including low CD127 and high PD-1 expression (Fig. 5E). This phenotype closely paralleled that of the endogenous DbGP33-specific response of the clone 13-infected recipient mice (Fig. 5E). The analogous experiment for clone 13-primed KLRG1hi and KLRG1lo effector CD8 T cell subsets yielded similar results (Fig. 5F to H). Only the KLRG1lo subset gave rise to a persisting population during chronic infection, and this KLRG1lo subset could give rise to the phenotypic characteristics observed in the endogenous exhausted CD8 T cell response. Thus, exhausted CD8 T cells arise from the same subset of effector CD8 T cells that gives rise to memory CD8 T cells.
DISCUSSION
Recent studies have examined the lineage origin and differentiation pathways that give rise to long-lived, functional, memory CD8 T cells following acute infections (14, 21, 42). During chronic infections, this pathway is substantially altered and virus-specific CD8 T cells instead become dysfunctional or exhausted. Despite recent work defining many characteristics of exhausted CD8 T cells, the lineage origin and developmental pathways that give rise to exhausted CD8 T cells are poorly understood. Here, we found that exhausted CD8 T cells arise from the same subpopulation of effector CD8 T cells that gives rise to functional memory CD8 T cells following acute infection, rather than the more terminally differentiated subset of effector CD8 T cells. Furthermore, we demonstrated that during the first weeks of chronic infection, the virus-specific CD8 T cell population retains lineage flexibility: these cells can revert to a functional memory CD8 T cell differentiation program if removed from chronic infection. Prolonged exposure to chronic infection, however, skews these cells down the developmental pathway of exhaustion, where they soon become irreversibly committed.
Previous studies demonstrated that virus-specific CD8 T cells isolated on day 5 of infection with LCMV clone 13 were not yet committed to exhaustion. Brooks et al. found that these day 5 clone 13-primed CD8 T cells remained functional if removed from infection (9). These data suggested that unlike in vitro anergy, CD8 T cell exhaustion is not a consequence of altered T cell priming. Our current work confirms and extends these observations in several ways. We first demonstrated that early during infection, virus-specific CD8 T cell populations retained considerable differentiation plasticity. Not only were CD8 T cells primed during what would become a chronic infection able to generate high-quality memory CD8 T cells, but priming during an acute infection did not prevent subsequent exhaustion if those CD8 T cells were then placed into a chronic infection. Together, these observations indicate that key signals governing the fate decisions of exhaustion versus memory can occur after the effector phase. By 2 weeks of chronic infection, virus-specific CD8 T cells had partially lost memory CD8 T cell potential, and by 1 month these cells were fully committed to an irreversible state of exhaustion and antigen-dependent maintenance. It was interesting that at day 15 following acute infection, virus-specific CD8 T cells could still develop into fully exhausted CD8 T cells. This observation suggests that more flexibility in differentiation potential is retained early after acute infection than during chronic infection. Together with the findings of recent studies indicating that fully formed primary memory CD8 T cells do not become exhausted during a new chronic infection (37), these observations suggest that large populations of preformed memory CD8 T cells have not yet developed by day 15 p.i., in agreement with other data (22). It is interesting to note that unlike primary memory CD8 T cells (37), secondary memory CD8 T cells can become exhausted (26). Perhaps the sensitivity to exhaustion is more tightly linked to the effector/effector memory state of differentiation than to central memory. However, our data indicate substantial flexibility in fate outcome for virus-specific CD8 T cells during the effector and contraction phases of the response.
A major question that emerges is whether the apparent differentiation plasticity observed for developing exhausted and memory CD8 T cells merely reflects preferential survival of different subpopulations of effector CD8 T cells. Indeed, the cellular origin of exhausted CD8 T cells remains unclear. During acute infection, memory CD8 T cells arise from a subpopulation of CD127hi KLRG1lo memory precursors, while another subpopulation of terminally differentiated (CD127lo KLRG1hi) effector CD8 T cells gradually dies off (18, 20). One possible model is that while memory CD8 T cells arise from the memory precursors, exhausted CD8 T cells could be the progeny of the KLRG1hi subset. However, our results indicate that exhausted CD8 T cells arise not from the KLRG1hi subset but from the same KLRG1lo population of memory precursors that can give rise to functional memory CD8 T cells. These observations have several important implications. First, they suggest that the differentiation flexibility observed for the CD8 T cells early in the response to acute or chronic infection does not simply reflect population dynamics and the selective survival of different previously defined subsets. Second, these data have implications for the relationship between senescence and exhaustion. Both senescent and exhausted CD8 T cells have poor proliferative potential, but it has been unclear whether senescence and exhaustion represented distinct or overlapping biological processes (3). Previous studies have indicated at least a partial lack of overlap in expression of markers associated with senescence and exhaustion, such as CD57 and PD-1 (5, 11, 33, 36). While in some human studies PD-1 and KLRG1 were found on the same population of virus-specific CD8 T cells (8), in mouse models, when PD-1 expression is very high, KLRG1 expression is low (41). The studies described here indicate that the terminally differentiated or senescent subset of effector CD8 T cells does not efficiently give rise to exhausted CD8 T cells. This observation suggests that terminal differentiation is not necessarily a precursor to exhaustion. However, whether the opposite is true or whether exhaustion and senescence can occur as divergent pathways of differentiation from a common precursor remains to be further explored.
There are potential therapeutic implications of the data presented in this study. Progressive loss of memory potential during chronic infections implies that early intervention during chronic infection might have a beneficial impact on the development of robust immunity to the pathogen. Indeed, early antiretroviral intervention during acute HIV infection leads to preservation of antiviral T cell responses (31, 32). Moreover, Alter et al. found that this highly active antiretroviral treatment-mediated preservation of antiviral immunity was gradually lost as treatment initiation was delayed into the chronic phase of infection (4). A number of other studies have demonstrated a beneficial antiviral effect of early drug treatment during hepatitis C virus (HCV) infection (12, 16, 34), though the impact on preservation of T cell immunity in these cases is not always clear. Our results now provide a potential explanation for these observations in terms of changes in the differentiation potential of virus-specific CD8 T cells at early versus later stages of chronic infection. Moreover, identification of a specific subpopulation of antiviral CD8 T cells early in the response as the precursor of both exhausted and memory CD8 T cells suggests that therapeutic targeting of this subset in combination with antivirals might prove highly synergistic. Indeed, IL-7 treatment during the early phases of LCMV clone 13 infection has a major beneficial impact (28). We would predict that this IL-7 effect could operate by helping to prevent the memory precursor population from becoming exhausted. The introduction of new, powerful anti-HCV antivirals (6, 24) should provide the opportunity to test some of these ideas in humans.
In conclusion, these studies demonstrate the progressive nature of CD8 T cell exhaustion and illustrate the considerable flexibility of differentiation that is present in developing exhausted or memory CD8 T cells. Fate commitment of CD8 T cells toward memory versus terminal differentiation can occur very early in the effector phase following an acute infection (21). However, our studies reinforce the notion that while a commitment toward a fate may be specified, this differentiation process remains malleable for a period of time. Ultimately, however, exhausted CD8 T cells become irreversibly committed, antigen dependent, and unable to revert to the alternate fate. Finally, we identify the cellular origin of exhausted CD8 T cells and demonstrate that exhausted and memory CD8 T cells arise from the same precursor population. These observations have relevance for the timing of antipathogen therapeutic interventions and also suggest cellular targets for immunotherapies during chronic infections.
ACKNOWLEDGMENTS
We acknowledge and thank Alexandra Doms for technical assistance and the members of the E. J. Wherry lab for input and helpful suggestions.
This work was supported by funding from the NIH (AI071309, AI083022, AI082630 to E.J.W.).
Footnotes
Published ahead of print 23 May 2012
REFERENCES
- 1. Ahmed R, Jamieson BD, Porter DD. 1987. Immune therapy of a persistent and disseminated viral infection. J. Virol. 61:3920–3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ahmed R, Salmi A, Butler LD, Chiller JM, Oldstone MB. 1984. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160:521–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akbar AN, Henson SM. 2011. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat. Rev. Immunol. 11:289–295 [DOI] [PubMed] [Google Scholar]
- 4. Alter G, et al. 2003. Longitudinal assessment of changes in HIV-specific effector activity in HIV-infected patients starting highly active antiretroviral therapy in primary infection. J. Immunol. 171:477–488 [DOI] [PubMed] [Google Scholar]
- 5. Appay V, Almeida JR, Sauce D, Autran B, Papagno L. 2007. Accelerated immune senescence and HIV-1 infection. Exp. Gerontol. 42:432–437 [DOI] [PubMed] [Google Scholar]
- 6. Asselah T. 27 March 2012. A revolution in HCV treatment with direct-acting antivirals: from non response to eradication. J. Hepatol. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 7. Barber DL, et al. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682–687 [DOI] [PubMed] [Google Scholar]
- 8. Bengsch B, et al. 2010. Coexpression of PD-1, 2B4, CD160 and KLRG1 on exhausted HCV-specific CD8+ T cells is linked to antigen recognition and T cell differentiation. PLoS Pathog. 6:e1000947 doi:10.1371/journal.ppat.1000947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brooks DG, McGavern DB, Oldstone MBA. 2006. Reprogramming of antiviral T cells prevents inactivation and restores T cell activity during persistent viral infection. J. Clin. Invest. 116:1675–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cui W, Kaech SM. 2010. Generation of effector CD8+ T cells and their conversion to memory T cells. Immunol. Rev. 236:151–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dagarag M, Ng H, Lubong R, Effros RB, Yang OO. 2003. Differential impairment of lytic and cytokine functions in senescent human immunodeficiency virus type 1-specific cytotoxic T lymphocytes. J. Virol. 77:3077–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dominguez S, et al. 2006. Efficacy of early treatment of acute hepatitis C infection with pegylated interferon and ribavirin in HIV-infected patients. AIDS 20:1157–1161 [DOI] [PubMed] [Google Scholar]
- 13. Fuller MJ, Zajac AJ. 2003. Ablation of CD8 and CD4 T cell responses by high viral loads. J. Immunol. 170:477–486 [DOI] [PubMed] [Google Scholar]
- 14. Harty JT, Badovinac VP. 2008. Shaping and reshaping CD8+ T-cell memory. Nat. Rev. Immunol. 8:107–119 [DOI] [PubMed] [Google Scholar]
- 15. Huster KM, et al. 2004. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc. Natl. Acad. Sci. U. S. A. 101:5610–5615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jaeckel E, et al. 2001. Treatment of acute hepatitis C with interferon alfa-2b. N. Engl. J. Med. 345:1452–1457 [DOI] [PubMed] [Google Scholar]
- 17. Jamieson BD, Butler LD, Ahmed R. 1987. Effective clearance of a persistent viral infection requires cooperation between virus-specific Lyt2+ T cells and nonspecific bone marrow-derived cells. J. Virol. 61:3930–3937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Joshi NS, et al. 2007. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity 27:281–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kaech SM, Hemby S, Kersh E, Ahmed R. 2002. Molecular and functional profiling of memory CD8 T cell differentiation. Cell 111:837–851 [DOI] [PubMed] [Google Scholar]
- 20. Kaech SM, et al. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4:1191–1198 [DOI] [PubMed] [Google Scholar]
- 21. Kaech SM, Wherry EJ. 2007. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity 27:393–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kaech SM, Wherry EJ, Ahmed R. 2002. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2:251–262 [DOI] [PubMed] [Google Scholar]
- 23. Kalia V, Sarkar S, Ahmed R. 2010. CD8 T-cell memory differentiation during acute and chronic viral infections. Adv. Exp. Med. Biol. 684:79–95 [DOI] [PubMed] [Google Scholar]
- 24. Lee LY, Tong CYW, Wong T, Wilkinson M. 2012. New therapies for chronic hepatitis C infection: a systematic review of evidence from clinical trials. Int. J. Clin. Pract. 66:342–355 [DOI] [PubMed] [Google Scholar]
- 25. Mescher MF, et al. 2006. Signals required for programming effector and memory development by CD8+ T cells. Immunol. Rev. 211:81–92 [DOI] [PubMed] [Google Scholar]
- 26. Nolz JC, Harty JT. 2011. Protective capacity of memory CD8+ T cells is dictated by antigen exposure history and nature of the infection. Immunity 34:781–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Oldstone MB, Blount P, Southern PJ, Lampert PW. 1986. Cytoimmunotherapy for persistent virus infection reveals a unique clearance pattern from the central nervous system. Nature 321:239–243 [DOI] [PubMed] [Google Scholar]
- 28. Pellegrini M, et al. 2011. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell 144:601–613 [DOI] [PubMed] [Google Scholar]
- 29. Redmond WL, Hernández J, Sherman LA. 2003. Deletion of naive CD8 T cells requires persistent antigen and is not programmed by an initial signal from the tolerogenic APC. J. Immunol. 171:6349–6354 [DOI] [PubMed] [Google Scholar]
- 30. Redmond WL, Sherman LA. 2005. Peripheral tolerance of CD8 T lymphocytes. Immunity 22:275–284 [DOI] [PubMed] [Google Scholar]
- 31. Rosenberg ES, et al. 2000. Immune control of HIV-1 after early treatment of acute infection. Nature 407:523–526 [DOI] [PubMed] [Google Scholar]
- 32. Rosenberg ES, et al. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278:1447–1450 [DOI] [PubMed] [Google Scholar]
- 33. Sadagopal S, et al. 2010. Enhanced PD-1 expression by T cells in cerebrospinal fluid does not reflect functional exhaustion during chronic human immunodeficiency virus type 1 infection. J. Virol. 84:131–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Santantonio T, Fasano M, Sinisi E. 2005. Efficacy of a 24-week course of PEG-interferon α-2b monotherapy in patients with acute hepatitis C after failure of spontaneous clearance. J. Hepatol. 42:329–333 [DOI] [PubMed] [Google Scholar]
- 35. Shin H, Blackburn SD, Blattman JN, Wherry EJ. 2007. Viral antigen and extensive division maintain virus-specific CD8 T cells during chronic infection. J. Exp. Med. 204:941–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Voehringer D, et al. 2001. Viral infections induce abundant numbers of senescent CD8 T cells. J. Immunol. 167:4838–4843 [DOI] [PubMed] [Google Scholar]
- 37. West EE, et al. 2011. Tight regulation of memory CD8(+) T cells limits their effectiveness during sustained high viral load. Immunity 35:285–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wherry EJ. 2011. T cell exhaustion. Nat. Immunol. 131:492–499 [DOI] [PubMed] [Google Scholar]
- 39. Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. 2004. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc. Natl. Acad. Sci. U. S. A. 101:16004–16009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R, Ahmed R. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911–4927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wherry EJ, et al. 2007. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27:670–684 [DOI] [PubMed] [Google Scholar]
- 42. Williams MA, Bevan MJ. 2007. Effector and memory CTL differentiation. Annu. Rev. Immunol. 25:171–192 [DOI] [PubMed] [Google Scholar]
- 43. Zajac AJ, et al. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]