Abstract
Hantaviruses primarily infect endothelial cells (ECs) and nonlytically cause vascular changes that result in hemorrhagic fever with renal syndrome (HFRS) and hantavirus pulmonary syndrome (HPS). Acute pulmonary edema during HPS may be caused by capillary leakage and failure of lymphatic vessels to clear fluids. Uniquely regulated lymphatic ECs (LECs) control fluid clearance, although roles for lymphatics in hantavirus disease remain undetermined. Here we report that hantaviruses productively infect LECs and that LEC infection by HPS causing Andes virus (ANDV) and HFRS causing Hantaan virus (HTNV) are inhibited by αvβ3 integrin antibodies. Although αvβ3 integrins regulate permeabilizing responses directed by vascular endothelial growth factor receptor 2 (VEGFR2), we found that only ANDV-infected LECs were hyperpermeabilized by the addition of VEGF-A. However, VEGF-C activation of LEC-specific VEGFR3 receptors blocked ANDV- and VEGF-A-induced LEC permeability. In addition, ∼75% of ANDV-infected LECs became viable mononuclear giant cells, >4 times larger than normal, in response to VEGF-A. Giant cells are associated with constitutive mammalian target of rapamycin (mTOR) activation, and we found that both giant LECs and LEC permeability were sensitive to rapamycin, an mTOR inhibitor, and VEGF-C addition. These findings indicate that ANDV uniquely alters VEGFR2-mTOR signaling responses of LECs, resulting in giant cell and LEC permeability responses. This suggests that ANDV infection alters normal LEC and lymphatic vessel functions which may contribute to edematous fluid accumulation during HPS. Moreover, the ability of VEGF-C and rapamycin to normalize LEC responses suggests a potential therapeutic approach for reducing pulmonary edema and the severity of HPS following ANDV infection.
INTRODUCTION
Hantaviruses predominantly infect endothelial cells (ECs) which line vessels and nonlytically cause 2 vascular diseases: hemorrhagic fever with renal syndrome (HFRS) and hantavirus pulmonary syndrome (HPS) (48, 51, 59, 85, 86). Andes virus (ANDV) causes HPS, resulting in acute pulmonary edema and respiratory insufficiency (12, 14, 18, 22, 36, 47, 53, 59, 62, 86). The means by which hantaviruses cause vascular leakage and edema are likely to be multifactorial in nature, and mechanisms by which hantaviruses alter fluid barrier properties of the vasculature are still being discovered (28, 29, 34, 35, 37, 45, 63, 70, 74). Tissue and organ edema are prominent findings in hantavirus patients, and blood vessel ECs (BECs) form a primary fluid barrier that normally restricts fluid egress into tissues and permits blood and fluid recirculation (1, 19, 80). However, a discrete lymphatic vessel network plays a fundamental role in clearing fluid from tissues, and edema may also result from reduced lymphatic vessel function (5, 44, 73).
ANDV (HPS) and Hantaan virus (HTNV; HFRS) infection of BECs alters normal EC fluid barrier functions. Pathogenic hantaviruses bind and inactivate αvβ3 receptors on capillary endothelial cells (27, 31, 65), and days after infection, hantaviruses inhibit β3 integrin responses and cause BEC hyperpermeability in response to vascular endothelial growth factor A (VEGF-A) (28, 29, 34, 35, 56, 65). VEGF-A was originally found as a vascular permeability factor that potently causes localized vascular leakage and edema (19–21). β3 Integrins normally regulate VEGF-A directed permeability by forming an immunoprecipitable complex with VEGF receptor 2 (VEGFR2) (7), and knocking out β3 or antagonizing αvβ3 functions enhances VEGFR2-directed signaling responses and BEC permeability (68, 82).
VEGF-A is induced by hypoxia and causes high-altitude-induced pulmonary edema (6, 19, 23, 58, 77). VEGF-A induces the dissociation of VE-cadherin from interendothelial adherens junctions via a VEGFR2-Src-VE-cadherin signaling pathway and thereby regulates the primary fluid barrier of the endothelium (19, 24, 25, 49). HPS patients are acutely hypoxic (36, 39, 59, 62, 79), suggesting a link between pulmonary edema during HPS and enhanced endothelial cell VEGF-A responses (12, 15, 16, 38, 57, 64, 76). In fact, both HTNV and ANDV enhance VEGF-A-directed permeability responses, and inhibitors that antagonize this pathway block the hyperpermeability of hantavirus-infected BECs (28, 29, 34, 35, 63). Collectively, these findings tie altered VEGF-A responses following hantavirus infection to edema observed in HPS and HFRS patients.
Lymphatic vessels are lined by uniquely regulated lymphatic ECs (LECs) and lack pericytes, smooth muscle cells, and a basal membrane (4, 5, 9, 10, 73). Lymphatic vessels separate lymph from the interstitial space and normally drain fluids from tissues in order to prevent edema (9, 10, 73). Pulmonary lymphatic vessels play a fundamental role in providing a moist but relatively dry environment within the lung that facilitates efficient gas exchange (73). Lymphatic system dysfunction is a known cause of lymphedema which, in contrast to BECs, is regulated by both VEGF-A and VEGF-C responses (4, 9, 10, 17, 33, 44, 52). Only LECs express VEGFR3 receptors which specifically respond to VEGF-C effectors, and VEGFR3 forms heterodimeric complexes with VEGFR2 that provide for novel VEGF-A/VEGF-C responses of LECs (2, 5, 73). VEGF-C activation of LECs reportedly reduces tissue edema while mutations in VEGFR3 or inhibiting VEGFR3 responses are causes of lymphedema (4, 9, 10).
Currently there is little understanding of hantavirus interactions with LECs or regulation of lymphatic vessel fluid clearance functions. In this study, we demonstrate that pathogenic ANDV and HTNV as well as nonpathogenic Tula virus (TULV) productively infect LECs. We found that pathogenic hantavirus infection of LECs was specifically inhibited by antibodies to αvβ3 integrins. However, only ANDV infection enhanced LEC permeability and caused the formation of giant LECs in response to VEGF-A. Furthermore, the addition of VEGF-C or rapamycin, which inhibit discrete VEGFR2 signaling targets, blocked giant cell formation and LEC permeability. These findings indicate that the HPS-associated ANDV uniquely alters pulmonary LEC responses that control lymphatic vessel fluid clearance functions. Our results further suggest that VEGF-C and rapamycin are potential ANDV therapeutics that may enhance normal lymphatic vessel functions and fluid clearance during HPS.
MATERIALS AND METHODS
Cells and virus.
Vero E6 cells (ATCC CRL-1586) were grown in Dulbecco's modified Eagle medium (DMEM) containing 10% fetal calf serum (FCS; Sigma), penicillin (100 μg/ml), streptomycin sulfate (100 μg/ml), and amphotericin B (50 μg/ml) (GIBCO). Human lung lymphatic microvascular endothelial cells (HMVEC-LLy) were purchased from Clonetics and grown in endothelial growth medium-2MV (EGM-2MV; Lonza) supplemented with gentamicin (50 μg/ml), amphotericin B (50 μg/ml), and 10% FCS (Sigma). ANDV (CHI-7913) (56), HTNV (76-118), and nonpathogenic TULV (Tula/Moravia/MA 5302V/94) were cultivated in a biosafety level 3 (BSL3) facility as described previously (29) and determined to be free of mycoplasma (Roche). LEC and HMVEC-L monolayers were ANDV, HTNV, or TULV infected at a multiplicity of infection (MOI) of 0.5 or mock infected. Viral titers were determined by focus assay after immunoperoxidase staining of hantavirus nucleocapsid protein within cells as described previously (31, 32).
Reagents and antibodies.
Vitronectin was obtained from Chemicon, and human VEGF-A and fluorescein isothiocyanate (FITC)-dextran (40 kDa) were from Sigma. Recombinant human VEGF-C was from R&D Systems. Ang-1 (human) was obtained from Alexis Biochemicals. Rapamycin was obtained from Santa Cruz Biotechnology, and Src family kinase inhibitor dasatinib {Sprycel; N-(2-chloro-6-methylphenyl)-2-[[6-[4-(2-hydroxyethyl)-1-piperazinyl]-2-methyl-4-pyrimidinyl]amino]-5-thiazole carboxamide monohydrate} (35, 75) was purchased from Selleck Chemicals. Blocking antibodies to the α2 (AB 1936), α5β1 (AB1950), and αvβ3 (AB1976, LM609) integrin subunits were purchased from Chemicon. Goat anti-rabbit IgG-horseradish peroxidase (HRP) conjugate was from Kirkegaard & Perry Laboratories, Inc.
Rapamycin and dasatinib were evaluated for LEC cytotoxicity by trypan blue exclusion, and inhibitor concentrations used were based on prior studies of drug cytotoxicity (13, 41, 50, 71, 84). Briefly, serially diluted inhibitors were added to LECs, and after 24 h, 0.4% trypan blue was added to cells. Cell viability was determined by quantifying the percentage of cells excluding trypan blue. Concentrations of inhibitors resulting in <5% change in cell viability were used as maximum inhibitory concentrations in experiments.
Immunoperoxidase staining of hantavirus infected cells and giant cells.
In order to monitor hantavirus infection, rabbit polyclonal anti-nucleocapsid serum directed against the NY-1V nucleocapsid protein was used to detect ANDV-, HTNV-, and TULV-infected cells as described previously (31). Briefly, infected lymphatic endothelial cell monolayers were fixed with 100% methanol and incubated with anti-nucleocapsid serum (1:5,000) followed by goat anti-rabbit IgG-HRP secondary antibody (1:5,000; Amersham Biosciences). Nucleocapsid protein-expressing cells were identified by immunoperoxidase staining using 3-amino-9-ethylcarbazole (0.026% in 0.1 M sodium acetate [pH 5.2] and 0.03% H2O2). Cells >3 times normal LEC size were considered to be giant cells. The number of infected cells (10 fields, 1,500 cells in each duplicate well) and giant cells were quantitated by microscopy using NIH Image (31).
Antibody inhibited infection of LECs.
LECs were pretreated with antibodies for 1 h at 4°C. Antibodies (20 μg/ml to 5 μg/ml) were preadsorbed to cells in 50 μl of EGM-2MV with 2% FCS in duplicate wells of a 96-well plate. The monolayer was washed three times with phosphate-buffered saline (PBS), and ∼200 focus-forming units (FFU) of hantavirus was adsorbed to monolayers for l hour at 37°C. Unbound virus was removed, and the monolayer was washed three times and incubated for 24 h at 37°C prior to methanol fixation, immunoperoxidase staining (as described above), and quantitative analysis of infected cell foci.
Endothelial cell permeability assay.
A previously described EC permeability assay was used to assess hantavirus-induced permeability (28, 29, 34). Briefly, human LECs were plated on Costar Transwell plates (3-μm pores; Corning) and confluent monolayers were infected in triplicate with pathogenic ANDV or HTNV or nonpathogenic TULV at an MOI of 0.5. Three days postinfection, cells were starved overnight with basal EBM-2 with 0.5% bovine serum albumin. FITC-dextran (0.5 mg/ml) was added to the upper chamber in the presence or absence of VEGF-A (100 ng/ml) or VEGF-C (100 ng/ml) as described previously (29, 35). Where indicated, cells were treated with angiopoietin-1 (Ang-1; 50 ng/ml) or dasatinib (5 ng/ml) as described previously (29, 35) or with rapamycin (20 ng/ml, added 1 h prior to VEGF-A) for 1 h at 37°C prior to the addition of FITC-dextran. Monolayer permeability was evaluated by quantitating FITC-dextran in the lower chamber using a using a BioTek FLx800 fluorimeter (490-nm excitation, 530-nm emission) (28, 29, 34).
Statistical analysis.
The results are derived from two to five independent experiments and presented as the mean ± standard error of the mean (SEM) with P values of <0.05 considered to be significant. Multiple group comparisons were made by one-way analysis of variance (ANOVA). Two-way comparisons were performed by two-tailed, impaired Student's t test. All analyses were performed using GraphPad Prism software version 5.0.
RESULTS
Hantavirus infection of lymphatic ECs.
There is little understanding of lymphatic involvement in HPS or HFRS; however, hantaviruses cause edema and clearance of edematous fluid is regulated by lymphatic vessels and their LEC lining (73). Nucleocapsid protein is present in a reticular pattern within lymph nodes of HPS patients (86), suggesting a potential role for hantaviruses to infect LECs and alter normal lymphatic vessel functions. Here we investigated the ability of ANDV (HPS), HTNV (HFRS), and TULV (nonpathogenic) to infect LECs. We found that LECs were infected by all 3 hantaviruses tested and resulted in a similar number of nucleocapsid protein expressing LECs at 24 h postinfection. Hantavirus infection of LECs was productive, resulting in a maximal titer of 3 × 104 FFU/ml at 3 to 4 days postinfection for ANDV (Fig. 1). However, ANDV replication in LECs was more rapid and resulted in 1- to 2-log-higher titers than HTNV or TULV (Fig. 1). In comparison to infection of human microvascular pulmonary endothelial cells, maximum hantavirus titers in LECs were ∼1 to 2 logs lower (29). These results indicate that LECs are targets of hantavirus infection and further suggest the potential for hantaviruses to alter normal LEC functions.
Fig 1.
Hantavirus replication in LECs. Confluent LECs were infected in duplicate with pathogenic ANDV or HTNV or nonpathogenic TULV at a multiplicity of infection (MOI) of 0.5 or mock infected. Viral titers in the supernatant of infected LECs were detected by infecting Vero E6 cells with serial 10-fold dilution of supernatants collected from 1 to 5 days postinfection. One-day postinfection cells were immunoperoxidase stained for N protein, and the number of infected cells was quantitated. Data represents the results of three independent experiments (**, P < 0.01).
Pathogenic hantaviruses attach to human capillary endothelial cells by binding to inactive αvβ3 integrin conformers (65) while infection by nonpathogenic TULV is specifically inhibited by antibodies to α5β1 integrins (27, 31). Here we investigated whether integrins similarly direct hantavirus attachment to LECs. Pretreating LECs with antibody to αvβ3 specifically inhibited ANDV and HTNV (but not TULV) infection of LECs in a concentration-dependent manner, while blocking antibodies to α5β1 or α2 integrins had no effect on ANDV and HTNV infection of LECs (Fig. 2). In contrast, nonpathogenic TULV infection of LECs was inhibited by antibody to α5β1 but not antibodies to α2 or αvβ3 integrins (Fig. 2). These data strongly suggest that pathogenic ANDV and HTNV infection of LECs is mediated by αvβ3 integrins.
Fig 2.
Hantavirus infection of LECs is inhibited by integrin-specific antibodies. Duplicate wells of LECs were pretreated with blocking antibodies to αvβ3, α5β1, or α2 integrins (1 h at 4°C) prior to infection with 200 FFU of ANDV, HTNV, or TULV (1 h adsorption at 37°C). After washing, LECs were grown for 24 h, fixed, and immunoperoxidase stained for N protein as described previously (31). Infected foci were quantitated and presented as a function of mock-pretreated controls. Results were reproduced in four separate experiments.
ANDV infection results in unique LEC responses.
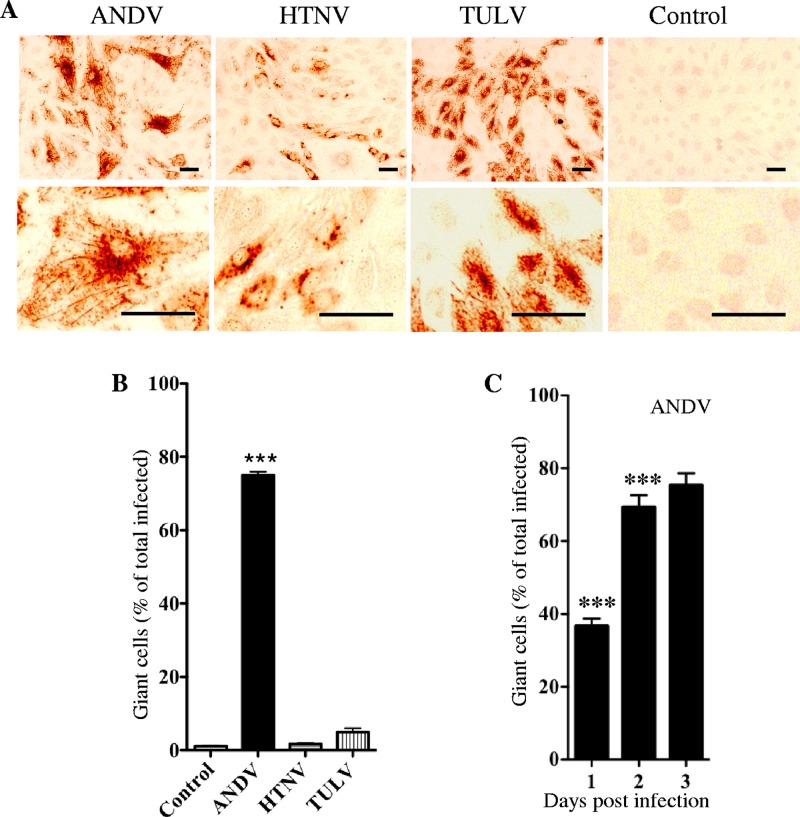
During the course of these studies, we noticed that only ANDV infection of LECs resulted in the appearance of giant LECs (mean, 245 ± 5.5 μm) that were ∼4 to 5 times the size of normal LECs (mean, 54.55 ± 5.6 μm; P < 0.0001) (Fig. 3A). Giant cells were predominantly mononuclear and viable by trypan blue exclusion. Three days postinfection, ∼75% of ANDV-infected LECs were giant cells while giant LECs were nearly absent in HTNV, TULV, or mock-infected monolayers (Fig. 3B). Although infected LECs uniformly express ANDV nucleocapsid protein at 24 h postinfection, the percentage of ANDV-infected giant LECs increased from 36% to 76% from 1 to 3 days postinfection, respectively (Fig. 3C). These findings indicate that ANDV infection selectively causes the formation of giant cells and suggest that ANDV dramatically alters normal LEC functions.
Fig 3.
Giant lymphatic endothelial cells in response to ANDV infection. (A) LECs were infected with indicated hantaviruses (MOI of 0.5) or mock infected. Three days postinfection, LECs were methanol fixed, immunoperoxidase stained for N protein (31), and visualized by light microscopy (×100; bar = 100 μm). (B) Hantavirus-infected giant LECs (∼4× normal size) were measured and quantitated by NIH Image and expressed as a percentage of total infected cells. Data represents results of six independent experiments (***, P < 0.001). (C) Quantitation of ANDV-infected giant LECs as a percentage of infected LECs from 1 to 3 days postinfection.
Altered VEGF-A and VEGF-C responses of hantavirus-infected LECs.
The role of αvβ3 integrins in regulating VEGFR2 responses suggested that pathogenic hantaviruses may alter normal LEC responses to VEGF-A (7, 66, 68, 82). LECs uniquely express VEGFR3 receptors and are regulated by both VEGF-C/VEGFR3 and VEGF-A/VEGFR2 responses elicited by VEGFR2/VEGFR3 heterodimers (5, 9, 10, 17, 73). We initially determined whether hantavirus infection of LECs alters the permeability of LECs in response to VEGF-A by monitoring LEC monolayer permeability to FITC-dextran (29). We found that only ANDV infection enhanced LEC permeability in response to VEGF-A (∼3-fold) compared to VEGF-A-treated uninfected LECs or LECs infected with HTNV or TULV (Fig. 4A). We observed no increase in LEC permeability for any hantaviruses in response to VEGF-C. However, we found that VEGF-C addition nearly completely inhibited the enhanced permeability of ANDV-infected LECs directed by VEGF-A (Fig. 4B). These findings indicate that ANDV induces specific changes in VEGF-A-directed LEC responses that are blocked by the VEGF-C stimulation of LEC-specific VEGFR3 receptors.
Fig 4.
LEC permeability responses to ANDV infection. (A) LECs were plated on vitronectin-coated Transwell inserts and were ANDV, HTNV, or TULV infected at an MOI of 0.5 in triplicate or mock infected. Three days postinfection, FITC-dextran was added to media in the upper chamber in the presence or absence of VEGF-A (100 ng/ml), and 3 h later, FITC-dextran in the lower chamber was quantitated by fluorimetry as described previously (29). Results are expressed as fold increases in permeability to FITC-dextran over VEGF-A-treated mock-infected controls. (B) Permeability of ANDV-infected cells to FITC-dextran was determined as in panel A above after VEGF-A or VEGF-C (100 ng/ml) was added as indicated. (C) LECs were infected with ANDV, and at 3 days postinfection, VEGF-A or VEGF-C was added as indicated (100 ng/ml). Three hours later, the presence of ANDV-infected giant LECs was quantitated and presented as a percentage of mock-treated ANDV-infected LECs. Data represents results of four independent experiments (**, P < 0.01 versus control).
The ability of VEGF-C to reduce LEC monolayer permeability suggests that VEGF-C may similarly regulate ANDV-induced giant LEC responses. ANDV-infected LECs were treated with VEGF-A, VEGF-C, or both growth factors as described above, and the presence of giant LECs was quantitated. We observed a 6-fold increase in the number of giant LECs after adding VEGF-A to the media (Fig. 4C). In contrast, VEGF-C addition dramatically reduced VEGF-A-directed giant cell responses following ANDV infection (Fig. 4C). The addition of VEGF-C alone also inhibited the basal level of giant LEC responses to ANDV infection by 2-fold compared to unstimulated ANDV-infected controls (Fig. 4C). These findings indicate that VEGF-C inhibits ANDV-induced hyperpermeability and giant cell responses. These findings suggest an association between the formation of giant LECs following ANDV infection and ANDV-enhanced LEC monolayer permeability in response to VEGF-A.
Role of rapamycin as a potential inhibitor of ANDV-induced permeability.
Similar to ANDV-directed LEC responses, continuous activation of the mammalian target of rapamycin (mTOR) causes giant cell formation and is associated with increased microvascular permeability in response to VEGFR2 (11, 43, 54, 61, 83, 84). Rapamycin inhibition of mTOR reduces microvascular hyperpermeability and blocks giant cell formation (54, 84). The similarity of ANDV and mTOR responses to VEGFR2 suggested analyzing rapamycin as a potential inhibitor of ANDV-induced LEC permeability and giant cell formation. Here we compared the ability of rapamycin to inhibit ANDV-induced permeability and giant cell formation to inhibitory responses of VEGF-C, the VEGFR2-Src pathway inhibitor, dasatinib, and angiopoietin-1 (Ang-1), a dominant inhibitor of VEGFR2-directed permeability (26, 35, 71, 78). We found that rapamycin reduced the number of ANDV-infected giant LECs 2- to 3-fold compared to LECs treated with VEGF-A alone (Fig. 5A) and similar to the addition of dasatinib, Ang-1, and VEGF-C. In parallel experiments, ANDV-infected LECs were hyperpermeabilized by the addition of VEGF-A; however, the addition of rapamycin, as well as VEGF-C, Ang-1, and dasatinib, dramatically decreased LEC permeability to control levels (Fig. 5B). These findings demonstrate that rapamycin inhibition of mTOR, a downstream consequence of VEGFR2 signaling, is sufficient to inhibit giant cell formation and LEC permeability responses. Additional upstream inhibitors of VEGFR2 responses (VEGF-C and dasatinib) similarly blocked giant cell and permeability responses, as did Ang-1, which inhibits VEGFR2 responses by activating discrete Tie-2 receptors. These findings suggest several potential ANDV therapeutics that act on discrete signaling targets as inhibitors of LEC giant cell formation and as a means of restoring LEC fluid barrier and clearance functions.
Fig 5.
Regulation of giant cell and LEC permeability. ANDV-infected giant LECs (A) and LEC permeability (B) were assayed as described in Fig. 4 and as described previously (31) in the presence or absence of VEGF-A (100 ng/ml), VEGF-C (100 ng/ml), dasatinib (5 nM), Ang-1 (50 ng/ml), or rapamycin (20 ng/ml). Data are derived from two independent experiments performed in triplicate with comparable results (**, P < 0.01 versus control).
DISCUSSION
ANDV causes HPS, an acute edematous syndrome with bilateral pulmonary infiltrates leading to respiratory distress and a 35 to 40% mortality rate (14, 18, 59, 72, 86). Pulmonary edema can result from vascular leakage or failure to clear alveolar fluid. Gas exchange is reduced by pulmonary edema, and normally, alveoli are kept relatively dry by an elaborate pulmonary lymphatic drainage system (73). Discrete endothelial cell types line capillary (BECs) and lymphatic (LECs) vessels and regulate the edematous accumulation of interstitial fluid. Hantaviruses alter functions of BECs following infection, leading to their increased permeability in response to VEGF-A. However, LECs are uniquely controlled by VEGF-C through VEGFR3 as well as VEGF-A through VEGFR2, and on LECs, these discrete VEGFRs form heterodimeric complexes that are both VEGF-A and VEGF-C regulated (10, 17, 33, 52). Hantavirus infection of LECs has not been described but has the potential to alter lymphatic vessel fluid clearance and play a fundamental role in tissue edema following hantavirus infection. Here we demonstrate that only the HPS causing ANDV alters LEC responses which regulate lymphatic vessel functions and, as a result, have the potential to contribute to pulmonary edema.
Our findings indicate that ANDV (HPS), HTNV (HFRS), and TULV (nonpathogenic) infect and successfully replicate in human pulmonary LECs. Infection of LECs by pathogenic ANDV and HTNV was blocked by antibodies to αvβ3, while antibodies to α5β1 integrins inhibited infection of LECs by nonpathogenic TULV. The use of LEC αvβ3 integrins by only pathogenic hantaviruses is consistent with the inhibition of αvβ3 integrin functions on BECs and the enhanced VEGFR2-directed permeability of BECs days after hantavirus infection (28, 30, 34, 65). αvβ3 forms an ectodomain complex with VEGFR2 that normally restricts VEGF-A/VEGFR2-directed BEC permeability (7, 67, 68, 82). However, little is currently known about the role of αvβ3 in regulating LEC VEGFR2/VEGFR3 functions (3, 8, 10, 17).
On LECs, VEGF-A activation reportedly inhibits VEGFR3, presumably through VEGFR2-VEGFR3 heterodimers (17, 33). Consistent with this finding, VEGFR3 activation regulates VEGFR2 responses and is associated with reduced tissue edema, while inhibiting VEGFR3 signaling is linked to congenital lymphedema (9, 10, 44). Although blocking antibodies to αvβ3 inhibited both HTNV and ANDV infection of LECs, we found that only HPS-associated ANDV enhanced LEC permeability in response to VEGF-A. Furthermore, VEGF-C failed to increase the permeability of hantavirus infected LECs. In fact, addition of VEGF-C and VEGF-A to ANDV-infected LECs inhibited LEC permeability responses directed by VEGF-A alone. This indicates that ANDV uniquely alters VEGFR2 responses of LECs and suggests novel ANDV interactions with LEC receptor complexes.
VEGFR2 signaling responses regulate vascular and lymphatic vessel functions (8, 20, 52) that are linked to the hantavirus disease process. VEGF-A acts on both BECs and LECs and uniquely regulates inter-LEC adherence through VE-cadherin and proliferative responses. In fact, acute hypoxia, which is a fundamental finding in HPS patients, induces VEGF-A production, and VEGF-A further induces the production of the hypoxia responsive transcription factor HIF1α, forming an amplification loop (23, 43, 50, 77) that causes high-altitude-induced edema (6, 23, 77). Although LEC responses of VEGFR2/VEGFR3 complexes are poorly understood (9, 10, 33, 44), our findings indicate that ANDV-infected LECs uniquely respond to VEGF-A and that this response is regulated by VEGF-C. Enhanced VEGFR2 responses of ANDV-infected cells may explain the hyperpermeability of ANDV-infected LECs, while LEC VEGFR2/VEGFR3 complexes rationalize their VEGF-C sensitivity.
Our results reveal a further connection between ANDV infection of LECs and lymphatic vessel dysfunction during HPS. In contrast to HTNV or TULV, ANDV infection of LECs resulted in the formation of giant LECs (150 to 250 μm) that are ∼4 to 5 times normal LEC size. ANDV-induced giant LECs provide a visual marker of altered LEC functions that may contribute to lymphatic vessel fluid clearance deficits in HPS. ANDV-induced giant LECs are analogous to giant ECs observed in response to a proliferation inhibitor (60), and giant cells are also a consequence of mutations in the tuberous sclerosis complex (TSC) that lead to constitutive mTOR activation (11, 54, 69). Similar to TSC-directed giant cell formation (11, 13, 69, 84), rapamycin inhibition of mTOR was found to block the formation of giant LECs and reduce LEC permeability following ANDV infection. Rapamycin inhibits VEGFR2-Akt-mTOR signaling by blocking downstream mTOR responses, and mTOR also regulates hypoxia and HIF1α-directed cellular responses (13, 41–43, 83, 84). Addition of VEGF-C, dasatinib, and angiopoietin-1 also inhibited giant cell formation and permeability responses of LECs consistent with their action at upstream points of VEGFR2-directed mTOR activation pathways (26, 35, 71). These findings are consistent with mTOR activation contributing to LEC dysfunction following ANDV infection and suggest a potential role for VEGFR2-directed mTOR signaling responses in HPS pulmonary edema. Clearly, further studies of VEGF-C and mTOR signaling pathways are needed to understand the mechanism by which ANDV uniquely affects these key LEC regulatory responses.
Human lymphatic endothelial cells from HPS patients contain hantavirus antigens (86), suggesting that in vitro LEC responses observed here may also contribute to HPS in vivo. However, the mechanisms by which hantaviruses cause acute pulmonary edema in HPS patients remains to be defined. Immune responses have been suggested to be involved in HPS pathogenesis (45), while the VEGF-directed hyperpermeability of hantavirus-infected human endothelial cells (29, 34, 35, 74) suggests roles for hypoxia-derived VEGF (12, 23, 57, 64) as a mechanism of pulmonary edema in HPS patients. A recent report demonstrates that depletion of T cells in ANDV-infected Syrian hamsters has no effect on the onset, symptoms, or severity of lethal pulmonary edema, suggesting that T cell responses may not be determinants of pathogenesis (37). However, another report on ANDV-infected Syrian hamsters suggests that the low level activation of several proinflammatory genes and Th1/Th2 responses in the lungs may contribute to pathogenesis (70). Although this study indicates only small systemic VEGF level changes (70), VEGF acts locally and induces permeability within 0.5 mm of its release (21) but does not act to systemically permeabilize the vasculature. Although pulmonary induction of VEGF and localized VEGF responses were not shown (70), Syrian hamsters, like HPS patients, are acutely hypoxic and in respiratory distress (12, 40, 81), suggesting the pulmonary induction of VEGF as contributing to HPS (18, 36, 59, 86) and HPS-like diseases (40). Consistent with localized permeability, HPS patient pleural fluids are initially transudative in nature (12); however, edemagenic constituents within patient edema fluids remain unstudied. The fact that hypoxic induction of VEGF alone is sufficient to cause high-altitude-induced pulmonary edema further suggests a role for hypoxia and VEGF responses in HPS (55, 57, 64). Hantavirus infection of patient LECs, which control fluid clearance from the lung, and the unique VEGF regulation of LECs further suggest the potential for additional edemagenic mechanisms to contribute to HPS in hypoxic patients.
Collectively, our findings demonstrate that ANDV-infected LECs provide additional sites for hantavirus replication and a rationale for lymphedema to contribute to HPS. Although it is unclear whether similar LEC responses are caused by other HPS- and HFRS-causing hantaviruses, our data demonstrate a unique interaction of ANDV with pulmonary LECs that has the potential to alter lymphatic vessel clearance functions and contribute to the severity of ANDV-induced pulmonary edema. Our studies further demonstrate that rapamycin and VEGF-C act on LECs to prevent ANDV-induced giant LEC and permeability responses. VEGF-C and rapamycin have known roles in reducing lymphedema, and rapamycin also reportedly stabilizes BECs by reducing capillary leakage induced by VEGFR2 (9–11, 13, 46, 52, 54, 84). As a result, our findings suggest that rapamycin and VEGF-C have the potential to be hantavirus therapeutics that reduce pulmonary edema by stabilizing the vasculature and restoring lymphatic vessel fluid clearance functions during HPS.
ACKNOWLEDGMENTS
We thank Valery Matthys and Nadine Dalrymple for helpful discussions and critical reviews of the manuscript.
This work was supported by National Institutes of Health grants R01AI47873, PO1AI055621, R21AI1080984, and U54AI57158 (Northeast Biodefense Center [director, W. I. Lipkin]).
Footnotes
Published ahead of print 13 June 2012
REFERENCES
- 1. Aird WC. 2004. Endothelium as an organ system. Crit. Care Med. 32:S271–S279 doi:10.1097/01.CCM.0000129669.21649.40 [DOI] [PubMed] [Google Scholar]
- 2. Alitalo K, Carmeliet P. 2002. Molecular mechanisms of lymphangiogenesis in health and disease. Cancer Cell 1:219–227 [DOI] [PubMed] [Google Scholar]
- 3. Avraamides CJ, Garmy-Susini B, Varner JA. 2008. Integrins in angiogenesis and lymphangiogenesis. Nat. Rev. Cancer 8:604–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bahram F, Claesson-Welsh L. 2010. VEGF-mediated signal transduction in lymphatic endothelial cells. Pathophysiology 17:253–261 [DOI] [PubMed] [Google Scholar]
- 5. Baluk P, et al. 2007. Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 204:2349–2362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Berger MM, et al. 2005. Hypoxia impairs systemic endothelial function in individuals prone to high-altitude pulmonary edema. Am. J. Respir. Crit. Care Med. 172:763–767 [DOI] [PubMed] [Google Scholar]
- 7. Borges E, Jan Y, Ruoslahti E. 2000. Platelet-derived growth factor receptor beta and vascular endothelial growth factor receptor 2 bind to the beta 3 integrin through its extracellular domain. J. Biol. Chem. 275:39867–39873 [DOI] [PubMed] [Google Scholar]
- 8. Breen EC. 2007. VEGF in biological control. J. Cell Biochem. 102:1358–1367 [DOI] [PubMed] [Google Scholar]
- 9. Breslin JW, et al. 2007. Vascular endothelial growth factor-C stimulates the lymphatic pump by a VEGF receptor-3-dependent mechanism. Am. J. Physiol. Heart Circ. Physiol. 293:H709–H718 [DOI] [PubMed] [Google Scholar]
- 10. Breslin JW, Yuan SY, Wu MH. 2007. VEGF-C alters barrier function of cultured lymphatic endothelial cells through a VEGFR-3-dependent mechanism. Lymphat Res. Biol. 5:105–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brugarolas JB, Vazquez F, Reddy A, Sellers WR, Kaelin WG., Jr 2003. TSC2 regulates VEGF through mTOR-dependent and -independent pathways. Cancer Cell 4:147–158 [DOI] [PubMed] [Google Scholar]
- 12. Bustamante EA, Levy H, Simpson SQ. 1997. Pleural fluid characteristics in hantavirus pulmonary syndrome. Chest 112:1133–1136 [DOI] [PubMed] [Google Scholar]
- 13. Cam H, Houghton PJ. 2011. Regulation of mammalian target of rapamycin complex 1 (mTORC1) by hypoxia: causes and consequences. Target Oncol. 6:95–102 [DOI] [PubMed] [Google Scholar]
- 14. Chang B, Crowley M, Campen M, Koster F. 2007. Hantavirus cardiopulmonary syndrome. Semin. Respir. Crit. Care Med. 28:193–200 [DOI] [PubMed] [Google Scholar]
- 15. Christou H, Yoshida A, Arthur V, Morita T, Kourembanas S. 1998. Increased vascular endothelial growth factor production in the lungs of rats with hypoxia-induced pulmonary hypertension. Am. J. Respir. Cell Mol. Biol. 18:768–776 [DOI] [PubMed] [Google Scholar]
- 16. Dehler M, Zessin E, Bartsch P, Mairbaurl H. 2006. Hypoxia causes permeability oedema in the constant-pressure perfused rat lung. Eur. Respir. J. 27:600–606 [DOI] [PubMed] [Google Scholar]
- 17. Dixelius J, et al. 2003. Ligand-induced vascular endothelial growth factor receptor-3 (VEGFR-3) heterodimerization with VEGFR-2 in primary lymphatic endothelial cells regulates tyrosine phosphorylation sites. J. Biol. Chem. 278:40973–40979 [DOI] [PubMed] [Google Scholar]
- 18. Duchin JS, et al. 1994. Hantavirus pulmonary syndrome: a clinical description of 17 patients with a newly recognized disease. The Hantavirus Study Group. N. Engl. J. Med. 330:949–955 [DOI] [PubMed] [Google Scholar]
- 19. Dvorak HF. 2006. Discovery of vascular permeability factor (VPF). Exp. Cell Res. 312:522–526 [DOI] [PubMed] [Google Scholar]
- 20. Dvorak HF, Brown LF, Detmar M, Dvorak AM. 1995. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 146:1029–1039 [PMC free article] [PubMed] [Google Scholar]
- 21. Dvorak HF, et al. 1991. Distribution of vascular permeability factor (vascular endothelial growth factor) in tumors: concentration in tumor blood vessels. J. Exp. Med. 174:1275–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Enria D, et al. 1996. Hantavirus pulmonary syndrome in Argentina. Possibility of person to person transmission. Medicina 56:709–711 [PubMed] [Google Scholar]
- 23. Forsythe JA, et al. 1996. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 16:4604–4613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gavard J. 2009. Breaking the VE-cadherin bonds. FEBS Lett. 583:1–6 [DOI] [PubMed] [Google Scholar]
- 25. Gavard J, Gutkind JS. 2006. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat. Cell Biol. 8:1223–1234 [DOI] [PubMed] [Google Scholar]
- 26. Gavard J, Patel V, Gutkind JS. 2008. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev. Cell 14:25–36 [DOI] [PubMed] [Google Scholar]
- 27. Gavrilovskaya IN, Brown EJ, Ginsberg MH, Mackow ER. 1999. Cellular entry of hantaviruses which cause hemorrhagic fever with renal syndrome is mediated by beta3 integrins. J. Virol. 73:3951–3959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gavrilovskaya IN, Gorbunova EE, Mackow ER. 2010. Pathogenic hantaviruses direct the adherence of quiescent platelets to infected endothelial cells. J. Virol. 84:4832–4839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gavrilovskaya IN, Gorbunova EE, Mackow NA, Mackow ER. 2008. Hantaviruses direct endothelial cell permeability by sensitizing cells to the vascular permeability factor VEGF, while angiopoietin 1 and sphingosine 1-phosphate inhibit hantavirus-directed permeability. J. Virol. 82:5797–5806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gavrilovskaya IN, Peresleni T, Geimonen E, Mackow ER. 2002. Pathogenic hantaviruses selectively inhibit beta3 integrin directed endothelial cell migration. Arch. Virol. 147:1913–1931 [DOI] [PubMed] [Google Scholar]
- 31. Gavrilovskaya IN, Shepley M, Shaw R, Ginsberg MH, Mackow ER. 1998. β3 Integrins mediate the cellular entry of hantaviruses that cause respiratory failure. Proc. Natl. Acad. Sci. U. S. A. 95:7074–7079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Geimonen E, et al. 2002. Pathogenic and nonpathogenic hantaviruses differentially regulate endothelial cell responses. Proc. Natl. Acad. Sci. U. S. A. 99:13837–13842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goldman J, et al. 2007. Cooperative and redundant roles of VEGFR-2 and VEGFR-3 signaling in adult lymphangiogenesis. FASEB J. 21:1003–1012 [DOI] [PubMed] [Google Scholar]
- 34. Gorbunova E, Gavrilovskaya IN, Mackow ER. 2010. Pathogenic hantaviruses Andes virus and Hantaan virus induce adherens junction disassembly by directing vascular endothelial cadherin internalization in human endothelial cells. J. Virol. 84:7405–7411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gorbunova EE, Gavrilovskaya IN, Pepini T, Mackow ER. 2011. VEGFR2 and Src kinase inhibitors suppress Andes virus-induced endothelial cell permeability. J. Virol. 85:2296–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hallin GW, et al. 1996. Cardiopulmonary manifestations of hantavirus pulmonary syndrome. Crit. Care Med. 24:252–258 [DOI] [PubMed] [Google Scholar]
- 37. Hammerbeck CD, Hooper JW. 2011. T cells are not required for pathogenesis in the Syrian hamster model of hantavirus pulmonary syndrome. J. Virol. 85:9929–9944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hanaoka M, et al. 2003. Vascular endothelial growth factor in patients with high-altitude pulmonary edema. J. Appl. Physiol. 94:1836–1840 [DOI] [PubMed] [Google Scholar]
- 39. Hjelle B, et al. 1994. A novel hantavirus associated with an outbreak of fatal respiratory disease in the southwestern United States: evolutionary relationships to known hantaviruses. J. Virol. 68:592–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hooper JW, Larsen T, Custer DM, Schmaljohn CS. 2001. A lethal disease model for hantavirus pulmonary syndrome. Virology 289:6–14 [DOI] [PubMed] [Google Scholar]
- 41. Huber S, et al. 2007. Inhibition of the mammalian target of rapamycin impedes lymphangiogenesis. Kidney Int. 71:771–777 [DOI] [PubMed] [Google Scholar]
- 42. Humar R, Kiefer FN, Berns H, Resink TJ, Battegay EJ. 2002. Hypoxia enhances vascular cell proliferation and angiogenesis in vitro via rapamycin (mTOR)-dependent signaling. FASEB J. 16:771–780 [DOI] [PubMed] [Google Scholar]
- 43. Ikeda H, et al. 2011. Increased Akt-mTOR signaling in lung epithelium is associated with respiratory distress syndrome in mice. Mol. Cell. Biol. 31:1054–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Karkkainen MJ, et al. 2000. Missense mutations interfere with VEGFR-3 signalling in primary lymphoedema. Nat. Genet. 25:153–159 [DOI] [PubMed] [Google Scholar]
- 45. Kilpatrick ED, et al. 2004. Role of specific CD8+ T cells in the severity of a fulminant zoonotic viral hemorrhagic fever, hantavirus pulmonary syndrome. J. Immunol. 172:3297–3304 [DOI] [PubMed] [Google Scholar]
- 46. Kobayashi S, et al. 2007. Rapamycin, a specific inhibitor of the mammalian target of rapamycin, suppresses lymphangiogenesis and lymphatic metastasis. Cancer Sci. 98:726–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Koster F, et al. 2001. Rapid presumptive diagnosis of hantavirus cardiopulmonary syndrome by peripheral blood smear review. Am. J. Clin. Pathol. 116:665–672 [DOI] [PubMed] [Google Scholar]
- 48. Lähdevirta J, Enger E, Hunderi OH, Traavik T, Lee HW. 1982. Hantaan virus is related to hemorrhagic fever with renal syndrome in Norway. Lancet ii:606. [DOI] [PubMed] [Google Scholar]
- 49. Lampugnani MG, Orsenigo F, Gagliani MC, Tacchetti C, Dejana E. 2006. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J. Cell Biol. 174:593–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Land SC, Tee AR. 2007. Hypoxia-inducible factor 1alpha is regulated by the mammalian target of rapamycin (mTOR) via an mTOR signaling motif. J. Biol. Chem. 282:20534–20543 [DOI] [PubMed] [Google Scholar]
- 51. Lee HW. 1982. Hemorrhagic fever with renal syndrome (HFRS). Scand. J. Infect. Dis. Suppl. 36:82–85 [PubMed] [Google Scholar]
- 52. Lohela M, Bry M, Tammela T, Alitalo K. 2009. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr. Opin. Cell Biol. 21:154–165 [DOI] [PubMed] [Google Scholar]
- 53. López N, Padula P, Rossi C, Lazaro ME, Franze-Fernandez MT. 1996. Genetic identification of a new hantavirus causing severe pulmonary syndrome in Argentina. Virology 220:223–226 [DOI] [PubMed] [Google Scholar]
- 54. Major P. 2011. Potential of mTOR inhibitors for the treatment of subependymal giant cell astrocytomas in tuberous sclerosis complex. Aging 3:189–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Manalo DJ, et al. 2005. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 105:659–669 [DOI] [PubMed] [Google Scholar]
- 56. Matthys VS, Gorbunova EE, Gavrilovskaya IN, Mackow ER. 2010. Andes virus recognition of human and Syrian hamster beta3 integrins is determined by an L33P substitution in the PSI domain. J. Virol. 84:352–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mukhopadhyay D, et al. 1995. Hypoxic induction of human vascular endothelial growth factor expression through c-Src activation. Nature 375:577–581 [DOI] [PubMed] [Google Scholar]
- 58. Nagy JA, Benjamin L, Zeng H, Dvorak AM, Dvorak HF. 2008. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis 11:109–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nolte KB, et al. 1995. Hantavirus pulmonary syndrome in the United States: a pathological description of a disease caused by a new agent. Hum. Pathology 26:110–120 [DOI] [PubMed] [Google Scholar]
- 60. Ohmi K, Yamashita S, Hashimoto Y, Nonomura Y. 1993. Induction of giant endothelial cells in culture by K-252a, a protein kinase inhibitor. Jpn. J. Pharmacol. 63:195–202 [DOI] [PubMed] [Google Scholar]
- 61. Paddenberg R, et al. 2007. Rapamycin attenuates hypoxia-induced pulmonary vascular remodeling and right ventricular hypertrophy in mice. Respir. Res. 8:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Padula PJ, et al. 1998. Hantavirus pulmonary syndrome outbreak in Argentina: molecular evidence for person-to-person transmission of Andes virus. Virology 241:323–330 [DOI] [PubMed] [Google Scholar]
- 63. Pepini T, Gorbunova EE, Gavrilovskaya I, Mackow JE, Mackow ER. 2010. Andes virus regulation of cellular microRNAs contributes to hantavirus-induced endothelial cell permeability. J. Virol. 84:11929–11936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pham I, et al. 2002. Hypoxia upregulates VEGF expression in alveolar epithelial cells in vitro and in vivo. Am. J. Physiol. Lung Cell. Mol. Physiol. 283:L1133–L1142 [DOI] [PubMed] [Google Scholar]
- 65. Raymond T, Gorbunova E, Gavrilovskaya IN, Mackow ER. 2005. Pathogenic hantaviruses bind plexin-semaphorin-integrin domains present at the apex of inactive, bent alphavbeta3 integrin conformers. Proc. Natl. Acad. Sci. U. S. A. 102:1163–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Reynolds AR, et al. 2004. Elevated Flk1 (vascular endothelial growth factor receptor 2) signaling mediates enhanced angiogenesis in beta3-integrin-deficient mice. Cancer Res. 64:8643–8650 [DOI] [PubMed] [Google Scholar]
- 67. Reynolds LE, et al. 2002. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat. Med. 8:27–34 [DOI] [PubMed] [Google Scholar]
- 68. Robinson SD, Reynolds LE, Wyder L, Hicklin DJ, Hodivala-Dilke KM. 2004. Beta3-integrin regulates vascular endothelial growth factor-A-dependent permeability. Arterioscler. Thromb. Vasc. Biol. 24:2108–2114 [DOI] [PubMed] [Google Scholar]
- 69. Ruvinsky I, Meyuhas O. 2006. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem. Sci. 31:342–348 [DOI] [PubMed] [Google Scholar]
- 70. Safronetz D, Haddock E, Feldmann F, Ebihara H, Feldmann H. 2011. In vitro and in vivo activity of ribavirin against Andes virus infection. PLoS One 6:e23560 doi:10.1371/journal.pone.0023560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Schittenhelm MM, et al. 2006. Dasatinib (BMS-354825), a dual SRC/ABL kinase inhibitor, inhibits the kinase activity of wild-type, juxtamembrane, and activation loop mutant KIT isoforms associated with human malignancies. Cancer Res. 66:473–481 [DOI] [PubMed] [Google Scholar]
- 72. Schmaljohn C, Hjelle B. 1997. Hantaviruses: a global disease problem. Emerg. Infect. Dis. 3:95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Schraufnagel DE. 2010. Lung lymphatic anatomy and correlates. Pathophysiology 17:337–343 [DOI] [PubMed] [Google Scholar]
- 74. Shrivastava-Ranjan P, Rollin PE, Spiropoulou CF. 2010. Andes virus disrupts the endothelial cell barrier by induction of vascular endothelial growth factor and downregulation of VE-cadherin. J. Virol. 84:11227–11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sillaber C, et al. 2009. Immunosuppression and atypical infections in CML patients treated with dasatinib at 140 mg daily. Eur. J. Clin. Invest. 39:1098–1109 [DOI] [PubMed] [Google Scholar]
- 76. Stenmark KR, Fagan KA, Frid MG. 2006. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ. Res. 99:675–691 [DOI] [PubMed] [Google Scholar]
- 77. Tang N, et al. 2004. Loss of HIF-1alpha in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell 6:485–495 [DOI] [PubMed] [Google Scholar]
- 78. Thurston G, et al. 2000. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat. Med. 6:460–463 [DOI] [PubMed] [Google Scholar]
- 79. Toro J, et al. 1998. An outbreak of hantavirus pulmonary syndrome, Chile, 1997. Emerg. Infect. Dis. 4:687–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. van Hinsbergh VW, van Nieuw Amerongen GP. 2002. Endothelial hyperpermeability in vascular leakage. Vascul. Pharmacol. 39:171–172 [DOI] [PubMed] [Google Scholar]
- 81. Wahl-Jensen V, et al. 2007. Temporal analysis of Andes virus and Sin Nombre virus infections of Syrian hamsters. J. Virol. 81:7449–7462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Weis SM, Cheresh DA. 2012. αv integrins in angiogenesis and cancer. Cold Spring Harb. Perspect. Med. 1:a006478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wouters BG, Koritzinsky M. 2008. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat. Rev. Cancer. 8:851–864 [DOI] [PubMed] [Google Scholar]
- 84. Xue Q, et al. 2009. Rapamycin inhibition of the Akt/mTOR pathway blocks select stages of VEGF-A164-driven angiogenesis, in part by blocking S6Kinase. Arterioscler. Thromb. Vasc. Biol. 29:1172–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Yanagihara R, Silverman DJ. 1990. Experimental infection of human vascular endothelial cells by pathogenic and nonpathogenic hantaviruses. Arch. Virol. 111:281–286 [DOI] [PubMed] [Google Scholar]
- 86. Zaki S, et al. 1995. Hantavirus pulmonary syndrome: pathogenesis of an emerging infectious disease. Am. J. Pathol. 146:552–579 [PMC free article] [PubMed] [Google Scholar]