Abstract
Virus-like particles (VLPs) can be generated from Chikungunya virus (CHIKV), but different strains yield variable quantities of particles. Here, we define the genetic basis for these differences and show that amino acid 234 in E2 substantially affects VLP production. This site is located within the acid-sensitive region (ASR) known to initiate a major conformational change in E1/E2. Selected other mutations in the ASR, or changes in pH, also increased VLP yield. These results demonstrate that the ASR of E2 plays an important role in regulating particle generation.
TEXT
The reemergence of Chikungunya virus (CHIKV) in Kenya in 2004 and its subsequent spread throughout Africa, Asia, and Europe has stimulated efforts to develop an effective vaccine for the resultant debilitating disease. We have found that expression of CHIKV structural proteins gives rise to virus-like particles (VLPs) that resemble replication-competent alphavirus. This CHIKV VLP-based vaccine efficiently induced high-titer neutralizing antibodies against homologous and heterologous CHIKV strains in monkeys, and VLP-immunized animals showed complete protection against challenge with a high titer of a heterologous CHIKV strain (1). Interestingly, we also found that CHIKV 37997 strain VLPs (VLP37997) could be produced efficiently but that yields of CHIKV OPY-1 strain (VLPOPY-1) were low, despite the fact that the two strains are highly related, with 95% amino acid sequence similarity. Understanding the mechanism for production of CHIKV VLPs will allow for improved vaccines against CHIKV and can potentially apply to other pathogenic alphavirus VLP vaccines.
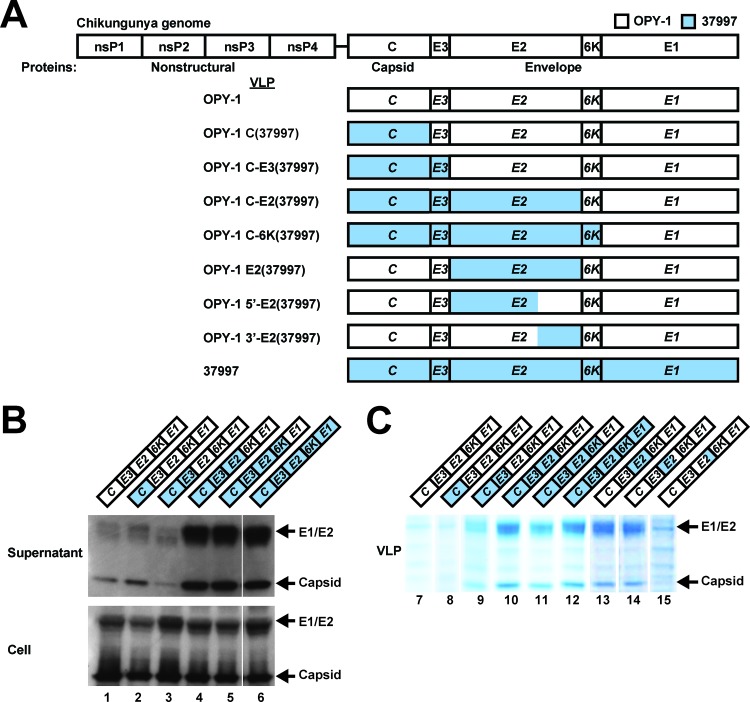
To identify the viral gene responsible for increased VLP generation, we prepared chimeric VLP expression vectors that introduced different segments of the 37997 strain into OPY-1. Using the C-E3-E2-6K-E1 region from an OPY-1 expression vector (VLPOPY-1), we inserted either capsid (C) alone, C-E3, C-E3-E2, or C-E3-E2-6K regions from the 37997 strain (Fig. 1A) using an overlap extension PCR as described previously (7). These chimeric vectors were expressed in 293F cells. VLP synthesis was determined by Western blotting directly in the supernatants without purification of particles. Release of VLPs into the supernatants differed significantly among these chimeras (Fig. 1B, top panel) despite comparable expression of the capsid and E1/E2 in cell lysates (Fig. 1B, bottom panel). Notably, the yield of VLPs increased more than 10-fold when the E2 region from the 37997 strain was included in the vector (Fig. 1B, top panel, lane 1 versus lane 4). To determine which region was responsible for this increase, the different polypeptide regions of 37997 were inserted into the OPY-1 expression vector. This analysis revealed that the E2 region alone mediated the increase in VLP production. Replacement of E2 enhanced VLP yield more than 80-fold as determined by Coomassie blue staining of sucrose density sedimentation-purified particles (Fig. 1C, lane 7 versus lane 13). To map the subregion responsible for this effect, we prepared chimeras that further subdivided E2. The NH2-terminal E2 domain (E2 amino acids [aa] 1 to 290) or the COOH-terminal E2 domain (E2 aa 291-423) was replaced in the VLPOPY-1 expression vector. VLP production in transfected cells revealed that the NH2-terminal region (aa 1 to 290) was necessary and sufficient for efficient VLP synthesis [VLPOPY-1 5′-E2(37997)] (Fig. 1C, lane 14).
Fig 1.
Characterization of chimeric CHIKV VLPs. (A) Schematic representation of the CHIKV genome and the chimeric CHIKV capsid-envelope (C–E) expression vectors used for VLP production from strains 37997 (blue) and OPY-1 (white). The CHIKV genome consists of the nonstructural polyproteins nsP1, nsP2, nsP3, and nsP4 and the structural polyproteins capsid (C) and envelope (E; E3, E2, 6K, and E1) (top). (B) Expression of chimeric VLP vectors in 293F cells. 293F cells (4.5 × 107) were transfected with the 293fectin transfection reagent and 30 μg of each VLP plasmid as previously described (1). Western blotting was performed on the supernatant directly without purification of particles (top) or cell lysate fractions (bottom) 48 h after transfection using antisera reactive with CHIKV as a primary antibody and goat anti-mouse immunoglobulins linked to horseradish peroxidase as a secondary antibody. The levels of expression of capsid and E1/E2 from all of the chimeric VLPs in cell lysates were within 1.1-fold of each other. (C) VLP yield from chimeric expression vectors. VLPs from supernatants of the indicated transfected 293F cells were purified using OptiPrep buoyant density gradient centrifugation as previously described (1).
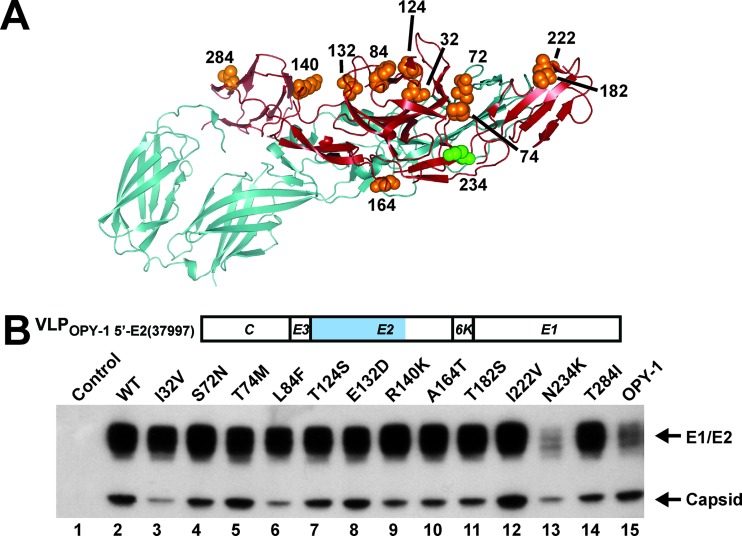
The sequences of 37997 and OPY-1 differ in this region by 12 amino acids (Fig. 2A). To determine the specific amino acid residues critical for VLP generation, site-specific mutations were introduced individually at these sites from the OPY-1 strain into the NH2-terminal E2 domain of the 37997 strain (Fig. 2B). Eleven of the 12 mutants synthesized VLPs at levels similar to those for VLPOPY-1 5′-E2(37997) (Fig. 2B, lane 2 versus lanes 3 to 12 and 14). In contrast, the N234K mutation from OPY-1 produced a >3-fold reduction in VLP release (Fig. 2B, lane 13), suggesting that this amino acid residue plays a critical role in the regulation of VLP synthesis.
Fig 2.
Structural models for the CHIKV OPY-1 E1/E2 complex compared to the CHIKV E2 37997 sequence, and effect of the single amino acid mutation N234K on CHIKV VLP production. (A) Location and structural orientation of relevant amino acid differences between CHIKV E1/E2 in the OPY-1 and 37997 strains. The CHIKV E1/E2 (OPY-1 strain) was modeled based on Protein Data Bank (PDB) code 3N43 and displayed using PyMOL (http://www.pymol.org). E2 is shown in red, and E1 is shown in light blue. The green spheres indicate the E2 234 site; amino acids shown as orange spheres show the differences in amino acids between OPY-1 and 37997 in E2 aa 1 to 290. (B) Expression of mutant VLPs produced from transfected 293F cells by Western blotting. The indicated amino acid sequence from the OPY-1 strain was introduced into the NH2-terminal E2 domain of chimeric VLPOPY-1 5′-E2 (37997): lane 1, control; 2, VLPOPY-1 5′-E2 (37997) (wild type [WT]); 3, I32V; 4, S72N; 5, T74M; 6, L84F; 7, T124S; 8, E132D; 9, R140K; 10, A164T; 11, T182S; 12, I222V; 13, N234K; 14, T284I; 15, VLPOPY-1. The supernatants harvested were analyzed by Western blotting as described in the legend to Fig. 1B. The yields of the mutant VLPs were between 0.9- and 1.1-fold (lane 2 versus lanes 3 to 12 and 14).
To determine whether modification of amino acid residue 234 from strain 37997 could improve VLP yield, this site was mutated from K to N in the OPY-1 expression vector (VLPOPY-1 K234N). Introduction of this single amino acid change into the OPY-1 vector increased VLP release by >10-fold compared to that of the parental VLPOPY-1 (Fig. 3A, lane 1 versus 2). Notably, since the release of capsid relative to E1/E2 with VLPOPY-1 plasmid (lane 1) was higher than with the VLP37997 plasmid (lane 4), we examined whether a plasmid encoding the capsid gene product (aa 1 to 261) alone could release capsid into the media. The release of capsid expressed from this plasmid into the medium was approximately 70% of levels released by the VLPOPY-1 plasmid (Fig. 3A, lane 5 versus 6), suggesting that production of capsid from strain OPY-1 occurs in the absence of envelope proteins. We performed buoyant density gradient centrifugation of supernatants from cells transfected with a plasmid encoding capsid gene product alone. The capsid proteins migrated at a density greater than that of VLPs, suggesting that the capsids do not form particles and likely represent aggregates released from dying transfected cells. At the same time, cell surface expression of envelope in the VLPOPY-1 K234N was similar to that of wild-type VLPOPY-1 (Fig. 3B), indicating that this mutation affected production without affecting E2 protein membrane expression. In fact, the yield of VLPOPY-1 K234N was more similar to that of wild-type VLP37997 or the swap mutant, VLPOPY-1 E2(37997). These data suggest that E2 234N plays a critical role in increasing VLP yield.
Fig 3.
Effect of a single amino acid mutation in E2 on CHIKV OPY-1 VLP production. (A) Expression of E2 mutants (left) and capsid (right) in transfected 293F cells by Western blotting. The VLP expression vectors with the indicated amino acid sequences from the 37997 strain were substituted into the E2 region of VLPOPY-1 or the OPY-1 capsid expression vector (aa 1 to 261) and were transfected as follows: lane 1, VLPOPY-1; 2, VLPOPY-1 K234N; 3, VLPOPY-1 E2(37997); 4, VLP37997; 5, capsidOPY-1; 6, VLPOPY-1. Supernatants were harvested 72 h after transfection and analyzed by Western blotting as described in the legend to Fig. 1B. (B) Envelope expression on the surface membranes of the indicated transfected cells 72 h after transfection was assessed by flow cytometry with a CHIKV E2 monoclonal antibody (m10-18, red line) or a control mouse monoclonal antibody (black line) as a primary antibody and a phycoerythrin secondary antibody and an amine-reactive dye, ViViD, to exclude the dead cell population. Monoclonal antibodies against CHIKV E2 were developed from mice immunized with VLP37997 based on methods described previously (19).
Based on the alignment of alphaviruses from the NCBI database (http://www.ncbi.nlm.nih.gov/Structure/cdd/cddsrv.cgi?uid=pfam00943), we found that these viruses conserve K at position 234 on E2 (15), except for a few isolated strains, such as the 37997 strain, suggesting that 234K plays an important role in the virus life cycle. Structural analysis of E2 suggested that 234K resides within the ASR (aa 159 to 171 and aa 231 to 258 of E2). The ASR has been observed in another alphavirus, Sindbis virus, and the structure was disordered by low pH (9, 15). This region is believed to be responsible for initiation of conformational changes in E1/E2 virus spikes. These irreversible conformational changes triggered by low pH cause the hydrophobic fusion peptide loop of E1 to interact with the cellular membrane and initiate fusion. Analysis of Semliki Forest virus has shown that E1/E2 conformation changes are caused by exposure to different pH conditions (18). Interestingly, pH has also been shown to be important for alphavirus budding. Budding of Semliki Forest virus became efficient when the infected cells were incubated at pHs greater than 7.5 compared to pHs below 7.0 (11). Therefore, we examined whether pH affected the yield of VLPOPY-1. We observed an ∼10-fold-increased yield when VLPs were generated at a pH higher than 7.0 (Fig. 4A, top left). In contrast, green fluorescent protein (GFP) expression levels in the cells as measured by flow cytometry were similar in different pH conditions, suggesting that pH had no effect on protein expression in the cells (data not shown).
Fig 4.
Effect of pH and amino acid mutations in the E2 ASR on VLP production. (A) Expression of VLPs in normal, basic pH buffers or adding E2 monoclonal antibody (m242) by Western blotting 48 h after transfection. At 24 h after transfection, Tris-HCl buffer at pH 7.5, 8.0, or 8.8 was added to the culture medium (final concentration, 40 mM) to change the pH to 7.3, 7.5, and 7.9, respectively (top left) or to change the pH to 7.9 (top middle and right; +). The supernatants were analyzed by Western blotting as described in the legend to Fig. 1B. The yields of the VLPOPY-1 K234N and VLP37997 were within 1.1-fold of each other at a higher pH (+) versus at a normal pH (−) (lane 8 versus lane 9 and lane 10 versus lane 11). At 24 h after transfection of the VLPOPY-1 plasmid, a mouse control antibody or a mouse anti-E2 monoclonal antibody (m242) was added (bottom left and middle, antibody final concentration was 0.16 μg/ml, 0.63 μg/ml, 2.5 μg/ml, or 10 μg/ml, respectively). Supernatants were analyzed by Western blotting with a rabbit serum immunized with VLP37997 as a primary antibody and goat anti-rabbit immunoglobulins linked to horseradish peroxidase as a secondary antibody. At 24 h after transfection of the furin cleavage site mutant VLPs (E3 R64E), the Tris-HCl buffer was added to change the pH to 7.9 (+) (bottom right). The supernatants were analyzed by Western blotting as described in the legend to Fig. 1B. The yields of the mutant VLPs (E3 R64E) were within 1.1-fold of each other at a higher pH (+) versus at a normal pH (−) (lane 24 versus 25). (B) Structural model of the E1/E2 dimer (left) and p62 (E3-E2)/E1 dimer (right) indicating the ASR of CHIKV, modified from PDB code 3N43. The E2 aa 170, 233, 252, and 256 positions in OPY-1 are shown in blue. The E2 aa 234 position in OPY-1 is shown in white. The E2 domain B is shown in green, the E2 domain A is shown in cyan, the E2 domain C is shown in pink, the E2 β-ribbon connector is shown in purple, and the E2 ASR domain (aa 231 to 258) in the E2 β-ribbon connector is shown in red. The E1 is marked in yellow. The E3 region is marked in gray. (C) Yield of VLP in normal or basic pH buffer determined by Western blotting. At 24 h after transfection of the indicated plasmids, Tris-HCl buffer was added to change the pH to 7.9 (+) as described above. The supernatants were analyzed by Western blotting as described in the legend to Fig. 1B. The yields of all the mutant VLPs were within 1.3-fold of each other at a higher pH (+) versus at a normal pH (−) (lane 28 versus lane 29, lane 30 versus lane 31, lane 32 versus lane 33, and lane 34 versus lane 35).
We therefore hypothesized that 234K would affect VLP yield at different pHs and that the VLPOPY-1 K234N mutant and VLP37997 would be less sensitive to the pH change. To test this hypothesis, the yields of VLP mutants at pH 7.0 and 7.9 were compared (Fig. 4A, top middle and top right). The VLPOPY-1 yield increased more than 10-fold at pH 7.9 (Fig. 4A, lane 6 versus lane 7), while the VLPOPY-1 K234N mutant yield did not increase at this pH (lane 8 versus lane 9). Conversely, the yield of VLP37997 did not change at high pHs (lane 10 versus lane 11); however, the yield of the VLP37997 N234K mutant increased more than 4.0-fold under the same conditions (lane 12 versus lane 13). These data suggest that 234K plays an important role in pH-dependent VLP release.
We next added an anti-E2 monoclonal mouse antibody (Ab; m242) to determine whether VLP yield is affected by an antibody that prevents the E1/E2 conformational change (Fig. 4A, bottom left). The m242 Ab has high neutralizing activity against both the OPY-1 and 37997 strains. We found that addition of the m242 Ab (lanes 18 to 21) increased VLP production up to ∼4.5-fold in a dose-dependent manner. The m242 Ab probably binds to, or near, the ASR and could prevent the initiation of the E1/E2 conformational change. The stabilization of E1/E2 could therefore either stimulate the release or enhance the stability of VLPs and thus improve the yield. These data support the notion that a stabilization of the prefusion form of E1/E2 increases VLP yield by facilitating budding/release or by preventing premature degradation/fusion. To explore this mechanism further, we tested a mutant E3 expression vector with a noncleavable E3/E2 furin site (Fig. 4A, E3 R64E, bottom right). This mutation leads to the retention of E3. E3 prevents the conformational change in E1/E2 during transport to the cell membrane by clamping the ASR in place until furin cleaves and releases E3 during virus maturation (15). The yield of the furin mutant VLPs increased >4.0-fold compared to results for the WT (Fig. 4A, lane 22 versus lane 24), documenting that stabilization of E2/E1 by engagement of the ASR increases VLP yield. In addition, there was no difference in the yield of the mutant VLPs at pH 7.0 versus pH 7.9 (lane 24 versus lane 25), suggesting that the mutant was insensitive to the pH change. Dimers of p62/E1 are stable at low pHs, while dimers of E2/E1 dissociate more readily (16). Taken together, these data suggested that stabilization of E1/E2 increased the efficiency of VLP production. Interestingly, p62, the E3-E2 precursor, was not detectable in supernatants or cell lysates (Fig. 1B and C), suggesting that it is cleaved efficiently by furin in VLP plasmid-transfected cells.
We next used structural information about E1/E2 (15) to test whether additional alteration of this specific region of E2 affected VLP production (Fig. 4B). We found that VLPOPY-1 K233N and K252Q mutants increased the yield of VLPs >4.4-fold compared to that of VLPOPY-1 (Fig. 4C, lane 26 versus lanes 28 and 30). In addition, there was no difference in the yield of the mutant VLPs at pH 7.0 versus pH 7.9 (lane 28 versus lane 29 and lane 30 versus lane 31), suggesting that the mutants were insensitive to the pH change. We introduced another mutation, E2 H170M, into the OPY-1 strain, since this amino acid is important for E1/E2 conformational changes and interacts strongly with a salt bridge and one hydrogen bond between E2 and E1 (15). The mutant E2 H170M increased the VLP yield 3.8-fold compared to that for wild-type VLPOPY-1 (Fig. 4C, lane 26 versus lane 32), and the yield of VLP did not increase at a higher pH (Fig. 4C, lane 32 versus lane 33). Histidine amino acids are candidates to play important roles in pH-dependent conformational changes, because the pK is in the same range as the pH of the conformational transition (15). Therefore, we also mutated 256H and found that this modification increased the yield of VLPs 4.3-fold (lane 26 versus 34), but we saw no additional change at the higher pH (lane 34 versus 35). These data suggest that the insensitivity to pH in ASR plays an important role in increasing VLP yield.
It has been shown that alphavirus assembly and budding efficiency is related to several factors, such as palmitoylation of the COOH terminal of E1 and E2 (6, 13), interactions between E1 and E2 (20, 21) or between the cytoplasmic domain of E2 and capsid proteins (8, 17, 22), the requirement for cholesterol in the cell membrane (10, 12, 14), and pH (11). In this study, we have identified specific sequences in the E2 ASR responsible for robust CHIKV VLP yield and show that VLP release is sensitive to pH in vitro. These findings suggest that the proper conformation of the E1/E2, dependent on pH, is important for the efficient generation of VLPs.
Neutralizing antibody escape mutants have been characterized in several alphaviruses. Most of these rescue mutants have been found to contain E2 modifications on the outer side of domain B, on the wings in domain A and in the ASR (4, 15). In the CHIKV ASR mutants, modifications at the E2 amino acid positions 229, 231, 232, 233, and 234 were observed (4). These data suggested that neutralizing antibodies recognize the E2 aa 229 to 234 region in the ASR and that binding of the antibodies to these amino acids prevented the conformational change necessary for the virus to initiate the process of fusion and entry into the cells. The E2 aa 229 to 234 region in the ASR appeared critical for virus replication partly due to its role in inducing pH-dependent conformational changes, further supporting our observation that the K234N mutation increased VLP yields by preventing the conformational changes.
The most recent outbreak strain, OPY-1, is responsible for the first reports of fatalities during a CHIKV epidemic, although potential limitations in monitoring the outcomes of previous CHIKV outbreaks may have resulted in underreporting. The OPY-1 strain also caused significant pathogenicity compared with an Asian strain in a mouse model (5). Analysis of replication-competent viruses from the WT and E2-234 mutant of OPY-1 or 37997 strains in vitro and comparison of pathogenicity between these replication-competent viruses in animal models would be interesting. Although pathogenicity is affected by many factors, particle formation during viral replication would likely contribute to attenuation of viral pathogenicity.
VLPs are known to have advantages for vaccination, such as a good safety profile and the ability to induce high levels of immunogenicity (1–3). Thus, a VLP vaccine strategy may prove to be optimal, and the results described here further the development of alphavirus vaccines. Although ASR-mutant VLPs as immunogens for vaccine development require further evaluation with other alphaviruses, well-designed ASR-mutant VLPs such as VLP37997 would induce cross-neutralizing antibodies, as we have shown previously (1). These results could apply to development of VLP vaccines for many alphaviruses and further an understanding of their mechanisms of assembly and budding.
ACKNOWLEDGMENTS
We thank Jeffrey C. Boyington for advice regarding structure model analysis and Wei Shi for helping with the isolation of monoclonal antibodies against CHIKV. We also thank Ati Tislerics for help with manuscript preparation, Brenda Hartman for graphic arts, and members of the Nabel lab for helpful discussions.
This research was supported by the Intramural Research Program of the Vaccine Research Center, National Institute of Allergy and Infectious Diseases, U.S. National Institutes of Health.
The authors report no financial conflicts of interest.
Footnotes
Published ahead of print 30 May 2012
REFERENCES
- 1. Akahata W, et al. 2010. A virus-like particle vaccine for epidemic Chikungunya virus protects nonhuman primates against infection. Nat. Med. 16:334–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bachmann MF, et al. 1993. The influence of antigen organization on B cell responsiveness. Science 262:1448–1451 [DOI] [PubMed] [Google Scholar]
- 3. Chackerian B. 2007. Virus-like particles: flexible platforms for vaccine development. Expert Rev. Vaccines 6:381–390 [DOI] [PubMed] [Google Scholar]
- 4. Coffey LL, Vignuzzi M. 2011. Host alternation of chikungunya virus increases fitness while restricting population diversity and adaptability to novel selective pressures. J. Virol. 85:1025–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gardner J, et al. 2010. Chikungunya virus arthritis in adult wild-type mice. J. Virol. 84:8021–8032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ivanova L, Schlesinger MJ. 1993. Site-directed mutations in the Sindbis virus E2 glycoprotein identify palmitoylation sites and affect virus budding. J. Virol. 67:2546–2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kong WP, et al. 2003. Immunogenicity of multiple gene and clade human immunodeficiency virus type 1 DNA vaccines. J. Virol. 77:12764–12772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee S, et al. 1996. Identification of a protein binding site on the surface of the alphavirus nucleocapsid and its implication in virus assembly. Structure 4:531–541 [DOI] [PubMed] [Google Scholar]
- 9. Li L, Jose J, Xiang Y, Kuhn RJ, Rossmann MG. 2010. Structural changes of envelope proteins during alphavirus fusion. Nature 468:705–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lu YE, Cassese T, Kielian M. 1999. The cholesterol requirement for sindbis virus entry and exit and characterization of a spike protein region involved in cholesterol dependence. J. Virol. 73:4272–4278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lu YE, Eng CH, Shome SG, Kielian M. 2001. In vivo generation and characterization of a soluble form of the Semliki Forest virus fusion protein. J. Virol. 75:8329–8339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marquardt MT, Phalen T, Kielian M. 1993. Cholesterol is required in the exit pathway of Semliki Forest virus. J. Cell Biol. 123:57–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ryan C, Ivanova L, Schlesinger MJ. 1998. Effects of site-directed mutations of transmembrane cysteines in sindbis virus E1 and E2 glycoproteins on palmitylation and virus replication. Virology 249:62–67 [DOI] [PubMed] [Google Scholar]
- 14. Vashishtha M, et al. 1998. A single point mutation controls the cholesterol dependence of Semliki Forest virus entry and exit. J. Cell Biol. 140:91–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Voss JE, et al. 2010. Glycoprotein organization of Chikungunya virus particles revealed by X-ray crystallography. Nature 468:709–712 [DOI] [PubMed] [Google Scholar]
- 16. Wahlberg JM, Boere WA, Garoff H. 1989. The heterodimeric association between the membrane proteins of Semliki Forest virus changes its sensitivity to low pH during virus maturation. J. Virol. 63:4991–4997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wilkinson TA, Tellinghuisen TL, Kuhn RJ, Post CB. 2005. Association of sindbis virus capsid protein with phospholipid membranes and the E2 glycoprotein: implications for alphavirus assembly. Biochemistry 44:2800–2810 [DOI] [PubMed] [Google Scholar]
- 18. Wu SR, et al. 2007. The dynamic envelope of a fusion class II virus. Prefusion stages of Semliki Forest virus revealed by electron cryomicroscopy. J. Biol. Chem. 282:6752–6762 [DOI] [PubMed] [Google Scholar]
- 19. Yang ZY, et al. 2007. Immunization by avian H5 influenza hemagglutinin mutants with altered receptor binding specificity. Science 317:825–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yao J, Strauss EG, Strauss JH. 1998. Molecular genetic study of the interaction of Sindbis virus E2 with Ross River virus E1 for virus budding. J. Virol. 72:1418–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yao JS, Strauss EG, Strauss JH. 1996. Interactions between PE2, E1, and 6K required for assembly of alphaviruses studied with chimeric viruses. J. Virol. 70:7910–7920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao H, Lindqvist B, Garoff H, von Bonsdorff CH, Liljestrom P. 1994. A tyrosine-based motif in the cytoplasmic domain of the alphavirus envelope protein is essential for budding. EMBO J. 13:4204–4211 [DOI] [PMC free article] [PubMed] [Google Scholar]