Abstract
The role of the polyomavirus BK (BKV) large tumor antigen (L-Tag) as a target of immune response in patients with prostate cancer (PCa) has not been investigated thus far. In this study, we comparatively analyzed humoral and cellular L-Tag-specific responsiveness in age-matched patients bearing PCa or benign prostatic hyperplasia, expressing or not expressing BKV L-Tag-specific sequences in their tissue specimens, and in non-age-matched healthy individuals. Furthermore, results from patients with PCa were correlated to 5-year follow-up clinical data focusing on evidence of biochemical recurrence (BR) after surgery (prostate specific antigen level of ≥0.2 ng/ml). In peripheral blood mononuclear cells (PBMC) from patients with PCa with evidence of BR and BKV L-Tag-positive tumors, stimulation with peptides derived from the BKV L-Tag but not those derived from Epstein-Barr virus, influenza virus, or cytomegalovirus induced a peculiar cytokine gene expression profile, characterized by high expression of interleukin-10 (IL-10) and transforming growth factor β1 and low expression of gamma interferon genes. This pattern was confirmed by protein secretion data and correlated with high levels of anti-BKV L-Tag IgG. Furthermore, in PBMC from these PCa-bearing patients, L-Tag-derived peptides significantly expanded an IL-10-secreting CD4+ CD25+(high) CD127− FoxP3+ T cell population with an effector memory phenotype (CD103+) capable of inhibiting proliferation of autologous anti-CD3/CD28-triggered CD4+ CD25− T cells. Collectively, our findings indicate that potentially tolerogenic features of L-Tag-specific immune response are significantly associated with tumor progression in patients with BKV+ PCa.
INTRODUCTION
Prostate cancer (PCa) represents the first leading cause of cancer morbidity and the third of cancer death in men in developed countries, with a worldwide incidence rate of 14% of total newly diagnosed malignancies and a worldwide total cancer mortality rate of 6% (29). A contemporary model of PCa induction and progression should include the analysis of the contribution of inflammation to the development of preneoplastic or neoplastic lesions (24). Indeed, proliferative inflammatory atrophy (PIA) of the prostate has recently gained importance as potential precursor of prostatic intraepithelial neoplasia (PIN) and overt PCa (14, 15). This is due particularly to the prevalence of PIA in the peripheral zone of the organ, where histological transition between PIA and PIN usually occurs (42).
The low rate of mutations detected in tumor suppressor genes pRB1 and p53 in primary PCa cells (16) has suggested a possible role of inflammatory agents ubiquitous in the urinary tract with unique oncogenic functions, that is, of sequestering wild-type products of tumor suppressor genes. Polyomaviruses' main regulatory proteins, large tumor antigens (L-Tag), interfere with wt-p53 binding to cellular DNA during virus infection (4), thereby impairing p53 control on cell growth activity and possibly leading to oncogenic transformation in nonpermissive cells (6). As such, the human urotheliotrophic polyomavirus BK (BKV) has been suggested to prominently associate with the development of cancer (26, 27) and, in particular, of urinary tract malignancies (3, 12, 54).
There is a continuing debate on BKV expression in overt cancers (1, 5). However, the possibility of “hit-and-run” carcinogenic mechanisms induced by BKV cannot be excluded (13). Genetically rearranged BKV variants (22, 45), presumably difficult to detect by commonly used assays, might exist in the urinary tract and be responsible for neoplastic transformation in prostate cells (i.e., URO-1) (37). Therefore, BKV has been indicated as a potential cofactor in the earliest stages of PCa (13).
Detection and expression of BKV L-Tag sequences in preneoplastic prostate tissues (12) have prompted us to investigate the role of this viral antigen as a target of immune response. Thus, we have addressed here the humoral and cellular responsiveness to BKV L-Tag in patients with benign prostatic hyperplasia (BPH) or newly diagnosed PCa, and we have correlated the viral and immunological features with PCa status.
MATERIALS AND METHODS
Patients and clinical follow-up.
A total of 110 consecutive patients diagnosed for either PCa (n = 60; prostate specific antigen [PSA] level of >4 ng/ml and suspicious digital rectal examination and/or positive for early-diagnosed high-grade prostate intraepithelial neoplasm at biopsy) or benign prostate hyperplasia (BPH; n = 50; urinary obstructive symptoms and acute urinary retention according to the international prostate symptom score) were enrolled in the study at the Department of Urology of the University Hospital of Basel, Switzerland, and at the Division of Urology of the University Hospital of Zurich, Switzerland, after obtaining informed consent, following approval by Cantonal Ethical Committees of Basel and Zurich. Five-year follow-up after surgery was completed for 48/60 PCa patients (80%). Timing of biochemical recurrence (BR+), with early censoring if only one or two values were available, was established at the first detection ≥0.2 ng of PSA/ml. BR− patients were those showing clearly negative PSA values (<0.04 ng/ml) during complete follow-up. The data were collected according to the American Society for Therapeutic Radiation and Oncology (ASTRO) criteria (23), considering both actuarial and ASTRO-time analytical methods, since ASTRO censoring allowed us to define biochemical failure as three consecutive increases in PSA values in 3/48 PCa patients only.
Virus detection in tissue specimens.
Specimens from tissues excised during surgical procedures were formalin fixed and paraffin embedded. DNA was extracted from three sections (thickness, 5 μm) randomly picked within the tumor area (PCa) or within the atrophic-hyperplastic gland (BPH) using a QIAamp DNA minikit (Qiagen, Basel, Switzerland) according to the manufacturer's instructions. Molecular detection of polyomavirus BK was performed by quantitative reverse transcription-PCR (qRT-PCR) using a TaqMan assay targeting L-Tag. The assay, named T3a, consists of a primer-probe set that was designed to reliably measure BKV L-Tag subtypes Ia, Ic, III, IV, and VI (25). PCR amplification was set up according to standard real-time PCR protocols, using a Corbett Life Science Rotor-Gene 3000 instrument (Corbett Life Science, Sydney, Australia). Standard curves for the quantification of BKV L-Tag were generated using serial 10-fold dilutions of plasmid pBKV35-1 DNA (LGC Standards Sarl, Molsheim, France). To correct for the variable amounts of DNA in individual tissue specimens, amplification of control gene aspartoacylase was performed in each sample, as previously described (43). Patients were considered BKV L-Tag negative upon consecutive negative testing on all three randomly picked punches.
EIA for patient and donor serology.
BKV-specific antibody detection was performed by enzyme immunoassay (EIA) with a glutathione S-transferase (GST)-BKV fusion protein. The optical density at 492 nm (OD492) was measured using an automated plate reader (Tecan Group, Ltd., Männedorf, Switzerland). Affinity-purified GST was run as a negative control, and corresponding OD values were subtracted from GST-BKV L-Tag domain 1 and GST-BKV VP1 specific signals. The cutoff was defined as two standard deviations above the mean values of negative controls. Therefore, all OD values of <0.04 were considered negative (7, 33).
Peptides and peptide pools.
The BKV L-Tag peptide pool was provided by JPT Peptide Technology (Berlin, Germany). This PepMix contains a total of 170 15mer peptides spanning the entire antigen (691 amino acids, Swiss-Prot P14999) and tiled at an 11-amino-acid pace. Similarly, the negative control PepMix HIVgag peptide pool (123 peptides including the B gag motif) was also provided by JPT. As a positive control, a PepMix CEF (cytomegalovirus, Epstein-Barr virus, and influenza virus) pool of 23 8-11mer peptides recognized by CD8+ T cells and presented by 11 class I HLA-A and HLA-B alleles (11) was used (JPT, Berlin Germany). For in vitro expansion and proliferation assays, both HIV peptide pool (see above) and the human cytomegalovirus promiscuous epitope pp65340-355 (41) (Princeton Biomolecules, Langhorne, PA) were used as controls.
Ex vivo induction and detection of cytokine gene expression by qRT-PCR.
Peripheral blood mononuclear cells (PBMC) isolated from venous blood by Ficoll-Hypaque density gradient centrifugation were resuspended in RPMI medium supplemented with 100 μg of kanamycin/ml, 10 mM HEPES, 1 mM sodium pyruvate, 1 mM GlutaMAX, and nonessential amino acids (all from Gibco, Paisley, Scotland) (complete medium) and 5% human serum (Blutspendezentrum UniversitätsSpital, Basel, Switzerland), at a final concentration of 106 cells/ml, plated in 96 U-bottom well plates (200 μl/well), and incubated for overnight resting. The cells were then stimulated with either a test (L-Tag) or a control (CEF, HIV) peptide pool (1 μg/ml) or phytohemagglutinin (PHA; 1 μg/ml) and harvested after 3 h for RNA extraction (RNeasy minikit protocol; Qiagen, Basel, Switzerland) and cDNA synthesis (Invitrogen, Carlsbad, CA). Quantitative gene amplification (i.e., qRT-PCR) was performed, as previously described (40), by using an ABI Prism 7500 FAST sequence detection system with a TaqMan Universal PCR master mix reagents kit and “on demand” sets of primers and probes for cytokine gene expression (gamma interferon [IFN-γ], interleukin-10 [IL-10], and transforming growth factor β1 [TGF-β1]; Applied Biosystems, Rotkreuz, Switzerland). β-Actin was used as endogenous reference gene, and normalized data were analyzed by the 2−ΔΔCT method (34).
Cell cultures.
Monocytes (CD14+) sorted from PBMC by using magnetic beads (Miltenyi Biotech) were cultured from 5 to 7 days in RPMI complete medium supplemented with 10% fetal calf serum (Gibco), 0.004% β-mercaptoethanol, recombinant human IL-4 (rhIL-4; 1,000 U/ml), and recombinant human granulocyte-macrophage colony-stimulating factor (50 ng/ml) to generate immature dendritic cells (iDCs). To induce maturation, iDCs were overnight exposed to 1 μg of lipopolysaccharide (Abortus Aequi; Sigma-Aldrich, St. Louis, MO)/ml. CD4+ T cells positively sorted from PBMC by using magnetic beads were cultured in complete medium with 5% human serum in 24 flat-bottom well plates at a 106-cells/ml concentration in the presence of autologous irradiated mature dendritic cells (mDCs), previously pulsed for 2 h with peptide pools (10 μg/ml), either for priming (day 0) or restimulation (day 7). rhIL-2 (Hoffmann-La Roche, Basel, Switzerland) was added to cultures at 1, 1, and 5 ng/ml on days 3, 7, and 10, respectively.
Regulatory T cell cultures were established by ex vivo magnetic sorting of CD4+ C25+ CD127−/dim subset, as previously described (48). The cells were subsequently stimulated with L-Tag peptide pool (5 μg/ml) or control peptides, including HCMVpp65340-355 and HIV peptide pool, in the presence of 10 μg of anti-CD28 (BD Bioscience, Allschwil, Switzerland)/ml and 5 ng of rIL-2/ml. The cultures were similarly restimulated on day 7. IL-10-secreting regulatory T (Treg) cells were sorted on day 14 by cytokine secretion assay (Miltenyi Biotec). For suppression assays, CD4+ CD25+(high) CD127−, IL-10-secreting T cells were cultured in the upper chamber of 24-transwell plates (BD Bioscience) with a double amount (0.5:1) of autologous CD4+ CD25− T cells, stimulated with 1 mg of anti-CD3/CD28 (BD Bioscience)/ml seeded in the lower chamber, and cultured for 8 days. The CD4+ CD25− T cell proliferation was measured by using cell proliferation reagent WST-1 (Roche Applied Science) according to the manufacturer's instructions, and the absorbance was read at 450 nm with an enzyme-linked immunosorbent assay plate reader.
Regulatory T cell quantification by flow cytometry.
CD4+ T cells stimulated by peptide pools, as described above, were stained with anti-CD4-fluorescein isothiocyanate (FITC) and anti-CD25-allophycocyanin (APC) antibodies (BD Biosciences). Anti-FoxP3 PE antibodies and FoxP3 Fix/Perm buffer were used for intracellular staining according to the manufacturer's protocol (eBioscience, Vienna, Austria). Separation into CD25 bright (CD25high) and CD25 dim (CD25low) cells was carried out using a fluorescence cutoff defined in healthy donors. Anti-CD103 PerCP (eBioscience) and anti-IL-10 APC intracellular staining (BD Bioscience) was additionally carried out for the identification of IL-10-secreting activated Treg cells. The data were acquired on a FACSCalibur flow cytometer equipped with CellQuest software (Becton Dickinson, San Jose, CA).
Cytokine measurement.
A FlowCytomix sample kit (eBioscience) was used to measure IFN-γ, IL-10, and TGF-β1 protein release in culture supernatants of peptide-stimulated T cells. Samples were run on a FACSCalibur flow cytometer equipped with CellQuest software (Becton Dickinson), analyzed with BMS FlowCytomix software (eBioscience), and referred to a standard curve for quantification.
Statistical analysis.
Statistical analysis was performed with GraphPad Prism (v5.1) and SAS/STAT (v9.1). The data were reported as means ± the standard deviations (SD) or mean ± the standard errors (SE), with median values and ranges where appropriate. Distributions of categorical markers were analyzed by both χ2 and Fisher exact tests. When comparing groups following a normal distribution (Shapiro-Wilk test), t tests (either pooled or Cochran method) were used. An F test was used to calculate variance among groups of samples. If a non-normal distribution was indicated, nonparametric tests such as Mann-Whitney U tests and Spearman's ρ correlation analyses were used. P values of <0.05 (95% confidence interval [CI]) were considered statistically significant.
RESULTS
Clinical profiles of patients.
The average age of patients enrolled in the study (n = 110) was 64 ± 7.7 years (range, 49 to 90 years) for PCa (n = 60) and 67 ± 7.4 years (range, 48 to 81 years) for age-matched BPH (n = 50), respectively.
Of 60 PCa patients, 1 was diagnosed with clinical stage pT1b, 10 with pT2a, 2 with pT2b, the majority (n = 30; 51%) with clinical stage pT2c, 4 with pT3a, and 3 with pT3b. Ten (17%) patients had tumors whose stage at diagnosis could not be assessed. Average serum PSA value was 17.4 ± 36.2 ng/ml (median, 6.7 ng/ml; range, 1.6 to 206 ng/ml) in PCa patients before surgery (n = 50, 83%) and 5.6 ± 5.7 ng/ml (median, 3.9 ng/ml; range, 0.4 to 26 ng/ml) in BPH patients (n = 36, 72%). The Gleason score of the tumor specimens (n = 55, 92%) ranged from 5 to 9 according to immunohistochemical analysis (Tables 1 and 2).
Table 1.
Patient PSA levels, Gleason score, L-Tag molecular testing results, and IgG serology for PCa samplesa
| PCa sample | PSA level (n = 50) | Gleason score (sum of patterns) (n = 55) | L-Tag DNA copies/105 cellsb (n = 43) | L-Tag IgG (OD492)c (n = 60) |
|---|---|---|---|---|
| PCa 1 | 7.50 | 7 (3 + 4) | NT | + |
| PCa 2 | 5.22 | 9 (5 + 4) | NT | + |
| PCa 3 | 4.60 | 7 (4 + 3) | + | + |
| PCa 4 | 5.30 | 7 (4 + 3) | – | + |
| PCa 5 | 4.80 | 7 (3 + 4) | – | + |
| PCa 6 | 10.70 | 7 (3 + 4) | NT | + |
| PCa 7 | 7.64 | 7 (4 + 3) | – | + |
| PCa 8 | 5.50 | 6 (3 + 3) | + | + |
| PCa 9 | 4.10 | 7 (3 + 4) | + | + |
| PCa 10 | 7.50 | 8 (3 + 5) | – | + |
| PCa 11 | 1.60 | 6 (3 + 3) | NT | – |
| PCa 12 | NA | 6 (3 + 3) | NT | – |
| PCa 13 | 7.20 | 7 (3 + 4) | – | – |
| PCa 14 | 7.80 | 7 (3 + 4) | – | + |
| PCa 15 | 10.80 | 9 (4 + 5) | NT | + |
| PCa 16 | 4.78 | 6 (3 + 3) | + | – |
| PCa 17 | NA | NA | NT | – |
| PCa 18 | 4.17 | 9 (4 + 5) | + | + |
| PCa 19 | 25.60 | 7 (4 + 3) | – | + |
| PCa 20 | 4.70 | 7 (4 + 3) | – | – |
| PCa 21 | 5.20 | 7 (3 + 4) | – | – |
| PCa 22 | NA | 7 (3 + 4) | NT | + |
| PCa 23 | 4.05 | 7 (3 + 4) | – | – |
| PCa 24 | 4.40 | 7 (3 + 4) | – | – |
| PCa 25 | 35.00 | 7 (4 + 3) | + | + |
| PCa 26 | 61.30 | 6 (3 + 3) | – | + |
| PCa 27 | NA | NA | + | + |
| PCa 28 | 9.20 | 7 (3 + 4) | – | – |
| PCa 29 | 4.80 | 6 (3 + 3) | – | – |
| PCa 30 | NA | 7 (4 + 3) | NT | + |
| PCa 31 | 6.59 | 7 (3 + 4) | – | + |
| PCa 32 | 8.73 | 6 (3 + 3) | NT | + |
| PCa 33 | 6.60 | 7 (3 + 4) | + | + |
| PCa 34 | 3.44 | 7 (3 + 4) | + | + |
| PCa 35 | 6.43 | 6 (3 + 3) | – | + |
| PCa 36 | 10.00 | 6 (3 + 3) | NT | + |
| PCa 37 | 8.80 | 7 (3 + 4) | + | + |
| PCa 38 | 7.46 | NA | NT | + |
| PCa 39 | 10.90 | 7 (4 + 3) | + | + |
| PCa 40 | 106.00 | 9 (4 + 5) | – | + |
| PCa 41 | NA | 5 (2 + 3) | – | – |
| PCa 42 | 10.60 | 7 (3 + 4) | NT | – |
| PCa 43 | 8.50 | 6 (3 + 3) | – | + |
| PCa 44 | 7.20 | 7 (4 + 3) | – | + |
| PCa 45 | 11.52 | 6 (3 + 3) | + | + |
| PCa 46 | 2.44 | 9 (4 + 5) | + | + |
| PCa 47 | NA | NA | NT | + |
| PCa 48 | 48.00 | 7 (4 + 3) | + | + |
| PCa 49 | NA | 6 (3 + 3) | + | + |
| PCa 50 | NA | 7 (3 + 4) | – | + |
| PCa 51 | NA | NA | NT | + |
| PCa 52 | 4.70 | 7 (3 + 4) | – | + |
| PCa 53 | 2.56 | 6 (3 + 3) | + | + |
| PCa 54 | 6.78 | 7 (3 + 4) | NT | + |
| PCa 55 | 4.40 | 7 (3 + 4) | – | + |
| PCa 56 | 6.50 | 6 (3 + 3) | – | – |
| PCa 57 | 4.50 | 7 (4 + 3) | + | + |
| PCa 58 | 4.60 | 6 (3 + 3) | + | + |
| PCa 59 | 206.00 | 9 (4 + 5) | – | + |
| PCa 60 | 4.11 | 6 (3 + 3) | NT | + |
NA, not available; NT, not tested. For L-Tag BKV, the plus or minus indicates L-Tag DNA detection in tissue specimens that is above or below the cutoff (50 copies/105 cells), respectively. For L-Tag IgG, the plus or minus indicates IgG activity against L-Tag that is above or below the cutoff (OD492 > 0.04), respectively. Samples for patients sharing L-Tag BKV+ specimens and IgG+ activity against L-Tag (IgG ≥ 0.04: n = 17/18, P = 0.005 [χ2]) are indicated in boldface.
BKV+, n = 18; BKV−, n = 25.
IgG ≥ 0.04, n = 46; IgG < 0.04, n = 14.
Table 2.
Patient PSA levels, L-Tag molecular testing results, and IgG serology for BPH samplesa
| BPH sample | PSA level (n = 36) | L-Tag DNA copies/105 cellsb (n = 38) | L-Tag IgG (OD492)c (n = 50) |
|---|---|---|---|
| BPH 1 | 12.75 | – | + |
| BPH 2 | NA | – | + |
| BPH 3 | 4.74 | NT | + |
| BPH 4 | NA | – | – |
| BPH 5 | NA | – | + |
| BPH 6 | 2.96 | + | + |
| BPH 7 | NA | – | + |
| BPH 8 | 18.60 | – | + |
| BPH 9 | 4.65 | NT | + |
| BPH 10 | 2.07 | – | + |
| BPH 11 | NA | – | + |
| BPH 12 | 26.00 | + | + |
| BPH 13 | 1.79 | + | – |
| BPH 14 | 1.26 | – | + |
| BPH 15 | 3.93 | – | + |
| BPH 16 | NA | – | + |
| BPH 17 | NA | NT | + |
| BPH 18 | NA | NT | + |
| BPH 19 | NA | NT | + |
| BPH 20 | 4.40 | – | – |
| BPH 21 | 17.00 | + | + |
| BPH 22 | 1.16 | – | + |
| BPH 23 | 6.40 | + | – |
| BPH 24 | 1.36 | + | – |
| BPH 25 | 6.34 | NT | + |
| BPH 26 | 4.8 | NT | + |
| BPH 27 | 10.10 | + | + |
| BPH 28 | 3.78 | – | + |
| BPH 29 | 4.26 | – | – |
| BPH 30 | 1.66 | + | + |
| BPH 31 | NA | + | – |
| BPH 32 | 1.11 | – | + |
| BPH 33 | NA | – | + |
| BPH 34 | 1.67 | – | – |
| BPH 35 | NA | NT | – |
| BPH 36 | 5.40 | – | – |
| BPH 37 | 2.12 | – | + |
| BPH 38 | 2.30 | – | + |
| BPH 39 | 8.14 | + | + |
| BPH 40 | NA | – | + |
| BPH 41 | NA | NT | + |
| BPH 42 | 4.25 | – | – |
| BPH 43 | 1.24 | – | + |
| BPH 44 | 8.90 | NT | + |
| BPH 45 | 2.66 | – | + |
| BPH 46 | 2.30 | + | – |
| BPH 47 | 2.85 | NT | + |
| BPH 48 | 3.50 | + | + |
| BPH 49 | 0.36 | NT | + |
| BPH 50 | 15.70 | – | + |
NA, not available; NT, not tested. For L-Tag BKV, the plus or minus indicates L-Tag DNA detection in tissue specimens that is above or below the cutoff (50 copies/105 cells), respectively. For L-Tag IgG, the plus or minus indicates IgG activity against L-Tag that is above or below the cutoff (OD492 > 0.04), respectively. Samples for patients sharing L-Tag BKV+ specimens and IgG+ activity against L-Tag (IgG ≥ 0.04: n = 7/12, P = 0.005 [χ2]) are indicated in boldface.
BKV+, n = 12; BKV−, n = 26.
IgG ≥ 0.04, n = 38; IgG < 0.04, n = 12.
L-Tag DNA detection in tissue specimens and BKV specific humoral response.
The presence of polyomavirus BK was analyzed by BKV L-Tag DNA detection in surgically excised PCa (n = 43/60, 72%) and BPH (n = 38/50, 76%) specimens. Positive samples (hereafter referred to as BKV+) were identified among both PCa (mean = 451.4 copies/105cells; n = 18/43, 42%) and BPH (mean = 391.5 copies/105cells; n = 12/38, 32%) with no significant quantitative differences among groups of patients (P = 0.72) (Fig. 1A). No significant association (χ2) with cancer (P = 0.18) or Gleason score (GS+ ≥7; P = 0.82) was observed. However, high PSA levels (PSA ≥ 4 ng/ml) were more frequently detectable in sera from patients with BKV+ PCa (P < 0.05).
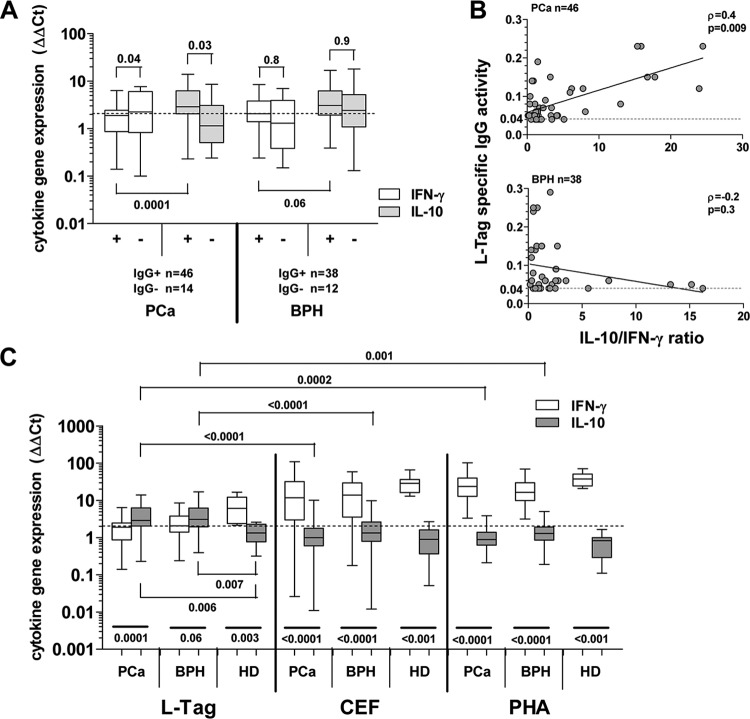
Fig 1.
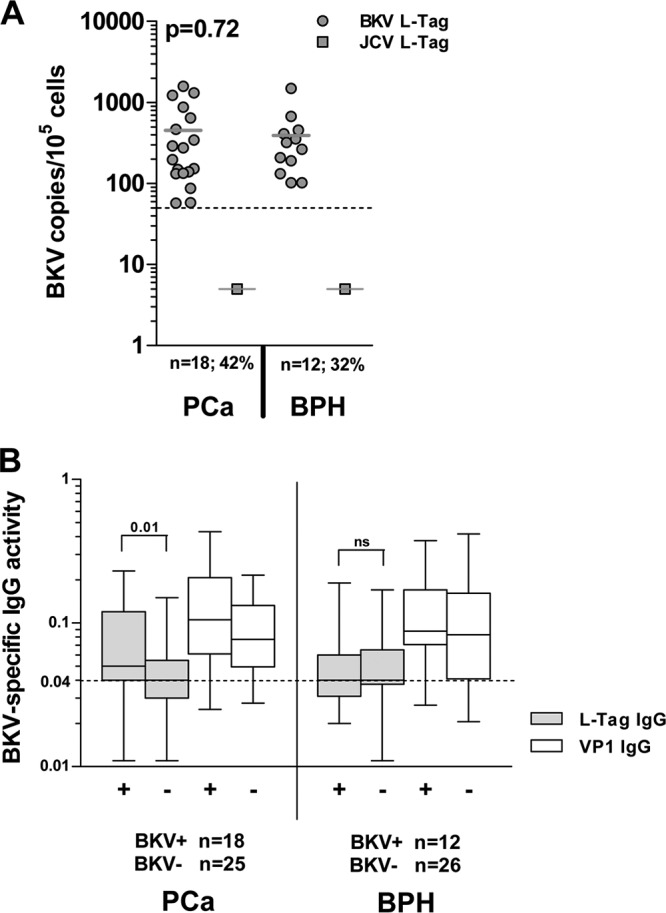
BKV L-Tag DNA detection in tissue specimens and L-Tag- or VP1-specific IgG response. (A) BKV L-Tag DNA was detectable in either PCa (n = 18/43, 42%) or BPH (n = 12/38, 32%) surgically excised lesions. Quantitative data, expressed as copy number/105 cells, were comparatively analyzed in positive PCa and BPH tissues. To emphasize the specificity of BKV detection in prostate specimens, JCV L-Tag sequence was also investigated. Rare samples displayed JCV L-Tag sequences in patients with PCa (n = 1/40, 2%) or in patients with BPH (n = 1/36, 3%) with copy numbers markedly below 50 copies/105 cells, the arbitrary limit for BKV L-Tag DNA detection selected in our study (cutoff; dotted line). (B) BKV L-Tag and VP1 IgG titer in patients with either PCa or BPH, stratified according to BKV L-Tag DNA molecular testing in their tissues (positive lesions, BKV+ or +; negative lesions, BKV− or −). Cutoff (dotted line) was set at 0.04, the lowest OD492 sufficient to detect antibody activity. Boxes and whiskers representing the 95% CI are also shown.
Humoral response against BKV VP1 and L-Tag was analyzed in sera from patients with PCa and BPH. Upon sample stratification based on BKV L-Tag DNA tissue testing, we noted that the levels of L-Tag-specific IgG (OD) were significantly higher in patients bearing BKV+ than BKV− PCa (P = 0.01), whereas they were similarly low in patients with BKV+ or BKV− BPH. In contrast, anti-VP1 IgG levels were similar in patients with PCa or BPH, irrespective of their BKV molecular status (Fig. 1B). Overall, detectable evidence of humoral responsiveness to L-Tag (OD ≥ 0.04) was observed in 17/18 (94%) patients with BKV+ PCa and in 7/12 (58%) patients with BKV+ BPH with a significant association (χ2) between BKV+ lesions and L-Tag IgG only in patients with PCa (P = 0.005) (Table 1).
Cytokine gene expression pattern in PBMC from patients with PCa or BPH upon ex vivo BKV L-Tag peptide pool stimulation.
Humoral response data, showing a high level of L-Tag IgG response in patients bearing BKV+ PCa, prompted us to explore cellular immune responsiveness to this antigen. Freshly isolated PBMC from 60 patients with PCa and 50 age-matched patients with BPH were ex vivo stimulated for 3 h with an L-Tag peptide pool, and IFN-γ and IL-10 cytokine gene expression were used as a readout. An HIV peptide pool with B gag motif was used as a negative control to provide a baseline, allowing the calculation of cytokine gene expression fold changes.
In L-Tag IgG-seronegative patients (PCa, n = 14; BPH, n = 12 [Tables 1 and 2]), regardless of underlying disease, L-Tag peptide pool stimulated cytokine gene expression was usually low (Fig. 2A). However, in L-Tag IgG+ patients with PCa (n = 46), L-Tag peptide pool induced a significantly higher IL-10 gene expression, (P = 0.03) but rather a decreased IFN-γ gene expression (P = 0.04) compared to seronegative patients. In contrast, in L-Tag IgG+ patients with BPH (n = 38), L-Tag peptide pool did not induce significantly higher IFN-γ (P = 0.8) and IL-10 (P = 0.9) gene expression compared to seronegative patients. Accordingly, these tests also showed that the L-Tag peptide pool induced a higher IL-10 than IFN-γ gene expression in L-Tag IgG+ patients with PCa (n = 46; P = 0.0001) and, to a lesser, nonsignificant extent in L-Tag IgG+ patients with BPH (n = 38; P = 0.06) (Fig. 2A).
Fig 2.
Cytokine gene expression upon ex vivo BKV L-Tag peptide pool stimulation in PBMC from patients bearing PCa or BPH or from healthy donors (HD). (A) IFN-γ (white boxes) and IL-10 (gray boxes) cytokine gene expression was measured upon ex vivo BKV L-Tag peptide pool stimulation of PBMC from patients with PCa and PBH, stratified according to their L-Tag-specific IgG activity (IgG+ ≥ 0.04). HIV peptide pool stimulation was used as background to compute fold changes in specific gene expression (cytokine gene relative quantification [2−ΔΔCT]). An arbitrary cutoff was set at 2-fold (dotted line). Boxes and whiskers representing the 95% CI show the significance of differential gene expression for each cytokine based on patient group stratifications. (B) Correlation (Spearman ρ) between cytokine gene expression, as detected upon ex vivo L-Tag peptide pool stimulation of PBMC from BKV L-Tag-seropositive patients with PCa or BPH and L-Tag-specific IgG. IL-10/IFN-γ gene expression ratios induced by L-Tag stimulation were plotted against L-Tag-specific IgG activity for all IgG+ PCa (n = 46; upper quadrant) and IgG+ BPH (n = 38; lower quadrant) patients. Cutoff (dotted line) was set at an OD492 of 0.04 (see the legend to Fig. 1). (C) Boxes and whiskers representing the 95% CI for IFN-γ (white boxes) and IL-10 (gray boxes) cytokine gene expression, as observed in PBMC from L-Tag IgG+ patients with PCa (n = 46), age-matched patients with BPH (n = 38), and non-age-matched but gender-matched healthy donors (HD; n = 8) after ex vivo stimulation with a BKV L-Tag peptide pool, a cytomegalovirus/Epstein-Barr virus/influenza virus peptide pool (CEF), or PHA. HIV peptide pool stimulation was used as the background to compute fold changes in specific gene expression (cytokine gene relative quantification [2−ΔΔCT]). An arbitrary cutoff was set at 2-fold (dotted line).
To investigate the relationship eventually occurring between the BKV L-Tag-specific humoral response and the L-Tag-specific gene expression pattern, the L-Tag-specific IgG activity was plotted against the IL-10/IFN-γ gene expression ratio. A significant direct correlation was indeed observed in patients with PCa (Spearman, ρ = 0.4, P = 0.009) but not in patients with BPH (ρ = −0.2, P = 0.3) (Fig. 2B).
These results led us to comparatively investigate cytokine gene expression induced by the L-Tag peptide pool, the CEF peptide pool, or PHA in L-Tag-seropositive patients with PCa or BPH and in seropositive gender- but not age-matched healthy donors. In contrast to PBMC from IgG+ patients with PCa and BPH (see above), L-Tag peptide pool stimulation of PBMC from L-Tag IgG+ healthy donors induced an IFN-γ gene expression significantly higher than IL-10 gene expression (n = 8; P = 0.003). Most importantly, the CEF peptide pool and PHA induced a similar, significantly higher IFN-γ than IL-10 gene expression in PBMC from patients with PCa or BPH (P < 0.0001) or from health donors (HD; P < 0.001) (Fig. 2C).
Notably, IL-10 gene expression induced by L-Tag peptide pool was significantly higher than IL-10 gene expression induced by CEF or PHA in PBMC from L-Tag IgG+ patients with PCa (P < 0.0001 and P = 0.0002, respectively) or BPH (P < 0.0001 and P = 0.001, respectively) (Fig. 2C). In PBMC from healthy donors, IL-10 gene expression was similarly negligible irrespective of the stimuli used. Remarkably, IFN-γ gene expression could be induced by CEF of PHA to comparably high extents in PBMC from L-Tag IgG+ patients with PCa or BPH or from healthy donors (Fig. 2C), thereby suggesting a specificity of the cytokine gene expression pattern induced by L-Tag stimulation in PBMC from L-Tag IgG+ patients.
BKV L-Tag peptide pool-stimulated cytokine gene expression pattern in PBMC from patients bearing BKV+ PCa or BPH.
We then evaluated in detail the L-Tag-specific cellular immune response in patients bearing BKV+ or BKV− PCa or BPH. Analysis of these data confirmed that a highly significant increase in IL-10 gene expression upon L-Tag peptide pool stimulation was detectable in PBMC from patients bearing a BKV+ PCa compared to those from patients bearing BKV− PCa (P < 0.0001). In contrast, IFN-γ gene expression induced by the L-Tag peptide pool was modest but detectable in PBMC from patients with BKV− PCa and significantly higher than that detectable in similarly stimulated PBMC from patients bearing BKV+ PCa (P = 0.02) (Fig. 3A). On the other hand, expression of IFN-γ and IL-10 genes upon L-Tag peptide pool stimulation was similar in PBMC from patients bearing BKV+ or BKV− BPH.
Fig 3.
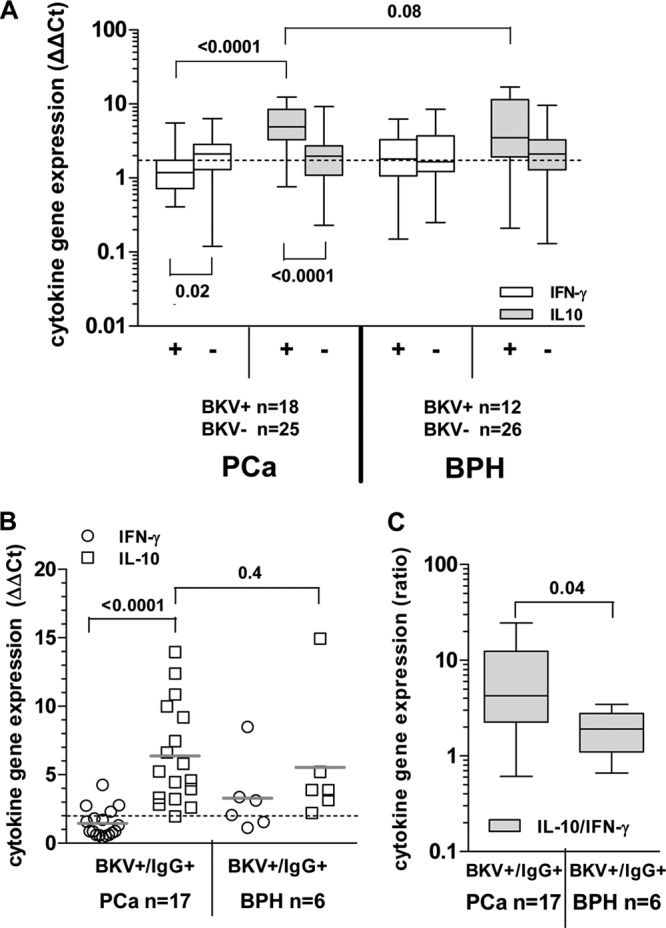
Cytokine gene expression pattern upon ex vivo BKV L-Tag peptide pool stimulation in L-Tag IgG+ patients bearing BKV+ PCa or BPH. (A) IFN-γ (white boxes) and IL-10 (gray boxes) cytokine gene expression was analyzed upon ex vivo BKV L-Tag peptide pool stimulation of PBMC from patients with PCa and PBH, stratified according to their BKV L-Tag DNA molecular testing (positive lesions, BKV+ or +; negative lesions, BKV− or −). HIV peptide pool stimulation was used as background to compute fold changes in specific gene expression (cytokine gene relative quantification [2−ΔΔCT]). An arbitrary cutoff was set at 2-fold (dotted line). Boxes and whiskers representing the 95% CI show the significance of differences observed for each cytokine gene expression based on patient group stratifications. (B) IFN-γ (white circles) and IL-10 (white squares) gene expression was measured upon L-Tag peptide pool stimulation (cutoff, 2-fold; dotted line) of PBMC from BKV+/IgG+ patients with PCa (n = 17) or BPH (n = 6). (C) IL-10/IFN-γ gene expression ratio observed after L-Tag peptide stimulation of PBMC from BKV+/IgG+ patients bearing BKV+ PCa or BKV+ BPH. Boxes and whiskers representing the 95% CI show differences in cytokine expression pattern between BKV+/IgG+ patient groups.
An analysis limited to patients with evidence of BKV L-Tag-specific IgG also showed a highly significant (P < 0.0001) predominance of IL-10 versus IFN-γ gene expression in patients bearing BKV+ prostate lesions. In contrast, the IFN-γ and IL-10 gene expression detectable upon L-Tag-triggered PBMC stimulation was similar in L-Tag IgG+ patients with BKV+ BPH (Fig. 3B).
Indeed, although IL-10 gene expression did not significantly differ between patients with BKV+ PCa or BKV+ BPH (P > 0.05) (Fig. 3A and B), most interestingly, the IL-10/IFN-γ gene expression ratio was significantly (P = 0.04) higher in BKV-specific IgG+ patients bearing BKV+ PCa (n = 17) than in BKV-specific IgG+ patients with BKV+ BPH (n = 6) (Fig. 3C).
Comparative analysis of L-Tag-induced cytokine gene expression in patients bearing BKV+ PCa with or without evidence of biochemical recurrence (BR).
The results obtained in the first part of our study suggested that a BKV-specific immune response is characterized by specific features, particularly in patients bearing BKV+ PCa, compared to patients with BPH, let alone healthy donors. These data raised the obvious issue of the association of this response pattern with PCa clinical course.
Therefore, considering their peculiar clinical condition, we analyzed in detail BKV L-Tag peptide-induced cytokine gene expression in PBMC from patients bearing BKV+ cancers with biochemical evidence of recurrence (BR+). BR was observed in 9/48 (19%) patients with PCa with complete follow-up, after 6 weeks (n = 6), 24 weeks (n = 1), 48 weeks (n = 1), and 96 weeks (n = 1). Two patients were excluded from the study because BKV molecular testing on tissue lesions was not available (Table 3). Despite the limited number of BR+ PCa patients, a significant association between BR status and BKV+ lesions, irrespective of serological status, was observed. Indeed, in 7/16 patients bearing BKV+ PCa, evidence of BR was detectable within a 96 weeks of follow-up, compared to none of 17 patients with BKV− PCa (Fisher exact test, P = 0.004).
Table 3.
Analysis of L-Tag IgG+ patients bearing BKV+ PCa with or without BRa
| Patientb | BR (PSA ng/ml)c | L-Tag DNA (copies/105 cells) | L-Tag IgG (OD492) |
|---|---|---|---|
| IgG+ BKV+ BR+** PCa (n = 7) | |||
| PCa 2 | 6 | NT | + |
| PCa 8 | 6 | + | + |
| PCa 18 | 6 | + | + |
| PCa 27 | 96 | + | + |
| PCa 37 | 6 | + | + |
| PCa 48 | 48 | + | + |
| PCa 49 | 24 | + | + |
| PCa 57 | 6 | + | + |
| PCa 60 | 6 | NT | + |
| IgG+ BKV+ BR−*** PCa (n = 8) | |||
| PCa 9 | – | + | + |
| PCa 16 | – | + | – |
| PCa 25 | – | + | + |
| PCa 33 | – | + | + |
| PCa 34 | – | + | + |
| PCa 39 | – | + | + |
| PCa 46 | – | + | + |
| PCa 53 | – | + | + |
| PCa 58 | – | + | + |
| IgG+ BKV+ BPH (n = 7) | |||
| BPH 6 | NA | + | + |
| BPH 12 | NA | + | + |
| BPH 21 | NA | + | + |
| BPH 27 | NA | + | + |
| BPH 30 | NA | + | + |
| BPH 39 | NA | + | + |
| BPH 48 | NA | + | + |
NA, not available; NT, not tested. BR, biochemical recurrence upon 5-year follow-up.
BR+, PSA ≥ 0.2 ng/ml; BR−, PSA < 0.04 ng/ml. Timing of biochemical recurrence is expressed in weeks in boldface type.
Then, we comparatively analyzed cytokine gene expression upon L-Tag stimulation in patients with BKV+ PCa stratified based on evidence of BR. In order to evaluate a more homogeneous test group, we excluded the only BR− patient with BKV+ PCa, seronegative for L-Tag-specific IgG (Table 3).
In BKV L-Tag-specific IgG+ patients with BKV+ PCa (n = 15), a distinctive L-Tag-induced gene signature prominently emerged. Indeed, significantly higher IL-10/IFN-γ (P < 0.01) and TGF-β1/IFN-γ (P = 0.02) gene expression ratios were observed in PBMC from patients bearing a BKV+ PCa with BR evidence (n = 7) compared to those detectable in PBMC from patients bearing a BKV+ PCa with no BR evidence (n = 8) (Fig. 4A). Importantly, in the former group, the extent of L-Tag-specific IgG response was significantly correlated with both the IL-10/IFN-γ (ρ = 0.8, P = 0.02) and the TGF-β1/IFN-γ (ρ = 0.7, P = 0.05) gene expression ratios induced in PBMC by L-Tag peptide stimulation (data not shown).
Fig 4.
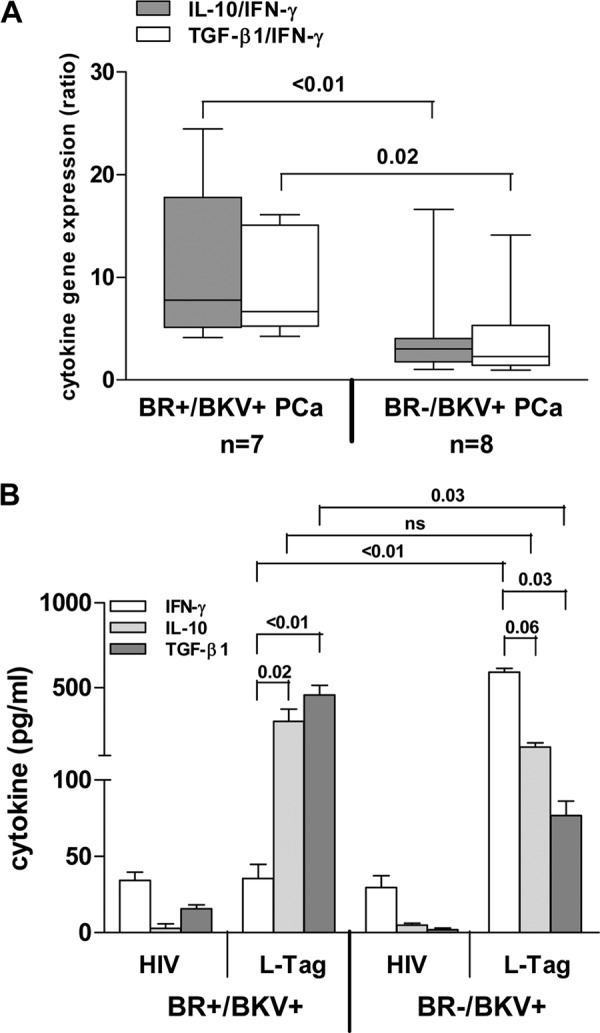
L-Tag peptide pool induced cytokine gene expression in L-Tag IgG+ patients bearing BKV+ PCa with or without evidence of biochemical recurrence (BR). (A) IL-10/IFN-γ (gray boxes) and TGF-β1/IFN-γ (white boxes) gene expression ratios, as detected after L-Tag peptide-specific ex vivo stimulation of PBMC from L-Tag IgG+ patients bearing BKV+ PCa with (n = 7) or without (n = 8) BR evidence. HIV peptide pool stimulation was used as a background to compute gene expression fold changes (2−ΔΔCT; cutoff, 2-fold). Boxes and whiskers representing the 95% CI show significant differences for both IL-10/IFN-γ and TGF-β1/IFN-γ gene expression ratios according to BR patient stratifications. (B) IFN-γ (white bars), IL-10 (light gray bars), and TGF-β1 (dark gray bars) protein production from 2-week in vitro-expanded PBMC from L-Tag IgG+ patients bearing BKV+ PCa with (n = 7) or without (n = 8) BR. HIV peptide pool in vitro stimulation was used as a control. Histograms ± the standard errors are reported.
To verify these findings at the protein level, we cultured CD4+ T cells from patients bearing BKV+ PCa with (n = 7) or without (n = 8) evidence of BR in the presence of autologous DC pulsed with either HIV derived or L-Tag peptide pools, and we analyzed IFN-γ, IL-10, and TGF-β1 release upon specific stimulation. In keeping with gene expression data (see above), we found that L-Tag-specific stimulation in BR+ patients with BKV+ cancers induced a significantly (P < 0.01) lower IFN-γ production and a significantly (P = 0.03) higher TGF-β1 production compared to cells from BR− patients bearing BKV+ PCa (Fig. 4b). Although IL-10 release did not significantly differ in the two groups, the IL-10/IFN-γ protein ratio was significantly (P < 0.01) higher in BR+ patients bearing BKV+ PCa.
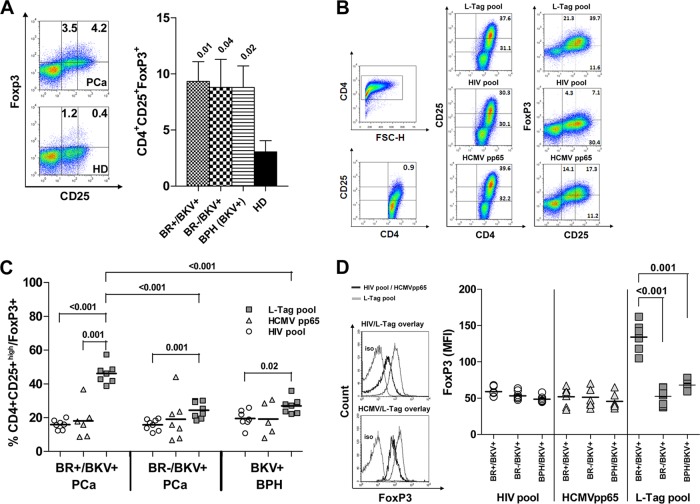
L-Tag peptide pool stimulation expands CD4+ CD25+(high) FoxP3+ T cells in patients bearing BKV+ PCa with BR.
The peculiar gene expression pattern induced by L-Tag in patients bearing BKV+ PCa, and, particularly, in BR+ patients, prompted us to investigate CD4+ regulatory (CD25+ FoxP3+) T cells in PBMC from these patients, both ex vivo and after L-Tag peptide pool-specific stimulation. In particular, to adequately control our study, we focused on cells from L-Tag-specific IgG+ patients bearing BKV+ PCa with or without BR (n = 7 and n = 8, respectively) and from L-Tag-specific IgG+ patients bearing BKV+ BPH (n = 7) (Table 3).
Peripheral blood CD4+ T cells from these patients were stained on their surfaces with anti-CD25 monoclonal antibody (MAb) and intracellularly with anti-Foxp3 MAb. No differences were detectable between patients with PCa, irrespective of BR, and patients with BPH regarding CD4+ CD25+ FoxP3+ T cells ex vivo frequency (Fig. 5A).
Fig 5.
BKV L-Tag-specific expansion of CD4+ T cell with a CD25+(high) FoxP3+ regulatory phenotype in L-Tag IgG+ patients bearing BR+ BKV+ PCa. (A) Representative fluorescence-activated cell sorting (FACS) density plot analysis of ex vivo FoxP3 expression in CD4+ CD25+ T cells in PBMC from a PCa patient (upper panel) and a healthy donor (lower panel). The histogram shows means ± the standard errors of ex vivo-detected frequencies of CD4+ CD25+ FoxP3+ T cells in L-Tag IgG+ patients with BR+/BKV+ PCa (dotted bar), BR−/BKV+ PCa (checkered bar) and BKV+ BPH (striped bar) compared to healthy donors (black bar). (B) Gating strategies for CD4+ T cell with a CD25+(high) FoxP3+ phenotype, as detectable upon peptide stimulation. (C) Percentages of CD4+ CD25+(high) FoxP3+ T cells in PBMC from L-Tag IgG+ patients with BR+/BKV+ PCa (n = 7), BR−/BKV+ PCa (n = 8), or BKV+ BPH (n = 7) upon HIV peptide pool (circles) or L-Tag peptide pool (squares) stimulation. HCMVpp65340-355 promiscuous peptide (triangles) was also used as an additional control on BR+/BKV+ PCa (n = 6), BR−/BKV+ PCa (n = 7) or BKV+ BPH (n = 5) L-Tag IgG+ patients. (D) Mean fluorescence intensity (MFI) of FoxP3 intracellular staining of cultured cells from L-Tag IgG+ patients with BR+/BKV+ PCa, BR−/BKV+ PCa or BKV+ BPH, after HIV peptide pool (circles), HCMVpp65340-355 promiscuous peptide (triangles), or L-Tag peptide pool (squares) in vitro stimulation. Overlaid histograms refer to FoxP3 MFI upon L-Tag peptide pool and HIV peptide pool (above) or L-Tag peptide pool and HCMVpp65340-355 promiscuous peptide (below) stimulations compared to isotype staining.
CD4+ T cells from the different groups of patients under investigation were then cultured for 2 weeks in the presence of autologous mDC pulsed with the L-Tag peptide pool, the HIV peptide pool, or the HCMVpp65340-355 promiscuous peptide. CD4+ CD25+(high) populations in cultured cells were identified as shown in Fig. 5B. After L-Tag peptide pool stimulation, cultures from BR+ patients bearing BKV+ PCa contained significantly higher percentages of CD4+ CD25+(high) FoxP3+ cells compared to similarly stimulated cultures from BR− patients bearing BKV+ PCa (P < 0.001) or to cultures from patients bearing BKV+ BPH (P < 0.001) (Fig. 5C). In contrast, HCMVpp65340-355 promiscuous peptide failed to expand CD4+ CD25+(high) FoxP3+ cells to extents significantly higher than HIV pool peptides in cells from any of the patient population under investigation. Furthermore, FoxP3+ protein expression upon L-Tag, but not HCMVpp65340–355 or HIV pool peptide stimulation, was significantly enhanced, as evaluated by mean fluorescence intensity (MFI) measurement, in cells from BR+ patients bearing BKV+ PCa in comparison to cells from BR− patients bearing BKV+ PCa (P < 0.001) or from patients bearing BKV+ BPH (P = 0.001) (Fig. 5D).
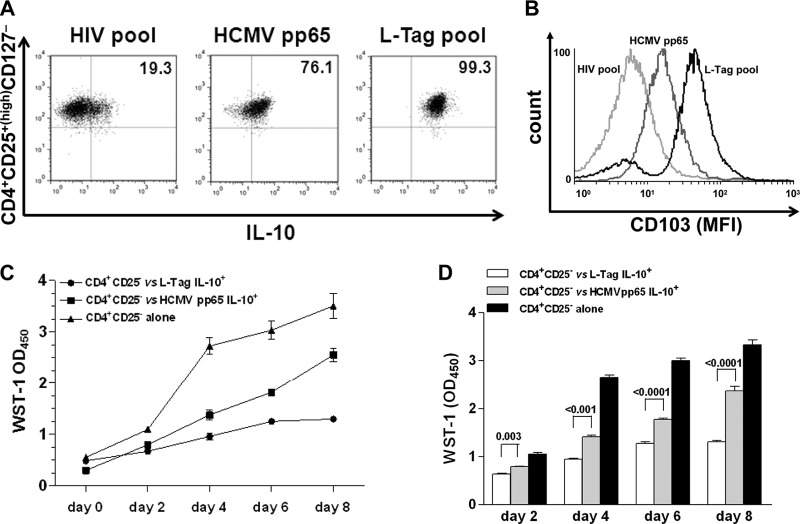
Functional effector memory phenotype (CD103) and regulatory activity of BKV L-Tag in vitro-generated CD4+ CD25+(high) CD127− T cells.
To investigate the potential functional relevance of the phenotypic modifications observed upon L-Tag stimulation, PBMC from BR+ patients bearing BKV+ PCa (n = 3) were cultured in the presence of L-Tag peptide pool, HCMVpp65340-355 promiscuous peptide and HIV peptide pool, as detailed in Materials and Methods. CD4+ CD25+(high) T cells were then sorted as CD127− and incubated in the presence of the antigens used for initial stimulation. High percentages of L-Tag and HCMVpp65340-355 stimulated CD4+ CD25+(high) CD127− T cells were able to produce IL-10, as detectable by intracellular staining (99.1% ± 0.2% and 78.9% ± 3.2%, respectively) (Fig. 6A). However, L-Tag-stimulated CD4+ CD25+(high) CD127− T cells showed a higher expression of CD103, a Treg effector memory marker (35), compared to HCMVpp65340-355 or HIV peptide pool-stimulated cells (Fig. 6B). Most importantly, L-Tag expanded CD4+ CD25+(high) CD127−, IL-10-secreting T cells were able to inhibit the proliferation of autologous anti-CD3/CD28 stimulated CD4+ CD25− T cells in coculture assays at a 0.5:1 ratio to a significantly higher extent than HCMVpp65340-355 expanded CD4+ CD25+(high) CD127− T cells (P < 0.0001) (Fig. 6C and D).
Fig 6.
Generation of BKV L-Tag-specific functional CD4+ CD25+(high) FoxP3+ T cells with effector-memory regulatory phenotype and suppressive activity. (A) Intracellular IL-10 protein production in CD4+ CD25+(high) CD127− T cells from one representative L-Tag IgG+ patient bearing a BKV+ PCa with BR, out of three studied, after L-Tag peptide pool stimulation (right quadrant) compared to HCMVpp65340-355 promiscuous peptide stimulation (middle quadrant) and negative control HIV peptide pool stimulation (left quadrant). (B) Expression of the regulatory T cell activation marker CD103 in IL-10-secreting CD4+ CD25+(high) CD127− T cells (see panel A) from one representative L-Tag IgG+ patient bearing a BKV+ PCa with BR, out of three tested. Overlaid histograms refer to L-Tag peptide pool-stimulated cells (black line), HCMVpp65340-355-stimulated cells (dark gray line), or negative control HIV peptide pool-stimulated cells (light gray line). (C) Proliferation index of anti-CD3/CD28-stimulated CD4+ CD25− T cells from one representative L-Tag IgG+ patient bearing a BKV+ PCa with BR, out of three tested, cocultured with autologous L-Tag peptide pool (circles) or HCMVpp65340-355 induced IL-10-secreting CD4+ CD25+(high) CD127− T cells (squares) or cultured alone (triangles) over 8 days. WST-1(OD450) values were plotted against days of incubation in cocultures performed at a 0.5:1 ratio. (D) Means ± standard errors of the proliferation index of anti-CD3/CD28 stimulated CD4+ CD25− T cells from the three IgG+ patients with BR+/BKV+ PCa tested, cocultured as described above.
DISCUSSION
BKV infection has repeatedly been suggested to be associated with cancers of the genitourinary tract. However, possibly due to conflicting results regarding detection of specific sequences and proteins in human cancers, its oncogenic role is controversial (1).
In a recent past, we have analyzed in detail cellular immune responses to BKV L-Tag-derived antigenic epitopes (39). Prompted by these studies, we have now addressed key features of BKV L-Tag-specific immune responsiveness in patients with PCa and the association of these features with the clinical course.
Our data reveal that in patients with PCa and, in particular, in patients showing biochemical evidence of tumor recurrence (BR+), immune responsiveness to BKV L-Tag is differentially characterized by a number of conspicuous features, compared to patients with no evidence of BR, or patients with BPH.
Indeed, in patients with BR+ PCa, a high titer of L-Tag-specific IgG is significantly associated with a high IL-10/IFN-γ gene expression ratio as observed after ex vivo PBMC stimulation with L-Tag peptides. Furthermore, in these patients, L-Tag peptide stimulation of PBMC in vitro results in a significantly higher expansion of a CD4+ CD25+(high) FoxP3+ population compared to patients bearing BKV+ PCa without BR evidence or with patients with BKV+ BPH. Notably, our data indicate that BKV L-Tag gene expression is detectable with similar frequency and at similar copy numbers in PCa and BPH tissues. Thus, we did not detect a preferential BKV gene expression in PCa (5) but rather a different pattern of L-Tag-specific immune responses.
At difference with capsid proteins, polyomavirus L-Tag is not present in viral particles but is only produced in infected cells and localizes in their nuclei. Therefore, while humoral response to capsid proteins is widely detectable after infection, induction of L-Tag-specific humoral responses is less likely to take place, unless cell death repeatedly occurs, which has been suggested to be the case in cancer tissues (38). Alternatively, we might hypothesize that only BKV abortive infections, e.g., those possibly leading to cancer transformation of infected cells, would permit L-Tag exposure to the immune system, thus favoring the induction of an antibody response (38). Indeed, our data show a significant association between L-Tag-specific IgG levels and L-Tag molecular detection in patients with PCa but not in patients with BPH. In contrast, VP1 IgG levels were similar in patients with PCa and BPH, irrespective of BKV detection in prostate lesions, partially reflecting humoral responses observed against Merkel cell polyomavirus (MCV) VP1 in Merkel cell carcinoma (MCC), a cancer associated with MCV infection (50).
Cellular immune responsiveness to BKV L-Tag is also characterized by a number of peculiar features. In particular, in PBMC from patients with PCa, the L-Tag peptide-specific response is characterized by an IL-10 gene expression significantly higher than that of IFN-γ gene. In PBMC from patients with BPH a similar trend is detectable, whereas in cells from healthy donors, IFN-γ gene expression is significantly higher than that of IL-10 gene. This gene expression pattern is unique to BKV L-Tag-stimulated cells because in PBMC from patients with PCa or BPH or from healthy donors, other viral peptides (CEF pool) or PHA similarly induce an IFN-γ gene expression significantly higher than that of IL-10 gene. Therefore, the skewed responsiveness to BKV L-Tag cannot be merely attributed to a physiological decline in immune fitness, which frequently occurs in elderly individuals.
Taken together, these data suggest that the analysis of systemic BKV seroprevalence, as determined by IgG activity against viral capsid proteins, fails to support an association between BKV infection and PCa (38). Instead, humoral and, most of all, cellular responsiveness to BKV L-Tag skewed toward an immunoregulatory gene expression profile and characterized by a high IL-10/IFN-γ gene expression ratio, appears to be associated with PCa. Most conspicuously, this pattern of responsiveness is typically detectable in cells from patients with recurrent PCa.
Interestingly, recent data indicate that PBMC from patients with MCC might fail to produce IFN-γ in response to specific L-Tag peptides (28). In this case as well, infection by MCV was found to induce L-Tag-specific humoral responses predominantly in cancer patients. However, L-Tag-stimulated IL-10 production was not explored.
It is tempting to speculate that the skewed cytokine gene expression signature typically detectable upon L-Tag peptide stimulation could be due to immunoregulatory activities of cells preferentially secreting IL-10. However, whereas IFN-γ is only produced by T and NK cells, IL-10 can be produced by several cell types, including tumor cells (10, 21), virus-infected cells (9), different types of activated antigen-presenting cells, and T cells.
We found similar numbers of circulating cells expressing classical CD4+ CD25+(high) FoxP3+ Treg phenotype in patients with PCa or BPH, irrespective of their BR and L-Tag molecular status. However, our data indicate that in vitro stimulation of PBMC from patients with recurrent BKV+ PCa, but not from patients with BKV+ PCa and no BR evidence or from patients with BKV+ BPH, results in the significant expansion of cells characterized by a typical Treg phenotype. These lymphocytes uniformly express IL-10, detectable by intracellular staining, which is consistent with a predominant role in the elicitation of the skewed cytokine gene expression profile induced by BKV L-Tag in PBMC from patients bearing BKV+ PCa. Admittedly, the sole expression and/or release of IL-10 does not represent a reliable signature for regulatory T cell involvement (44) since it can also be induced in effector cells to reduce inflammation at the site of acute viral infections (49). However, the detection of this cytokine in combination with both TGF-β1 and permanent FoxP3 expression is usually associated with regulatory functions (2, 52). Most importantly, L-Tag-induced CD4+ CD25+(high) FoxP3+ CD103+ T cells also display a remarkable capacity to inhibit the proliferation of autologous anti-CD3/CD28-stimulated CD4+ CD25− lymphocytes compared to HCMVpp65-stimulated CD4+ CD25+(high) FoxP3+ CD103+/− T cells, in accordance with recently published reports (46, 56). Collectively, these data hint at mechanisms potentially underlying our findings in ex vivo-activated PBMC.
Immunoregulatory activities driven by viral antigens and promoting the establishment of chronic infections have already been described (18, 20). These functions can be elicited by recruiting either CD4+ or CD8+ Treg cells, depending on the type of infection (30). The involvement of both Treg subsets is dictated by HLA-restricted virus-specific stimulation taking place at the site of infection and presupposes both the expression of viral antigens and of Treg cell-derived cytokines, such as IL-10 and TGF-β1 (18, 30, 31). Moreover, circulating tumor-associated antigen-specific T cells with a regulatory phenotype [CD25+(high) FoxP3+] and the ability of secreting IL-10 and exerting suppressive functions have also been described in metastatic melanoma patients (51).
Still unclear is the molecular background preferentially favoring the expansion of these cells in patients bearing BKV+ PCa. It is relevant, however, that prostate tissues appear to be characterized by features consistent with an immunosuppressive microenvironment. Infiltrating CD4+ and CD8+ T lymphocytes are predominantly characterized by regulatory (32, 36) and functionally exhausted (PD-1+, B7-H1+) (8, 17, 47) phenotypes. Furthermore, enhanced suppressive function of adaptive CD4+ Treg cells has been observed in the peripheral blood of patients with PCa and found to correlate with metastatic behavior (55).
We previously showed that indoleamine-2,3-deoxygenase (IDO) gene is frequently expressed at high levels in PCa (19). The specific gene product plays a key role in tryptophan metabolism, and its enhanced activities might result in both the depletion of an amino acid essential for lymphocyte metabolism and the generation of toxic metabolites (53). In addition, arginase production by macrophages infiltrating prostatic tissues has been shown to favor the induction of anergy in resident lymphocytes (8). Therefore, in patients bearing a BKV+ PCa, presentation of L-Tag to T cells might occur in conditions likely favoring the generation of immune responses characterized by a high IL-10/IFN-γ gene expression ratio.
Most obviously, our data do not allow the postulation of any causal relationship between features of L-Tag-specific immune responses, BKV infection, and cancer. Nevertheless, they suggest the existence of a complex interaction of potential clinical relevance.
Our study has a major limitation, however. Although it capitalizes on the analysis of specific immune responses in a substantial number of patients, the number of informative cases, e.g., patients bearing BKV+ lesions with or without BR, is relatively modest. Therefore, additional investigations confirming these results in a larger number of cases are obviously warranted.
In conclusion, our data provide important novel contributions to the analysis of the increasingly puzzling relationship between BKV infection and PCa by identifying subpopulations of patients deserving major attention in translational research and, most importantly, by highlighting subtle peculiarities of immune responses against BKV L-Tag in patients with PCa, with previously unsuspected associations with the clinical course of this disease.
ACKNOWLEDGMENTS
This study was partially supported by the Foundation for Research at the Medical Faculty, University of Zurich (grant to G.S.), and the Swiss National Science Foundation (grant 320000-122429/1 to M.P. and grant 320030-120320 to G.C.S.).
We thank Gaetano Mauro for helping us in statistical data analysis and Damina Balmer for her critical comments.
Footnotes
Published ahead of print 30 May 2012
REFERENCES
- 1. Abend JR, Jiang M, Imperiale MJ. 2009. BK virus and human cancer: innocent until proven guilty. Semin. Cancer Biol. 19:252–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baecher-Allan C, Viglietta V, Hafler DA. 2004. Human CD4+ CD25+ regulatory T cells. Semin. Immunol. 16:89–98 [DOI] [PubMed] [Google Scholar]
- 3. Balis V, et al. 2007. Prevalence of BK virus and human papillomavirus in human prostate cancer. Int. J. Biol. Markers 22:245–251 [DOI] [PubMed] [Google Scholar]
- 4. Bargonetti J, Reynisdottir I, Friedman PN, Prives C. 1992. Site-specific binding of wild-type p53 to cellular DNA is inhibited by SV40 T antigen and mutant p53. Genes Dev. 6:1886–1898 [DOI] [PubMed] [Google Scholar]
- 5. Bergh J, et al. 2007. No link between viral findings in the prostate and subsequent cancer development. Br. J. Cancer 96:137–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bocchetta M, et al. 2008. The SV40 large T antigen-p53 complexes bind and activate the insulin-like growth factor-I promoter stimulating cell growth. Cancer Res. 68:1022–1029 [DOI] [PubMed] [Google Scholar]
- 7. Bodaghi S, et al. 2009. Antibody responses to recombinant polyomavirus BK large T and VP1 proteins in young kidney transplant patients. J. Clin. Microbiol. 47:2577–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bronte V, et al. 2005. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J. Exp. Med. 201:1257–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burdin N, Peronne C, Banchereau J, Rousset F. 1993. Epstein-Barr virus transformation induces B lymphocytes to produce human interleukin 10. J. Exp. Med. 177:295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen Q, Daniel V, Maher DW, Hersey P. 1994. Production of IL-10 by melanoma cells: examination of its role in immunosuppression mediated by melanoma. Int. J. Cancer 56:755–760 [DOI] [PubMed] [Google Scholar]
- 11. Currier JR, et al. 2002. A panel of MHC class I restricted viral peptides for use as a quality control for vaccine trial ELISPOT assays. J. Immunol. Methods 260:157–172 [DOI] [PubMed] [Google Scholar]
- 12. Das D, Shah RB, Imperiale MJ. 2004. Detection and expression of human BK virus sequences in neoplastic prostate tissues. Oncogene 23:7031–7046 [DOI] [PubMed] [Google Scholar]
- 13. Das D, Wojno K, Imperiale MJ. 2008. BK virus as a cofactor in the etiology of prostate cancer in its early stages. J. Virol. 82:2705–2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. De Marzo AM, Marchi VL, Epstein JI, Nelson WG. 1999. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am. J. Pathol. 155:1985–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Marzo AM, et al. 2007. Inflammation in prostate carcinogenesis. Nat. Rev. Cancer 7:256–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dong JT. 2006. Prevalent mutations in prostate cancer. J. Cell Biochem. 97:433–447 [DOI] [PubMed] [Google Scholar]
- 17. Ebelt K, et al. 2009. Prostate cancer lesions are surrounded by FOXP3+, PD-1+, and B7-H1+ lymphocyte clusters. Eur. J. Cancer 45:1664–1672 [DOI] [PubMed] [Google Scholar]
- 18. Ebinuma H, et al. 2008. Identification and in vitro expansion of functional antigen-specific CD25+ FoxP3+ regulatory T cells in hepatitis C virus infection. J. Virol. 82:5043–5053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feder-Mengus C, et al. 2008. High expression of indoleamine 2,3-dioxygenase gene in prostate cancer. Eur. J. Cancer 44:2266–2275 [DOI] [PubMed] [Google Scholar]
- 20. Garba ML, Pilcher CD, Bingham AL, Eron J, Frelinger JA. 2002. HIV antigens can induce TGF-beta(1)-producing immunoregulatory CD8+ T cells. J. Immunol. 168:2247–2254 [DOI] [PubMed] [Google Scholar]
- 21. Gastl GA, et al. 1993. Interleukin-10 production by human carcinoma cell lines and its relationship to interleukin-6 expression. Int. J. Cancer 55:96–101 [DOI] [PubMed] [Google Scholar]
- 22. Gosert R, et al. 2008. Polyomavirus BK with rearranged noncoding control region emerge in vivo in renal transplant patients and increase viral replication and cytopathology. J. Exp. Med. 205:841–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gretzer MB, Trock BJ, Han M, Walsh PC. 2002. A critical analysis of the interpretation of biochemical failure in surgically treated patients using the American Society for Therapeutic Radiation and Oncology criteria. J. Urol. 168:1419–1422 [DOI] [PubMed] [Google Scholar]
- 24. Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: the next generation. Cell 144:646–674 [DOI] [PubMed] [Google Scholar]
- 25. Hoffman NG, Cook L, Atienza EE, Limaye AP, Jerome KR. 2008. Marked variability of BK virus load measurement using quantitative real-time PCR among commonly used assays. J. Clin. Microbiol. 46:2671–2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Imperiale MJ. 2000. The human polyomaviruses, BKV and JCV: molecular pathogenesis of acute disease and potential role in cancer. Virology 267:1–7 [DOI] [PubMed] [Google Scholar]
- 27. Imperiale MJ. 2001. Oncogenic transformation by the human polyomaviruses. Oncogene 20:7917–7923 [DOI] [PubMed] [Google Scholar]
- 28. Iyer JG, et al. 2011. Merkel cell polyomavirus-specific CD8 and CD4 T-cell responses identified in Merkel cell carcinomas and blood. Clin. Cancer Res. 17:6671–6680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jemal A, et al. 2011. Global cancer statistics. CA Cancer J. Clin. 61:69–90 [DOI] [PubMed] [Google Scholar]
- 30. Joosten SA, Ottenhoff TH. 2008. Human CD4 and CD8 regulatory T cells in infectious diseases and vaccination. Hum. Immunol. 69:760–770 [DOI] [PubMed] [Google Scholar]
- 31. Karlsson I, et al. 2007. FoxP3+ CD25+ CD8+ T-cell induction during primary simian immunodeficiency virus infection in cynomolgus macaques correlates with low CD4+ T-cell activation and high viral load. J. Virol. 81:13444–13455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kiniwa Y, et al. 2007. CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin. Cancer Res. 13:6947–6958 [DOI] [PubMed] [Google Scholar]
- 33. Leuenberger D, et al. 2007. Human polyomavirus type 1 (BK virus) agnoprotein is abundantly expressed but immunologically ignored. Clin. Vaccine Immunol. 14:959–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 35. Mellor AL, Munn DH. 2011. Physiologic control of the functional status of Foxp3+ regulatory T cells. J. Immunol. 186:4535–4540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Miller AM, et al. 2006. CD4+ CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J. Immunol. 177:7398–7405 [DOI] [PubMed] [Google Scholar]
- 37. Monini P, et al. 1995. DNA rearrangements impairing BK virus productive infection in urinary tract tumors. Virology 214:273–279 [DOI] [PubMed] [Google Scholar]
- 38. Paulson KG, et al. 2010. Antibodies to Merkel cell polyomavirus T antigen oncoproteins reflect tumor burden in Merkel cell carcinoma patients. Cancer Res. 70:8388–8397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Provenzano M, et al. 2006. Characterization of highly frequent epitope-specific CD45RA+/CCR7+/− T lymphocyte responses against p53-binding domains of the human polyomavirus BK large tumor antigen in HLA-A*0201+ BKV-seropositive donors. J. Transl. Med. 4:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Provenzano M, et al. 2006. MHC-peptide specificity and T-cell epitope mapping: where immunotherapy starts. Trends Mol. Med. 12:465–472 [DOI] [PubMed] [Google Scholar]
- 41. Provenzano M, et al. 2009. A HCMV pp65 polypeptide promotes the expansion of CD4+ and CD8+ T cells across a wide range of HLA specificities. J. Cell Mol. Med. 13:2131–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Putzi MJ, De Marzo AM. 2000. Morphologic transitions between proliferative inflammatory atrophy and high-grade prostatic intraepithelial neoplasia. Urology 56:828–832 [DOI] [PubMed] [Google Scholar]
- 43. Randhawa PS, et al. 2002. Quantitation of viral DNA in renal allograft tissue from patients with BK virus nephropathy. Transplantation 74:485–488 [DOI] [PubMed] [Google Scholar]
- 44. Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. 2001. Type 1 T regulatory cells. Immunol. Rev. 182:68–79 [DOI] [PubMed] [Google Scholar]
- 45. Rubinstein R, Schoonakker BC, Harley EH. 1991. Recurring theme of changes in the transcriptional control region of BK virus during adaptation to cell culture. J. Virol. 65:1600–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schwele S, et al. 2012. Cytomegalovirus-specific regulatory and effector T cells share TCR clonality-possible relation to repetitive CMV infections. Am. J. Transplant. 12:669–681 [DOI] [PubMed] [Google Scholar]
- 47. Sfanos KS, et al. 2009. Human prostate-infiltrating CD8+ T lymphocytes are oligoclonal and PD-1+. Prostate 69:1694–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stockis J, Colau D, Coulie PG, Lucas S. 2009. Membrane protein GARP is a receptor for latent TGF-beta on the surface of activated human Treg. Eur. J. Immunol. 39:3315–3322 [DOI] [PubMed] [Google Scholar]
- 49. Sun J, Dodd H, Moser EK, Sharma R, Braciale TJ. 2011. CD4+ T cell help and innate-derived IL-27 induce Blimp-1-dependent IL-10 production by antiviral CTLs. Nat. Immunol. 12:327–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Touze A, et al. 2011. High levels of antibodies against Merkel cell polyomavirus identify a subset of patients with Merkel cell carcinoma with better clinical outcome. J. Clin. Oncol. 29:1612–1619 [DOI] [PubMed] [Google Scholar]
- 51. Vence L, et al. 2007. Circulating tumor antigen-specific regulatory T cells in patients with metastatic melanoma. Proc. Natl. Acad. Sci. U. S. A. 104:20884–20889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. 2004. Loss of functional suppression by CD4+ CD25+ regulatory T cells in patients with multiple sclerosis. J. Exp. Med. 199:971–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Weber WP, et al. 2006. Differential effects of the tryptophan metabolite 3-hydroxyanthranilic acid on the proliferation of human CD8+ T cells induced by TCR triggering or homeostatic cytokines. Eur. J. Immunol. 36:296–304 [DOI] [PubMed] [Google Scholar]
- 54. Weinreb DB, et al. 2006. Polyoma virus infection is a prominent risk factor for bladder carcinoma in immunocompetent individuals. Diagn. Cytopathol. 34:201–203 [DOI] [PubMed] [Google Scholar]
- 55. Yokokawa J, et al. 2008. Enhanced functionality of CD4+ CD25high FoxP3+ regulatory T cells in the peripheral blood of patients with prostate cancer. Clin. Cancer Res. 14:1032–1040 [DOI] [PubMed] [Google Scholar]
- 56. Zabransky DJ, et al. 2012. Phenotypic and functional properties of helios+ regulatory T cells. PLoS One 7:e34547 doi:10.1371/journal.pone.0034547 [DOI] [PMC free article] [PubMed] [Google Scholar]