Abstract
Previous studies have described the role of p53 isoforms, including p53β and Δ133p53α, in the modulation of the activity of full-length p53, which regulates cell fate. In the context of influenza virus infection, an interplay between influenza viruses and p53 has been described, with p53 being involved in the antiviral response. However, the role of physiological p53 isoforms has never been explored in this context. Here, we demonstrate that p53 isoforms play a role in influenza A virus infection by using silencing and transient expression strategies in human lung epithelial cells. In addition, with the help of a panel of different influenza viruses from different subtypes, we also show that infection differentially regulates the expressions of p53β and Δ133p53α. Altogether, our results highlight the role of p53 isoforms in the viral cycle of influenza A viruses, with p53β and Δ133p53α acting as regulators of viral production in a p53-dependent manner.
INTRODUCTION
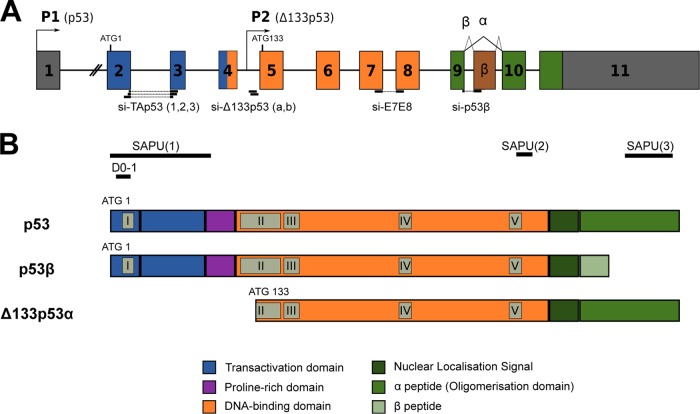
In response to stress, p53 is rapidly accumulated in the nucleus, thus regulating gene expression to regulate cell fate (10). In addition to full-length p53 (also named p53α), the TP53 gene expresses 12 p53 protein isoforms, due to the use of alternative promoters, splicing sites, and translational initiation sites. Hence, four N-terminal forms can be combined into three distinct C-terminal forms (2, 9, 15, 16). Among them, Δ133p53α and p53β are produced by two different mechanisms (Fig. 1). The internal TP53 promoter (P2) regulates the transcription of Δ133p53 mRNA, initiated in intron 4 and encoding Δ133p53 protein isoforms, which lack the entire transactivation domain and part of the DNA-binding domain (2). On the other hand, p53β mRNA retains part of intron 9 by alternative splicing and encodes the p53β protein isoform, the oligomerization domain of which is replaced by 10 new amino acids (2).
Fig 1.
Genetic and structural organization of human p53 isoforms. (A) The human TP53 gene contains a proximal promoter, P1, and an internal promoter, P2 ( ), regulating the expressions of p53 and Δ133p53 transcripts, respectively. In addition, alternative splicing (∧) has been described for the 3′ part of TP53 gene, which results in the partial inclusion of intron 9, encoding the β forms. Bold lines indicate locations of sequences targeted by the different siRNAs. (B) Among the human p53 protein isoforms, three are studied in this report. The canonical p53 protein (or full-length p53) contains an N-terminal transactivation domain (blue), a central DNA-binding domain (orange), and a C terminus with a nuclear localization signal and an oligomerization domain (green). Compared to p53, Δ133p53α lacks the entire transactivation domain and part of the DNA-binding domain, while p53β exhibits new residues in place of the oligomerization domain. Gray boxes indicate a domain conserved throughout evolution. Bold lines indicate the epitope regions recognized by the different antibodies used in this study. ATG, initiation site of translation.
), regulating the expressions of p53 and Δ133p53 transcripts, respectively. In addition, alternative splicing (∧) has been described for the 3′ part of TP53 gene, which results in the partial inclusion of intron 9, encoding the β forms. Bold lines indicate locations of sequences targeted by the different siRNAs. (B) Among the human p53 protein isoforms, three are studied in this report. The canonical p53 protein (or full-length p53) contains an N-terminal transactivation domain (blue), a central DNA-binding domain (orange), and a C terminus with a nuclear localization signal and an oligomerization domain (green). Compared to p53, Δ133p53α lacks the entire transactivation domain and part of the DNA-binding domain, while p53β exhibits new residues in place of the oligomerization domain. Gray boxes indicate a domain conserved throughout evolution. Bold lines indicate the epitope regions recognized by the different antibodies used in this study. ATG, initiation site of translation.
It was reported previously that p53β can directly bind p53 target gene promoters and modulate p53 transcriptional activity on a p53-responsive promoter in a promoter-dependent manner (2). In contrast, Δ133p53α acts as a modulator of full-length p53 in response to stress, inhibiting p53-mediated apoptosis and G1 cell cycle arrest without inhibiting p53-mediated G2 cell cycle arrest, suggesting that Δ133p53α promotes p53-dependent cell survival in response to stress (1, 3). Moreover, in normal human fibroblasts, Δ133p53α inhibits, while p53β promotes, p53-mediated replicative senescence (6). In accordance with roles in p53-suppressive functions, several studies reported a deregulation of p53 isoform expression in human cancers (13). Since multiple interplays between p53 and viruses have been described, Rohaly and colleagues recently investigated the role of a short form of p53 (Δp53), lacking part of the p53 DNA-binding domain, in simian virus 40 (SV40) replication (22). Interestingly, physiological p53 isoforms, including Δ133p53α and p53β, have never been explored in the context of a viral infection.
Influenza A viruses belong to the family Orthomyxoviridae of enveloped viruses and contain a segmented genome of single-stranded negative RNA (21). These viruses are some of the few RNA viruses to undergo replication and transcription within the host cell nucleus, thus having direct access to nuclear host factors and machineries to successfully support their viral replication cycle (7). Several studies have underlined the interplay between influenza viruses and different signaling pathways, notably the apoptotic pathways (5, 11, 12, 29). Recently, in a study based on a transcriptional profiling of influenza virus-infected human cells, we have shown that the p53 pathway was massively downregulated in response to influenza A virus infection (26), indicating that the p53 pathway is of particular interest to better understand influenza virus interactions with the host cell. Only a few studies have reported interplays between influenza viruses and p53 (19, 20, 31). Using p53-null cell lines, the ectopic expression of a dominant negative p53 mutant, or treatment with p53 inhibitors, several studies have identified p53 as an antiviral protein. However, the mechanisms underlying this activity are still poorly characterized (19, 25–27).
Based on current knowledge of the role of p53 isoforms in the activities of full-length p53, p53 isoforms might play a role in viral infection, in particular in response to infection by influenza viruses. In infected human lung epithelial cells, we studied the impact of p53 isoform expression on viral replication but also the impact of different influenza A viruses on Δ133p53α and p53β expression. Our results show distinct roles of the Δ133p53α and p53β isoforms in the viral cycle of influenza viruses, which may help us to further understand the antiviral activity of p53.
MATERIALS AND METHODS
Cell lines, viruses, and infection.
Human lung epithelial A549 (wild-type 53; ATCC CCL-185) and H1299 (p53 null; ATCC CRL-5803) cells were maintained at 37°C in Dulbecco's modified Eagle's medium (DMEM; Life Technologies) supplemented with 10% heat-inactivated fetal calf serum, 2 mM l-glutamine, and 0.5% gentamicin, under a 5% CO2 atmosphere.
Influenza viruses A/New Caledonia/20/99 (H1N1), A/Moscow/10/99 (H3N2), A/Turkey/582/2006 (H5N1), A/Finch/England/2051/94 (H5N2), and A/Chicken/Italy/2076/99 (H7N1) were produced in Madin-Darby canine kidney (MDCK) cells (ATCC CCL-34) in Eagle's minimum essential medium (EMEM; Life Technologies) supplemented with 2 mM l-glutamine, 100 U of penicillin/ml, 100 mg of streptomycin sulfate/ml, and 1 μg of trypsin/ml. Viruses were titrated to determine the 50% tissue culture infectious dose (TCID50) in MDCK cells by endpoint titration (log10 TCID50/ml) (18) Viral titers were measured in quadruplicate in two independent experiments and compiled to perform a statistical analysis (Student t test). Viral kinetics in A549 cells were described in a previous study (8). Influenza A virus strains A/New Caledonia/20/99 (H1N1), A/Moscow/10/99 (H3N2), A/Finch/England/2051/94 (H5N2), and A/Chicken/Italy/2076/99 (H7N1) were obtained from the French national influenza monitoring network GROG (Groupes Régionaux d'Observation de la Grippe, Lyon, France) and the WHO NIMR/MRC collaborative center (kindly provided by Alan Hay). Influenza A virus strain A/Turkey/582/2006 A(H5N1) was obtained from the National Reference Center of Turkey. H5N1, H5N2, and H7N1 viruses were manipulated separately in biosafety level 3 (BSL3) facilities (VirPath Lab).
Human lung epithelial A549 and H1299 cells (cell confluence, 80%) were infected at a multiplicity of infection (MOI) of 0.001, 0.1, or 4 TCID50/cell. After a 1-h viral adsorption period, cells were overlaid with DMEM with 2 mM l-glutamine and 0.5% gentamicin supplemented with 1 μg/ml trypsin (Roche Diagnostics) and further infected at 37°C. Cells were harvested for real-time quantitative PCR (RT-qPCR) or Western blot analysis at 4 and 8 h postinfection (hpi) or at 24 hpi, respectively.
siRNA targeting p53 isoforms in A549 cells.
The silencing of p53 isoforms was performed with A549 cells (cell confluence, 50%) seeded in antibiotic-free medium and directly transfected with 50 nM either nonspecific (si-NC, negative control) (catalog number 0R-0030-Neg05; Eurogentec) or p53-targeted small interfering RNAs (siRNAs) (see Table S1 in the supplemental material), using 4 μl of Oligofectamine (Life Technologies) according to the manufacturer's instructions. Three pools of siRNAs were designed: si-p53, which targets all p53 isoforms; si-Δ133, which targets only Δ133 forms (Δ133p53α, Δ133p53β, and Δ133p53γ); and si-β, which targets only β forms (p53β, Δ40p53β, and Δ133p53β) (1). Cells were transfected twice at 24-h intervals and infected after 48 h of treatment. The efficiency of siRNA-mediated knockdown after treatment was evaluated by Western blotting and/or RT-qPCR.
Transient expression of p53 isoforms in H1299 cells.
p53, Δ133p53α, and p53β transcripts were previously cloned into a pSV expression vector (pSV-p53, pSV-Δ133p53α, and pSV-p53β) (2). H1299 cells at 60% confluence were transfected with 1 μg of each construct (3 μg of total DNA adjusted with the pSV-empty plasmid), using 12 μl of Fugene (Roche). Cells were infected at 24 h posttransfection. The levels of expression of p53 isoforms were assessed by Western blotting.
Real-time quantitative PCR.
Total RNAs were extracted by using the RNeasy minikit (Qiagen). Reverse transcription was performed on 1 μg of total RNAs by using SuperScriptII (Life Technologies) at 42°C. The quantification of p53 isoform mRNA levels was carried out by real-time quantitative PCR on an MX3005P apparatus (Stratagene). Briefly, 10 ng of cDNAs was amplified by using 1× TaqMan Universal Master Mix (Applied Biosystems), 0.8 μM primers, and 0.4 μM probes (see Table S1B in the supplemental material). Primers and probes were designed to amplify total p53 mRNA levels expressed by the TP53 gene (all p53 isoforms), N-terminal Δ133 forms (Δ133p53α, Δ133p53β, and Δ133p53γ), or C-terminal β forms (p53β, Δ40p53β, and Δ133p53β) (1). The ΔΔCT method was used to determine the fold change of mRNA levels, using the human actin gene as a reference (23). The mRNA levels were measured in triplicate in three independent experiments and compiled to perform a statistical analysis (Student t test). A significant difference was considered to be a P value of <0.05.
The quantification of the matrix (M) viral genome segments released into supernatants by RT-qPCR after viral RNA (vRNA) extraction was previously described (4). Briefly, vRNAs were isolated with a QIAamp viral mRNA minikit (Qiagen) and were subjected to reverse transcription by using SuperScriptII (Life Technologies) at 42°C. cDNAs were amplified by using 1× TaqMan Universal Master Mix (Applied Biosystems), 1 μM primers, and 0.5 μM probe (see Table S1B in the supplemental material). The primers targeting the M gene and designed for the universal detection of influenza A viruses were previously described for classical RT-PCR (30). For each RT-qPCR run, 10-fold dilution series of a calibrated synthetic M RNA transcript were tested in duplicate and used for absolute quantification, presented as log10 RNA copies/ml. The vRNA levels were measured in triplicate in three independent experiments and compiled to perform a statistical analysis (Student t test). A significant difference was considered to be a P value of <0.05.
Western blotting.
Proteins were extracted by scraping and syringing cells in 1× NuPAGE LDS buffer (Invitrogen). Fifteen micrograms to 30 μg of protein extracts was separated on precast 10% NuPAGE gels (Invitrogen). Monoclonal mouse DO-1 antibody was used to detect p53 and p53β isoforms, since its epitope located in the transactivation domain is lacking in Δ133p53α (9). Polyclonal sheep SAPU antibody detects all p53 isoforms, while monoclonal rabbit KJC8 antibodies recognize only β forms. Monoclonal mouse Ku80 antibody (AbCam) was used as a loading control.
RESULTS AND DISCUSSION
Silencing of TP53 gene products increases viral production.
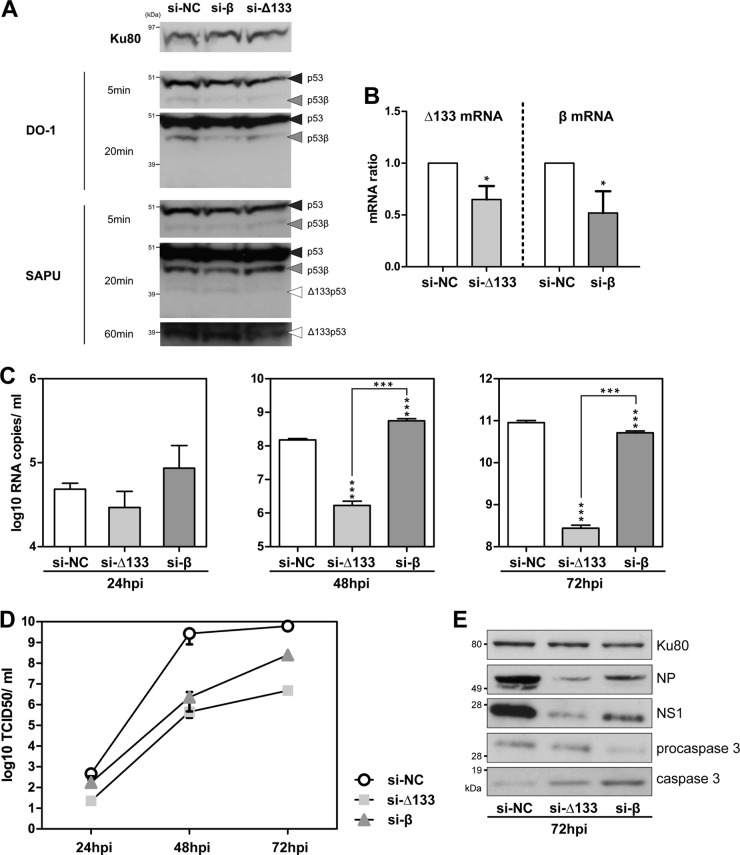
Since the identification of 12 p53 isoforms expressed by the TP53 gene, no study has investigated whether the antiviral role of p53 can be attributed to full-length p53 without the requirement of p53 isoforms or to the global expression of p53 isoforms. To address this question, we took advantage of human lung A549 cells, which endogenously express three p53 isoforms at the protein level: full-length p53 (or p53α), p53β, and Δ133p53α. The expression of each p53 isoform can be specifically repressed in A549 cells upon the transfection of p53 isoform-specific siRNAs, as previously described (Fig. 1; see also Fig. 3) (1, 16).
Fig 3.
Knockdown of Δ133p53 or p53β mRNA expression in A549 cells differentially modulates H1N1 influenza virus production. (A) Characterization of p53 isoform expression in A549 cells. To identify each p53 isoform, human lung A549 cells were treated with siRNAs (nonspecific si-NC, or siRNA-Δ133 or si-p53β, targeting Δ133p53 and p53β, respectively) and were analyzed by Western blotting with two p53 antibodies, DO-1 and SAPU (see Materials and Methods). SAPU antibody is sufficient to detect both p53β (47 kDa) and Δ133p53α (39 kDa). (B) Knockdown expression was verified by RT-qPCR using a set of primers and probes specific for the Δ133 or β form at 48 h posttransfection. (C to E) Forty-eight hours after siRNA treatment, cells were infected with influenza virus A/New Caledonia/20/99 (H1N1) at an MOI of 0.001, and the cells and supernatants were then harvested at 24, 48, and 72 hpi for analysis. Viral production was assessed by three different techniques: the quantification of M viral genome segments (log10 RNA copies/ml) released into the supernatants by RT-qPCR after vRNA extraction (measured in triplicate in 3 independent experiments) (C), the determination of infectious titers of supernatants (log10 TCID50/ml) by endpoint titration in MDCK cells (measured in quadruplicate in 2 independent experiments) (D), and analysis of influenza NP and NS1 expression levels by Western blotting at 72 hpi (E). Expression levels of procaspase 3 and cleaved caspase 3 were also monitored by Western blotting at 72 hpi. For panels B and C, * and *** indicate P values of <0.05 and <0.001, respectively, determined by the Student t test.
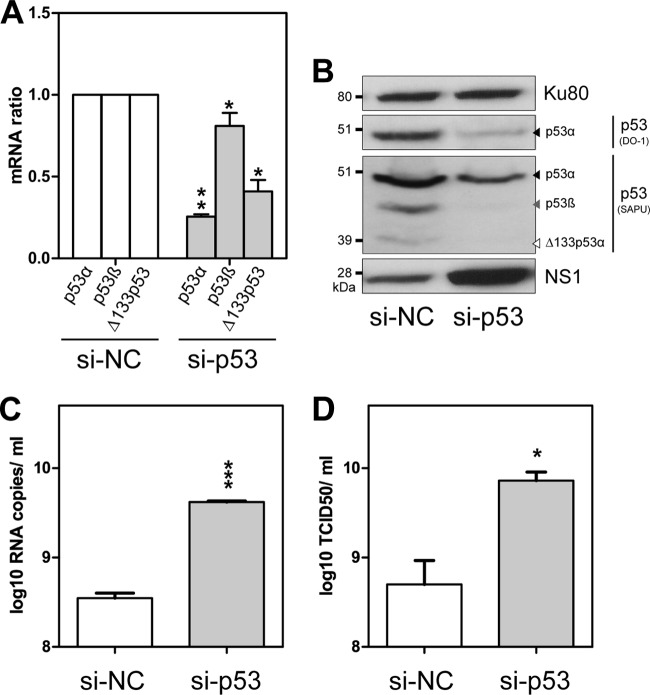
First, we evaluated the impact of the knockdown of all p53 isoforms on influenza virus production. A549 cells were transfected with either a pool of siRNA (si-p53) targeting all p53 isoforms or a nonspecific siRNA (si-NC), used as a negative control (see Table S1A in the supplemental material). After 48 h, the siRNA efficiency was verified by RT-qPCR for each p53 mRNA isoform (Fig. 2A). Transfected cells were then infected with influenza virus A/New Caledonia/20/99 (H1N1) at a multiplicity of infection (MOI) of 0.001. Viral supernatants and cell extracts were harvested at 48 h postinfection (hpi). Compared to cells treated with si-NC, the levels of expression of all p53 protein isoforms were reduced in cells transfected with si-p53 at 48 hpi, indicating that the knockdown by si-p53 was efficient for all p53 isoforms (Fig. 2B).
Fig 2.
Knockdown of total p53 expression in A549 cells increases viral production. Human lung A549 cells were transfected with either a nonspecific siRNA (negative-control si-NC) or a pool of siRNAs targeting all p53 isoforms, termed si-p53 (see Table S1A in the supplemental material). (A) After 48 h of treatment, the siRNA efficiency was verified by the quantification of p53α, p53β, and Δ133p53 mRNA levels by RT-qPCR. mRNA levels were measured in triplicate in two independent experiments and compiled to perform a statistical analysis. Cells were then infected at an MOI of 0.001 by influenza virus A/New Caledonia/20/99 (H1N1). Supernatants and cell lysates were collected at 48 h postinfection for further analysis, and the data are presented in panels B to D. (B) Analysis of p53 and influenza virus NS1 expression levels by Western blotting. Two different antibodies for the detection of p53 and p53 isoforms were used (DO-1 and SAPU); the characterization and annotation of p53 isoforms in A549 cells by Western blotting was performed with the help of specific siRNAs (Fig. 3). Ku80 was used as a loading control. (C) Quantification of M viral genome segments (log10 RNA copies/ml) released into the supernatants by RT-qPCR after vRNA extraction (measured in triplicate in 3 independent experiments). (D) Determination of infectious titers of supernatants (log10 TCID50/ml) by endpoint titration in MDCK cells (measured in quadruplicate in 2 independent experiments). For panels A, C, and D, *, **, and *** indicate P values of <0.05, <0.005, and <0.001, respectively, determined by the Student t test.
Viral production at 48 hpi was assessed by two methods: (i) the quantification of influenza M genome copies by RT-qPCR (Fig. 2C) and (ii) the measurement of infectious viral titers by endpoint titration in supernatants (Fig. 2D), as previously described (4, 18). We observed that the viral M genome copy number was significantly increased by about 10 times in the supernatant of si-p53-transfected cells compared to that in the supernatant of si-NC-transfected cells (Fig. 2C). This result was confirmed by infectious titers, with a more-than-1-log10 increase of the TCID50 under si-p53 conditions compared to si-NC conditions (Fig. 2D). In accordance with these results, an increased expression level of the NS1 viral protein was observed by Western blotting under si-p53 compared to si-NC conditions, also indicating an increased level of viral production in cells depleted of all p53 protein isoforms (Fig. 2B). Similar results were obtained with another influenza A virus subtype [A/Moscow/10/99 (H3N2)] (data not shown). Altogether, these results demonstrate that the global expression of the TP53 gene has an antiviral activity. Our data are consistent with previous reports showing that the p53 protein is involved in the cellular antiviral response against influenza virus infection (26, 27). In addition, in a high-throughput study, Shapira and colleagues previously found an antiviral role for p53 by using a pool of siRNAs targeting the 3′ untranslated region (3′UTR) of p53 mRNA common to all p53 isoforms (24). Overall, these data indicate that the TP53 gene products inhibit influenza virus production.
The Δ133p53 isoform modulates p53-dependent antiviral activity.
It was reported previously that variations in p53 isoform expression levels can modulate gene expression in p53-dependent and -independent manners, thus regulating cell fate in response to stress (1, 2, 6, 14). In order to evaluate the role of p53 isoforms in influenza virus production in cells expressing the wild-type TP53 gene, A549 cells expressing endogenous p53 isoforms were transfected with siRNAs targeting specifically either the Δ133p53 (si-Δ133) or the p53β (si-β) mRNA or the siRNA si-NC, used as a negative control (see Materials and Methods; see also Table S1A in the supplemental material). Hence, in A549 cells, the transfection of the siRNA si-Δ133 specifically represses the expression of Δ133p53α, while the transfection of the siRNA si-β specifically represses the expression of p53β. The efficiency of siRNA silencing in A549 cells was verified by Western blotting and RT-qPCR before infection (Fig. 3A and B). A549 cells were infected 48 h after siRNA transfection by influenza virus A/New Caledonia/20/99 (H1N1) at an MOI of 0.001, and supernatants were harvested at 24, 48, and 72 hpi. Upon transfection with si-Δ133, the numbers of M genome copies released into the supernatant significantly decreased at 48 hpi and 72 hpi compared to si-NC-transfected cells, with more than 89 and 342 times fewer genome copies, respectively (Fig. 3C). This result suggests that the level of viral production is decreased upon the depletion of Δ133p53 mRNAs. The measurement of titers of infectious virus released into the cell supernatant confirmed that cells transfected with si-Δ133 produced significantly fewer virions at 48 hpi and 72 hpi than si-NC-transfected cells (Fig. 3D). Consistently, Western blot analysis of si-Δ133-transfected cells extracted at 72 hpi showed reduced expression levels of the NS1 and NP viral proteins (Fig. 3E). We excluded the possibility of any artifactual interference between the siRNAs and viral production, as siRNA treatment had no effect on viral production in a p53-null cell line (see Fig. S1A in the supplemental material). The same results were observed for another virus subtype, influenza virus A/Moscow/10/99 (H3N2), with the depletion of Δ133p53 mRNAs being associated with a reduction in levels of viral production from 24 hpi in A549 cells (see Fig. S1 in the supplemental material). The knockdown of Δ133p53 expression was associated with a slight increase of the p53 (p53α) protein level (see Fig. S1E in the supplemental material). Altogether, our results show that the level of production of influenza viruses decreases upon the depletion of Δ133p53 mRNAs, suggesting a proviral effect of Δ133p53α on A549 cells.
Viral production is also altered in response to si-β transfection but to a lesser extent than in response to si-Δ133 transfection (Fig. 3). The depletion of p53β mRNAs was associated with statistically significant changes in the numbers of M genome copies released into the supernatant, with M genome copy numbers increasing at 48 hpi (3.7 times) and then decreasing at 72 hpi (1.7 times) compared to transfection with si-NC (Fig. 3C). The decrease in the level of viral production at 72 hpi was also confirmed by Western blot analysis. The level of the NS1 viral protein was reduced in cells transfected with si-β compared to cells transfected with si-NC (Fig. 3E). However, this pattern of viral production throughout time was not observed for determinations of viral titers (Fig. 3D). At 24, 48, and 72 hpi, lower infectious titers were measured in si-β-transfected cells than in si-NC-transfected cells. The discordance at 48 hpi of viral production, as determined by quantifications of viral genome copy numbers and titers of infectious virus, may be explained by an increased cell death of the si-β-transfected cells at 48 hpi, releasing viral genome copies into the supernatants before virion generation. Indeed, the increased level of cleaved caspase 3 associated with a reduction of procaspase 3 expression levels in si-β-transfected cells compared to si-NC-transfected cells suggests an induction of apoptosis in si-β-transfected cells infected with H1N1 (Fig. 3E). This may explain the increased release of genome copies without virion generation at 48 hpi (Fig. 3C). The general decrease in the level of virus production upon the depletion of p53β mRNAs was also observed with influenza virus A/Moscow/10/99 (H3N2) (see Fig. S1 in the supplemental material). Of note, infectious titers as well as M genome copy numbers and viral protein expression levels remained higher in si-β-transfected cells than in si-Δ133-transfected cells. Moreover, the knockdown of p53β expression did not change p53 (p53α) protein levels (see Fig. S1E in the supplemental material). Our results indicate that the viral production of influenza virus decreases upon the depletion of p53β mRNAs, suggesting that p53β plays a proviral role in influenza virus infection of A549 cells.
Altogether, our results indicate that the depletion of p53β mRNAs or of Δ133p53 mRNAs decreases viral production and suggest a possible proviral role of p53β and Δ133p53α in A549 cells expressing the wild-type TP53 gene.
The role of p53β and Δ133p53α in influenza virus production is p53 dependent.
To decipher the role of the p53β and Δ133p53α isoforms, we took advantage of human H1299 epithelial lung cells, which carry a partial homozygous deletion of the TP53 gene and thus lack the expression of all p53 isoforms. H1299 cells were transfected with different combinations of pSV vectors expressing the full-length p53 (pSV-p53), Δ133p53α (pSV-Δ133p53α), or p53β (pSV-p53β) protein. The expression of p53 isoforms was assessed by Western blot analysis at 24 h posttransfection (Fig. 4A). We verified that cell viability was not affected by transfection at 24 h posttreatment. Cells were infected by influenza virus A/New Caledonia/20/99 (H1N1) at an MOI of 0.001 at 24 h posttransfection, and supernatants were harvested at 24 hpi. The M genome copy numbers were first measured in H1299 cells transfected with an empty vector (pSV-empty), indicating a level of viral production similar to that in nontransfected cells (6 log10 RNA copies/ml) (Fig. 4B, lane A).
Fig 4.
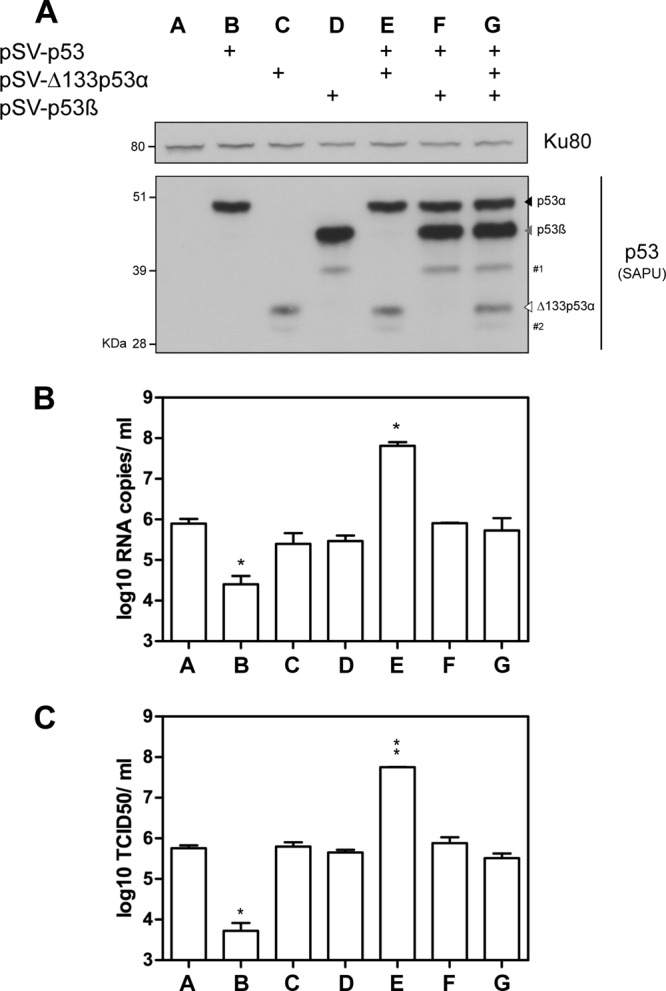
Transient coexpression of p53 isoforms in H1299 cells differentially impacts the level of viral production. Human lung H1299 cells (p53 null) were transfected with different combinations of pSV constructs expressing p53, Δ133p53α, or p53β. (A) The level of ectopic protein expression was verified at 24 h posttransfection by Western blotting using the SAPU antibody, which recognizes all p53 isoforms. Ku80 was used as a loading control. #1 and #2 indicate Δ40p53β and Δ160p53α (14), respectively. Transfected cells were then infected by influenza virus A/New Caledonia/20/99 (H1N1) at an MOI of 0.01. Supernatants and cells were harvested at 24 hpi, and viral production was assessed by two different techniques: RT-qPCR (log10 RNA copies/ml, measured in triplicate in 3 independent experiments) (B) and the determination of infectious titers of supernatants (log10 TCID50/ml) by endpoint titration in MDCK cells (measured in quadruplicate in 2 independent experiments) (C). For panels B and C, * and ** indicate P values of <0.05 and <0.005, respectively, determined by the Student t test.
Compared to pSV-empty-transfected cells, the number of M genome copies released into the supernatants was significantly lower in cells transfected with full-length p53, with about 33 times fewer genome copies (Fig. 4B, lane B versus A). A similar result was obtained when the infectious titers were quantified (Fig. 4C, lane B versus A). This observation is consistent with the antiviral role of the full-length p53 protein, as previously described (19, 20, 27). Unlike full-length p53 (p53α), the ectopic expression of Δ133p53α or p53β alone was not associated with significant differences in viral production compared to that in cells transfected with pSV-empty (Fig. 4B and C, lanes C and D versus A), suggesting that Δ133p53α or p53β does not alter influenza virus production in the absence of full-length p53.
We thus investigated whether Δ133p53α or p53β affects viral production in the presence of full-length p53. With this aim, H1299 cells were cotransfected with p53 and different combinations of vectors expressing the Δ133p53α (pSV-Δ133p53α) or p53β (pSV-p53β) protein. Compared to the cotransfection of p53 with pSV-empty, the cotransfection of p53 with p53β restored viral production (Fig. 4B and C, lane F versus B). This indicates that p53β inhibits the antiviral activity of p53.
Interestingly, the cotransfection of p53 with Δ133p53α significantly increased numbers of M genome copies and infectious particles released into supernatants by 200 times compared to transfection with the pSV-empty vector (Fig. 4B and C, lane E versus A) and by 1,000 times compared to the cotransfection of p53 with pSV-empty (Fig. 4B and C, lane E versus B). Accordingly, cells cotransfected with p53 and Δ133p53α produced about 100 times more virus than cells transfected with pSV-empty and about 20,000 times more virus than cells cotransfected with pSV-empty and p53. This indicates that when combined, p53 and Δ133p53α exert a synergistic proviral effect on influenza virus production (Fig. 4B and C, lane E versus B and C). Similar results were obtained with influenza virus A/Moscow/10/99 (H3N2) (see Fig. S2A and S2B in the supplemental material). Moreover, this is in accordance with siRNA experiments, where the depletion of Δ133p53 mRNAs in A549 cells expressing p53 significantly reduced viral production. Altogether, our results show that p53 combined with Δ133p53α increased influenza virus production. This may be explained by the fact that Δ133p53α promotes p53-dependent cell survival in response to stress (1–3, 6, 14). In addition, the cotransfection of p53β with p53 and Δ133p53α inhibited the proviral effect of p53 combined with Δ133p53α (Fig. 4B and C, lane G versus E), suggesting an interplay of p53β with p53 and Δ133p53α.
To our knowledge, this is the first time that a biological function issued from the combined expression of p53, p53β, and Δ133p53α is demonstrated. Previous studies showed that endogenous p53β can form a protein complex with p53 and that endogenous p53β directly binds promoters of p53 target genes in a promoter-dependent manner (2). Moreover, we have demonstrated that p53 and Δ133p53α can form a protein complex (1). We thus think that p53 transcriptional activity is modulated by the combined expression of p53β and Δ133p53α. In addition, these modulations could have an impact on the antiviral activity of p53, at the level of the interferon (IFN) response, as suggested by the different levels of Stat1 phosphorylation observed, depending on the expression of the p53β and Δ133p53α isoforms (see Fig. S2C in the supplemental material). However, other levels of regulation (cell cycle or apoptosis) could be involved. Further experiments will be required to decipher the interplay between p53 and its combined isoforms regarding the antiviral facet of p53.
Altogether, our results based on the ectopic expression of p53, p53β, and/or Δ133p53α demonstrate a proviral role of full-length p53 in the presence of the Δ133p53α protein, which can be modulated by p53β. Therefore, these results suggest that influenza virus production could be controlled by differentially regulating the expression of p53, p53β, and Δ133p53α.
Influenza A virus infection alters p53 isoform expression.
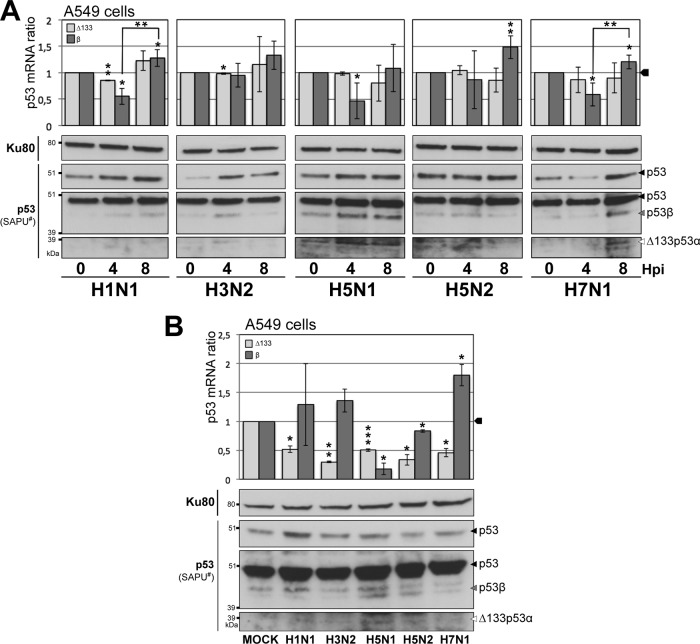
Altogether, silencing and ectopic expression experiments demonstrated the role of the p53β and Δ133p53α isoforms in regulating virus production. We then investigated the impact of influenza virus infection on p53 isoform expression at the mRNA and protein levels with human lung A549 cells infected with influenza A viruses of different origins and subtypes (H1N1, H3N2, H5N1, H5N2, and H7N1). This experiment was performed at the scale of 1 viral cycle (Fig. 5A) or after several viral cycles (Fig. 5B), by infecting cells at an MOI of 4 for 4 hpi and 8 hpi or at an MOI of 0.1 for 24 h, respectively. The expression of p53 was analyzed by Western blotting, while p53β and Δ133p53α isoform expressions were analyzed at the mRNA and protein levels by RT-qPCR and Western blotting, respectively.
Fig 5.
Influenza virus infection modulates both mRNA and protein levels of the Δ133p53 and p53β isoforms. (A) Human lung A549 cells were infected at an MOI of 4 with influenza viruses A/New Caledonia/20/99 (H1N1), A/Moscow/10/99 (H3N2), A/Turkey/582/2006 (H5N1), A/Finch/England/2051/94 (H5N2), and A/Chicken/Italy/2076/99 (H7N1) and were harvested at 4 and 8 h postinfection for analysis. The expression levels of the Δ133p53 and p53β isoforms were analyzed at the mRNA level by RT-qPCR (top) and at the protein level by Western blotting (bottom). (B) Human lung A549 cells were infected at an MOI of 0.1 with the same panel of influenza viruses used for panel A and were harvested at 24 h postinfection for analysis. The expressions of the Δ133p53 and p53β isoforms were analyzed at the mRNA level by RT-qPCR (top) and at the protein level by Western blotting (bottom), using SAPU antibody, which recognizes all p53 isoforms. In panels A and B, mRNA levels were measured in triplicate in two independent experiments and compiled to perform a statistical analysis (*, **, and *** indicate P values of <0.05, 0.005, and 0.001, respectively, determined by the Student t test). Primers and probes used for RT-qPCR are given in Table S1B in the supplemental material. The Ku80 protein was used as a loading control for Western blots. # indicates different film exposures with the SAPU antibody. The characterization and annotation of p53 isoforms in A549 cells in Western blots were performed with the help of specific siRNAs (Fig. 3; see also Table S1A in the supplemental material).
Each influenza virus subtype induced p53 protein accumulation at 4 hpi or 8 hpi at an MOI of 4 (Fig. 5A), while no significant accumulation of the full-length p53 protein was observed in response to influenza virus infection at 24 hpi at an MOI of 0.1 (except in H1N1-infected cells) (Fig. 5B). This suggests that p53 accumulation can occur at the level of 1 viral cycle. Expression levels of Δ133p53 mRNAs were significantly reduced at 4 hpi at an MOI of 4 only in H1N1-infected cells (Fig. 5A, top). Moreover, expression levels of Δ133p53 mRNAs were significantly reduced in infected cells at 24 hpi at an MOI of 0.1 for every viral subtype studied compared to mock-infected cells (Fig. 5B).
The relative expression levels of Δ133 mRNA were reduced by about 50% under each condition at 24 hpi, indicating a strong impact of viral infection on Δ133p53 expression at late stages of infection. At the protein level, the Δ133p53α protein was barely detectable at 0 hpi, but Δ133p53α accumulation was observed for two subtypes (H5N1- and H7N1-infected cells) (Fig. 5A, bottom), suggesting that the Δ133p53α protein is regulated at the posttranscriptional level notably in H5N1- and H7N1-infected cells.
Overall, Δ133p53α was significantly repressed at the mRNA level during late stages of infection in A549 cells, suggesting that the p53 internal promoter is repressed during influenza virus infection. Few studies have identified transcriptional factors that regulate p53 internal promoter activity. The full-length p53 protein itself has been identified as an inducer of Δ133p53α expression (1, 14). The generally decreased expression levels of Δ133p53α observed in this study could be explained by the inactivation of the full-length p53 protein during influenza virus infection, since a recent transcriptomic analysis showed that most of the p53 target genes are downregulated in response to influenza virus infection (26). Thus, the decreased expression levels of Δ133p53α in response to influenza virus infection may result from the inactivation of the p53 protein. In addition, a recent study showed the role of a p53-homologous protein, p63, in decreasing the expression level of Δ133p53α during keratinocyte differentiation, suggesting that a transcription factor other than p53 can regulate Δ133p53 mRNA expression during influenza virus infection (17).
Contrary to Δ133p53α, the impact of influenza virus infection on p53β expression was more drastic at early stages than at late stages of infection. Indeed, in H1N1-, H5N1-, and H7N1-infected cells, β mRNA levels were significantly decreased at 4 hpi and significantly increased at 8 hpi, suggesting a differential regulation of β mRNA expression during influenza virus infection (Fig. 5A, top). Even in H3N2- and H5N2-infected cells, where changes in β mRNA expression levels did not reach statistical significance, the same profile was observed: a decrease at 4 hpi followed by an increase at 8 hpi. Moreover, it is worth noting that the profile of β mRNA expression conserved throughout all influenza virus subtypes was also observed for total p53 mRNA levels in H3N2- and H5N1-infected cells (data not shown). Thus, for the p53β protein, decreased expression at the mRNA level was observed at 4 hpi, followed by an increased expression level at 8 hpi, suggesting a subtle regulation of p53β expression during the viral cycle. The regulation of p53β protein expression has not yet been characterized, and influenza virus infection seems to be an interesting tool to assess the specific regulation of endogenous p53β expression. In contrast, at 24 hpi at an MOI of 0.1, the variations of β mRNA or p53β protein levels were different from one subtype to another (Fig. 5B). While no variation was observed in H3N2-infected cells, the p53β protein accumulated in H1N1-infected cells without any variation in mRNA levels. In H5N2-infected cells, β mRNA levels, but not p53β protein levels, were significantly reduced. Finally, H7N1-infected cells showed a significant increase of β mRNA levels and a reduction of p53β protein expression levels, while H5N1-infected cells expressed significantly less β mRNA and high levels of the p53β protein. Altogether, these observations showed that all subtypes of influenza virus differentially regulate p53 isoform expression at the transcriptional and posttranscriptional levels. Previous findings indicated a possible direct interaction between the NS1 protein and p53 (28). We hypothesize that this interaction may play a role in the interplay between p53 isoforms and influenza viruses, which needs to be further investigated.
In conclusion, this study has explored, for the first time, the role of the Δ133p53α and p53β isoforms in the context of a viral infection. Our results showed an interplay between these two p53 isoforms and influenza A viruses, where (i) influenza virus infection modulates the expressions of Δ133p53α and p53β at the transcriptional and posttranscriptional levels and, reciprocally, (ii) a modulation of Δ133p53α and p53β expression levels affects viral production. A preliminary model emerges where the Δ133p53α and p53β isoforms act as regulators of influenza virus production in a full-length-p53-dependent manner. The composition, relative ratios, and kinetics of p53 isoform expression probably contribute to determining the level of the global p53-mediated antiviral response during the time course of infection. Future investigations will determine the relative contribution of each p53 isoform to the antiviral or proviral activity of p53 and explore the different biological processes implicated, such as the cell cycle, apoptosis, and the immune response. These approaches will contribute to further investigations of the cross talk between p53 isoforms but also to a better understanding of the role of p53 and its functional partners in influenza virus-host interactions.
Supplementary Material
ACKNOWLEDGMENTS
We thank Kenneth Fernandes for helpful advice and discussion. We also thank Vincent Moulès for experiments performed in the BSL3 facility.
O.T. was funded by Cancer Research UK; V.M. was funded by the Breast Cancer Campaign.
Footnotes
Published ahead of print 30 May 2012
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1. Aoubala M, et al. 2011. p53 directly transactivates Δ133p53α, regulating cell fate outcome in response to DNA damage. Cell Death Differ. 18:248–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bourdon JC, et al. 2005. p53 isoforms can regulate p53 transcriptional activity. Genes Dev. 19:2122–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen J, et al. 2009. p53 isoform delta113p53 is a p53 target gene that antagonizes p53 apoptotic activity via BclxL activation in zebrafish. Genes Dev. 23:278–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Duchamp MB, et al. 2010. Pandemic A(H1N1)2009 influenza virus detection by real time RT-PCR: is viral quantification useful? Clin. Microbiol. Infect. 16:317–321 [DOI] [PubMed] [Google Scholar]
- 5. Ehrhardt C, Ludwig S. 2009. A new player in a deadly game: influenza viruses and the PI3K/Akt signalling pathway. Cell. Microbiol. 11:863–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fujita K, et al. 2009. p53 isoforms delta133p53 and p53beta are endogenous regulators of replicative cellular senescence. Nat. Cell Biol. 11:1135–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Josset L, Frobert E, Rosa-Calatrava M. 2008. Influenza A replication and host nuclear compartments: many changes and many questions. J. Clin. Virol. 43:381–390 [DOI] [PubMed] [Google Scholar]
- 8. Josset L, et al. 2010. Gene expression signature-based screening identifies new broadly effective influenza A antivirals. PLoS One 5(10):pii=e13169. doi:10.1371/journal.pone.0013169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khoury MP, Bourdon JC. 2010. The isoforms of the p53 protein. Cold Spring Harb. Perspect. Biol. 2(3):a000927 doi:10.1101/cshperspect.a000927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levine AJ, Oren M. 2009. The first 30 years of p53: growing ever more complex. Nat. Rev. Cancer 9:749–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lowy RJ. 2003. Influenza virus induction of apoptosis by intrinsic and extrinsic mechanisms. Int. Rev. Immunol. 22:425–449 [DOI] [PubMed] [Google Scholar]
- 12. Ludwig S, Pleschka S, Planz O, Wolff T. 2006. Ringing the alarm bells: signalling and apoptosis in influenza virus infected cells. Cell. Microbiol. 8:375–386 [DOI] [PubMed] [Google Scholar]
- 13. Machado-Silva A, Perrier S, Bourdon JC. 2010. p53 family members in cancer diagnosis and treatment. Semin. Cancer Biol. 20:57–62 [DOI] [PubMed] [Google Scholar]
- 14. Marcel V, et al. 2010. p53 regulates the transcription of its delta133p53 isoform through specific response elements contained within the TP53 P2 internal promoter. Oncogene 29:2691–2700 [DOI] [PubMed] [Google Scholar]
- 15. Marcel V, et al. 2010. Delta160p53 is a novel N-terminal p53 isoform encoded by delta133p53 transcript. FEBS Lett. 584:4463–4468 [DOI] [PubMed] [Google Scholar]
- 16. Marcel V, et al. 23 September 2011. Biological functions of p53 isoforms through evolution: lessons from animal and cellular models. Cell Death Differ. doi:10.1038/cdd.2011.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marcel V, et al. 11 November 2011. Diverse p63 and p73 isoforms regulate Δ133p53 expression through modulation of the internal TP53 promoter activity. Cell Death Differ. doi:10.1038/cdd.2011.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moulès V, et al. 2011. Importance of viral genomic composition in modulating glycoprotein content on the surface of influenza virus particles. Virology 414:51–62 [DOI] [PubMed] [Google Scholar]
- 19. Muñoz-Fontela C, et al. 2008. Transcriptional role of p53 in interferon-mediated antiviral immunity. J. Exp. Med. 205:1929–1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muñoz-Fontela C, et al. 2011. p53 serves as a host antiviral factor that enhances innate and adaptive immune responses to influenza A virus. J. Immunol. 187:6428–6436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Palese P, Shaw M. 2007. Orthomyxoviridae: the viruses and their replication, p 1647–1689 In Knipe DM, et al. (ed), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 22. Rohaly G, Korf K, Dehde S, Dornreiter I. 2010. Simian virus 40 activates ATR-delta p53 signaling to override cell cycle and DNA replication control. J. Virol. 84:10727–10747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3:1101–1108 [DOI] [PubMed] [Google Scholar]
- 24. Shapira SD, et al. 2009. A physical and regulatory map of host-influenza interactions reveals pathways in H1N1 infection. Cell 139:1255–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shen Y, et al. 2009. Influenza A virus induces p53 accumulation in a biphasic pattern. Biochem. Biophys. Res. Commun. 382:331–335 [DOI] [PubMed] [Google Scholar]
- 26. Terrier O, et al. 2011. Cellular transcriptional profiling in human lung epithelial cells infected by different subtypes of influenza A viruses reveals an overall down-regulation of the host p53 pathway. Virol. J. 8:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Turpin E, et al. 2005. Influenza virus infection increases p53 activity: role of p53 in cell death and viral replication. J. Virol. 79:8802–8811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang X, et al. 2010. The non-structural (NS1) protein of influenza A virus associates with p53 and inhibits p53-mediated transcriptional activity and apoptosis. Biochem. Biophys. Res. Commun. 395:141–145 [DOI] [PubMed] [Google Scholar]
- 29. Watanabe T, Watanabe S, Kawaoka Y. 2010. Cellular networks involved in the influenza virus life cycle. Cell Host Microbe 7:427–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Whiley DM, Sloots TP. 2005. A 5′-nuclease real-time reverse transcriptase-polymerase chain reaction assay for the detection of a broad range of influenza A subtypes, including H5N1. Diagn. Microbiol. Infect. Dis. 53:335–337 [DOI] [PubMed] [Google Scholar]
- 31. Zhirnov OP, Klenk HD. 2007. Control of apoptosis in influenza virus-infected cells by up-regulation of Akt and p53 signaling. Apoptosis 12:1419–1432 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.