Abstract
We analyzed Lynx rufus fecal parasites from California and Colorado, hypothesizing that bobcats shed zoonotic parasites around human landscapes. Giardia duodenalis, Cryptosporidium, Ancylostoma, Uncinaria, and Toxocara cati were shed. Toxoplasma gondii serology demonstrated exposure. Giardia and Cryptosporidium shedding increased near large human populations. Genotyped Giardia may indicate indirect transmission with humans.
TEXT
Pathogen spillover is a major disease source for humans and wildlife (8, 10, 16). Studies have suggested that wild felids might contribute to spillover of a variety of high-profile pathogens to humans (3, 5, 22, 24, 27, 39). However, owing to their secretive nature and the effort required to sample, few studies can draw significant inferences about wild felid pathogen exposure and shedding. In this study, we screened a large number of bobcat (Lynx rufus) fecal samples for zoonotic enteric parasites and evaluated determinants of shedding and exposure in relation to human-occupied landscapes. We hypothesized that bobcats become exposed and shed zoonotic parasites around human-occupied landscapes.
Bobcats are widespread in North America and persist across a continuum of natural to heavily urbanized habitats, often existing in sympatry with humans, domestic animals, and urban-adapted wildlife along urban boundaries (9, 28, 31). Fecal zoonotic pathogens shed by felids, and considered most likely to be detected in this study, include Toxoplasma gondii, Cryptosporidium, Giardia, roundworms, and hookworms. In certain circumstances, these cosmopolitan pathogens infect and cause disease to humans and cause outbreaks, primarily associated with environmental contamination (7, 13, 29, 34, 41). Comparably, domestic animals, urban wildlife, and, in some cases, humans are also permissive hosts for such common parasites (11, 17, 32, 40). Thus, along urban boundaries, abundant sympatric populations of permissive hosts may amplify the pathogen circulation and force of infection (18, 32).
We screened samples from three sites in California (Ventura County [VC]) and Colorado (Front Range [FR] and Western Slope [WS]) that vary in the degree of urbanization (Fig. 1). Bobcat sex, age (young, <2 years; adult, ≥2 years), and location were recorded at the time of capture (4). Fecal (n = 141) and matching blood (n = 73) samples were collected in the field, refrigerated, and then frozen until analyses. From a subset of WS animals (ca. 60%), fecal samples were collected from hunter-killed animals and thoracic fluid was collected instead of serum.
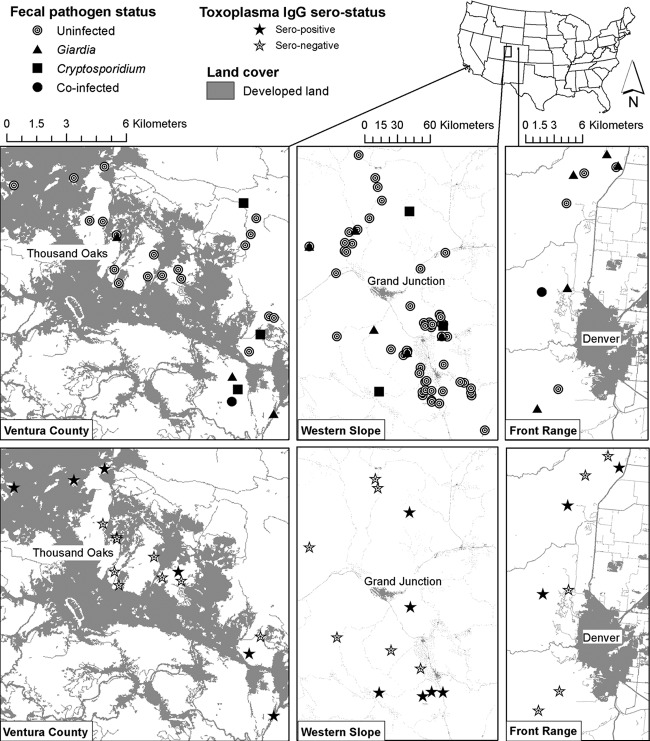
Fig 1.
Capture locations of bobcats and associated developed landscape features (primarily urban areas and major highways) across study locations in California (Ventura County [VC]) and Colorado (Front Range [FR] and Western Slope [WS]). VC is a highly urbanized landscape within the Santa Monica Mountains, north of Los Angeles, CA. FR is an urbanized area along the Front Range of the Rocky Mountains, northwest of the Denver metropolitan area and immediately adjacent to Boulder. WS is a primarily rural and exurban region along the Western Slope of the Rocky Mountains around Montrose and Grand Junction. Upper panels illustrate detection of Giardia, Cryptosporidium, and their cooccurrence in feces. Lower panels illustrate T. gondii IgG serostatuses of bobcats. T. gondii IgM antibodies, oocysts, and DNA (shedding) in feces were not detected in any of the bobcats.
Fecal flotation was used for detection of parasite eggs, cysts, and oocysts, and immunofluorescence assays were used for Giardia cysts and Cryptosporidium oocysts (36). PCR assays for Giardia sp., Cryptosporidium sp., and T. gondii were performed on fecal dilutions from fluorescence assays (19, 26, 30, 33, 37). Positive amplicons were sequenced in forward and reverse directions (ABI3100 genetic analyzer; Applied Biosystems). Sequences were compared with sequences from GenBank by BLAST, and new sequences were placed in GenBank. Additionally, IgM and IgG antibodies against T. gondii were detected using indirect enzyme-linked immunosorbent assays (ELISAs) on sera (20, 42) and modified agglutination tests on thoracic fluid (2).
Statistical analyses focused on the most-abundant pathogens and those with greatest zoonotic potential (T. gondii, Cryptosporidium, and Giardia). Estimates of prevalence (and 95% confidence intervals [CIs]) were determined using maximum likelihood. The effects of host (sex and age) and geographic (study site) determinants of shedding/serostatus were evaluated using individual odds ratios (based on conditional maximum likelihood estimation). Analyses were conducted using R (v.12.14.1; www.r-project.org) with the stats and epitools packages.
Fecal flotation identified a variety of parasites (Table 1). In support of our hypothesis, we found evidence of zoonotic parasite shedding (Giardia duodenalis, Cryptosporidium sp., Ancylostoma sp., Uncinaria sp., Toxascaris leonina, and Toxocara cati) or exposure (T. gondii) across all sites (Table 1). There are a few studies of enteric parasites of bobcats, but those that exist collectively report similar parasite communities (e.g., see references 12, 23, 38, and 43). The prevalences (1 to 13.5%) of the parasites examined here are also broadly comparable to those of adjacent domestic cat populations (6, 14), suggesting common exposure among the felids.
Table 1.
Enteric parasites identified through microscopic examination of fecal flotation filtrate across study sites
| Phylum | Group | Parasite(s) | No. of samples with indicated parasite in each location (n)a |
||
|---|---|---|---|---|---|
| VC (33) | WS (103) | FR (10) | |||
| Nematoda | Hookwormsb | Ancylostoma sp. and Uncinaria sp. | 2 | 2 | 1 |
| Roundwormsb | Toxascaris leonina | 1 | 3 | 5 | |
| Toxocara cati | 0 | 5 | 0 | ||
| Whipworms | Trichuris sp. | 2 | 0 | 0 | |
| Stomach worms | Physaloptera felis | 0 | 9 | 0 | |
| Lungworms | Eucoleus sp. | 0 | 1 | 1 | |
| Unidentified | Soil nematodes | 3 | 0 | 0 | |
| Platyhelminthes | Flatworms | Fluke eggs | 1 | 0 | 0 |
| Apicomplexa | Coccidias | Eimeria sp. | 1 | 0 | 0 |
| Isospora sp. | 0 | 7 | 0 | ||
| Cystoisospora felis | 0 | 2 | 0 | ||
VC, Ventura County; WS, Western Slope; FR, Front Range. Numbers in parentheses indicate numbers of fecal samples examined.
Zoonotic potential.
No fecal samples were positive for T. gondii oocysts or DNA. However, 17.8% (95% CI, 10.2 to 27.6) of bobcats were seropositive by IgG only, demonstrating past exposure and suggesting shedding patterns similar to domestic cats (25). Seroprevalence was not predicted by sex, but adults were 6.1 (95% CI, 1.2 to 61.8) times more likely to be seropositive than young animals. Bobcats from VC and FR, the more-urban sites, were 5.8 (1.2 to 33.2) and 6.3 (0.7 to 51.2) times more likely to be seropositive than bobcats from WS, respectively, but this effect was partially driven by more young animals being sampled from WS. In general, T. gondii exposure appears greater among wild felid populations than among domestic cats in the United States, suggesting that wild felids may be a source for domestic cats (4), although T. gondii can persist in regions in which wild felids are absent (1). The force of exposure to T. gondii for domestic cats, humans, and urban wildlife might be enhanced directly adjacent to the urban-wildland interface, where there is sympatry among wild and domestic felids and amplification of T. gondii through the food chain (21). For example, sympatry between pumas and domestic cats might have been the driver of the 1995 human toxoplasmosis outbreak in British Columbia (3).
Cryptosporidium parvum and Giardia duodenalis account for the majority of waterborne disease outbreaks in humans, with approximately 2 million and 750,000 annual cases in the United States, respectively (11, 15, 17, 35). Both pathogens have several species and subtypes, with some host specific and others zoonotic. Little is known about the shedding of these pathogens by bobcats. We found shedding across all sites (Fig. 2). Neither Giardia nor Cryptosporidium was predicted by sex or age. The prevalence of Giardia shedding was greater at FR than at either VC or WS, and there was a trend for greater shedding at VC than WS (Table 2 and Fig. 2a). Similarly, shedding of Cryptosporidium oocysts was greater at VC than WS, and a trend for greater shedding at FR than WS was noted (Table 2 and Fig. 2b). Overall, results indicate a greater probability of shedding of Giardia and Cryptosporidium at VC and FR, which are situated near large human population centers, than at the more-remote WS site.
Fig 2.
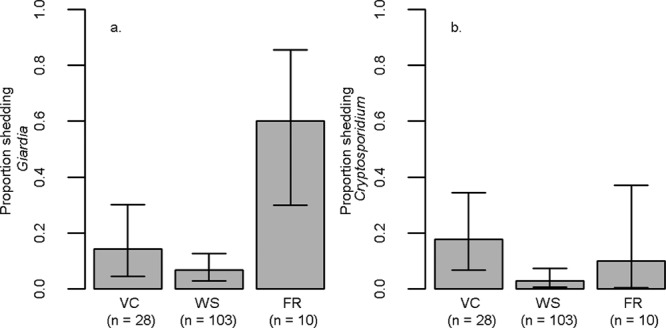
Proportions (maximum likelihood estimates ± 95% CIs) of bobcats shedding Giardia (a) and Cryptosporidium (b) across the study locations Ventura County (VC), Western Slope (WS), and Front Range (FR).
Table 2.
Individual odds ratios and 95% confidence intervals for predictors of Giardia sp. and Cryptosporidium sp. shedding in bobcatsa
| Compared parameters | Odds ratios and 95% CIs for predictors of shedding of: |
|||||
|---|---|---|---|---|---|---|
|
Giardia sp. |
Cryptosporidium sp. |
|||||
| Odds ratio | 95% CI |
Odds ratio | 95% CI |
|||
| Lower | Upper | Lower | Upper | |||
| Male vs female | 1.272 | 0.385 | 4.919 | 0.389 | 0.073 | 1.909 |
| Adult vs young | 1.600 | 0.456 | 7.162 | 1.685 | 0.303 | 17.303 |
| VC vs WS | 2.269 | 0.450 | 9.815 | 7.094 | 1.277 | 48.909 |
| FR vs VC | 8.320 | 1.326 | 63.870 | 0.141 | 0.020 | 0.783 |
| FR vs WS | 19.385 | 3.673 | 117.946 | 0.275 | 0.020 | 15.733 |
Based on conditional maximum likelihood estimations. The predictors include sex (male or female), age (young or adult), and location (Ventura County [VC], Western Slope [WS], or Front Range [FR]).
We typed four Giardia isolates as G. duodenalis assemblage A1, which is commonly found in humans and appears maintained in multispecies complexes, including domestic animals and wildlife (11). Though Cryptosporidium shedding patterns were similar to those of Giardia, we were unable to determine if positive samples were felid, human, or other animal in origin. A direct fluorescence assay is the gold standard for generic Giardia and Cryptosporidium identification, and our inability to identify more isolates to the species level was likely due to PCR inhibitors in feces or possibly due to the volume of sample available. Notably, a cluster of bobcats shedding Giardia and Cryptosporidium at VC corresponds to the highly urbanized Malibu creek watershed (Fig. 1). Given shedding patterns among sites and within VC as well as genetic identities, our results may suggest anthroponotic transmission of Giardia to bobcats, likely through contaminated water or other environmental sources.
An alternative interpretation for our results is the impact of climatic and ecological drivers among sites, particularly in comparing California and Colorado sites. VC is characterized by a Mediterranean climate and associated vegetative communities, whereas the Colorado study sites are cooler, semiarid, and dominated by coniferous woodlands. If climate- and habitat-related factors are primary determinants of enteric pathogens across these sites, we might expect Colorado sites (WS and FR) to be more similar to each other than to VC. However, the similarity of the most-urbanized sites (VC and FR) in the prevalence of pathogen shedding and the differences between these sites and the least-urbanized site (WS) suggest other factors to be important determinants of enteric pathogen shedding and exposure.
Our results are supportive of abundant populations of permissive hosts around urban areas leading to amplification of pathogen circulation and force of infection and exposure (18). We found support for our hypothesis that bobcats become exposed to and shed zoonotic parasites in fecal material around human-occupied landscapes. Future research evaluating parasite exposure and shedding, along with landscape genetic structure along urban-wildland gradients, among bobcats, other wildlife (particularly Puma concolor), domestic animals, and humans will contribute a better empirical understanding of multihost parasite dynamics, pathogen spillover, and human exposure risk.
Nucleotide sequence accession numbers.
New sequences were added to GenBank under accession numbers JQ688299 to JQ688300 for Giardia and JQ740600 to JQ740605 for Cryptosporidium.
ACKNOWLEDGMENTS
This study was supported by the National Science Foundation Ecology of Infectious Diseases research program (NSF EF-0723676).
We acknowledge J. Lewis, L. Serieys, L. Sweanor, K. Logan, A. Griffith, M. Brewer, J. Hawley, and A. Morris for assistance with sample collection and processing. We also thank Colorado Parks and Wildlife, the United States Geological Survey, and the National Park Service for their collaboration.
Any use of trade, product, or firm names is for descriptive purposes only and does not imply an endorsement by the U.S. Government.
Footnotes
Published ahead of print 20 June 2012
REFERENCES
- 1. Afonso E, Thulliez P, Gilot-Fromont E. 2006. Transmission of Toxoplasma gondii in an urban population of domestic cats (Felis catus). Int. J. Parasitol. 36:1373–1382 [DOI] [PubMed] [Google Scholar]
- 2. Al-Kappany YM, et al. 2011. Seroprevalence of Toxoplasma gondii and concurrent Bartonella spp., feline immunodeficiency virus, feline leukemia virus, and Dirofilaria immitis infections in Egyptian cats. J. Parasitol. 97:256–258 [DOI] [PubMed] [Google Scholar]
- 3. Aramini JJ, et al. 1999. Potential contamination of drinking water with Toxoplasma gondii oocysts. Epidemiol. Infect. 122:305–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bevins SN, et al. 2012. Three pathogens in sympatric populations of pumas, bobcats, and domestic cats: implications for infectious disease transmission. PLoS One 7:e31403 doi:10.1371/journal.pone.0031403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bevins SN, et al. 2009. Wild felids as hosts for human plague, western United States. Emerg. Infect. Dis. 15:2021–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bowman DD, Torre CJ, Mannella C. 2007. Survey of 11 western states for heartworm (Dirofilari immitis) infection, heartworm diagnostic and prevention protocols, and fecal examination protocols for gastrointestinal parasites. Vet. Ther. 8:293–304 [PubMed] [Google Scholar]
- 7. CDC 2007. Outbreak of cutaneous larva migrans at a children's camp—Miami, Florida, 2006. MMWR Morb. Mortal. Wkly. Rep. 56:1285–1287 [PubMed] [Google Scholar]
- 8. Cleaveland S, Laurenson MK, Taylor LH. 2001. Diseases of humans and their domestic mammals: pathogen characteristics, host range and the risk of emergence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 356:991–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crooks KR. 2002. Relative sensitivities of mammalian carnivores to habitat fragmentation. Conserv. Biol. 16:488–502 [Google Scholar]
- 10. Daszak P, Cunningham AA, Hyatt AD. 2000. Emerging infectious diseases of wildlife—threats to biodiversity and human health. Science 287:443–450 [DOI] [PubMed] [Google Scholar]
- 11. Feng YY, Xiao LH. 2011. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin. Microbiol. Rev. 24:110–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heidt GA, Rucker RA, Kennedy ML, Baeyens ME. 1988. Hematology, intestinal parasites, and selected antibodies from a population of bobcats (Felis rufus) in central Arkansas. J. Wildl. Dis. 24:180–183 [DOI] [PubMed] [Google Scholar]
- 13. Hill D, Dubey JP. 2002. Toxoplasma gondii: transmission, diagnosis and prevention. Clin. Microbiol. Infect. 8:634–640 [DOI] [PubMed] [Google Scholar]
- 14. Hill SL, et al. 2000. Prevalence of enteric zoonotic organisms in cats. J. Am. Vet. Med. Assoc. 216:687–692 [DOI] [PubMed] [Google Scholar]
- 15. Jaykus LA. 1997. Epidemiology and detection as options for control of viral and parasitic foodborne disease. Emerg. Infect. Dis. 3:529–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jones KE, et al. 2008. Global trends in emerging infectious diseases. Nature 451:990–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Karanis P, Kourenti C, Smith H. 2007. Waterborne transmission of protozoan parasites: a worldwide review of outbreaks and lessons learnt. J. Water Health 5:1–38 [DOI] [PubMed] [Google Scholar]
- 18. Keesing F, Holt RD, Ostfeld R. 2006. Effects of species diversity on disease risk. Ecol. Lett. 9:485–498 [DOI] [PubMed] [Google Scholar]
- 19. Lalle M, et al. 2005. Genetic heterogeneity at the β-giardin locus among human and animal isolates of Giardia duodenalis and identification of potentially zoonotic subgenotypes. Int. J. Parasitol. 35:207–213 [DOI] [PubMed] [Google Scholar]
- 20. Lappin MR, Jacobson ER, Kollias GV, Powell CC, Stover J. 1991. Comparison of serologic assays for the diagnosis of toxoplasmosis in nondomestic felids. J. Zoo Wildl. Med. 22:169–174 [Google Scholar]
- 21. Lelu M, Langlais M, Poulle ML, Gilot-Fromont E. 2010. Transmission dynamics of Toxoplasma gondii along an urban-rural gradient. Theor. Popul. Biol. 78:139–147 [DOI] [PubMed] [Google Scholar]
- 22. Littnan CL, Stewart BS, Yochem PK, Braun R. 2006. Survey for selected pathogens and evaluation of disease risk factors for endangered Hawaiian monk seals in the main Hawaiian Islands. Ecohealth 3:232–244 [Google Scholar]
- 23. Marchiondo AA, Karpowitz JF, Conder GA. 1986. Parasites of the bobcat (Lynx rufus pallescens) in Central and Southern Utah. Proc. Helminthol. Soc. Wash. 53:113–116 [Google Scholar]
- 24. Miller MA, et al. 2002. Coastal freshwater runoff is a risk factor for Toxoplasma gondii infection of southern sea otters (Enhydra lutris nereis). Int. J. Parasitol. 32:997–1006 [DOI] [PubMed] [Google Scholar]
- 25. Miller NL, Frenkel JK, Dubey JP. 1972. Oral infections with toxoplasma cysts and oocysts in felines, other mammals, and in birds. J. Parasitol. 58:928–937 [PubMed] [Google Scholar]
- 26. Morgan UM, et al. 2001. Molecular and phylogenetic characterisation of Cryptosporidium from birds. Int. J. Parasitol. 31:289–296 [DOI] [PubMed] [Google Scholar]
- 27. Moschidou P, et al. 2011. Mixed infection by feline astrovirus and feline panleukopenia virus in a domestic cat with gastroenteritis and panleukopenia. J. Vet. Diagn. Invest. 23:581–584 [DOI] [PubMed] [Google Scholar]
- 28. Ordenaña MA, et al. 2010. Effects of urbanization on carnivore species distribution and richness. J. Mammal. 91:1322–1331 [Google Scholar]
- 29. Pinelli E, Herremans T, Harms M, Hoek D, Kortbeek L. 2011. Toxocara and Ascaris seropositivity among patients suspected of visceral and ocular larva migrans in the Netherlands: trends from 1998 to 2009. Eur. J. Clin. Microbiol. Infect. Dis. 30:873–879 [DOI] [PubMed] [Google Scholar]
- 30. Read CM, Monis PT, Thompson RC. 2004. Discrimination of all genotypes of Giardia duodenalis at the glutamate dehydrogenase locus using PCR-RFLP. Infect. Genet. Evol. 4:125–130 [DOI] [PubMed] [Google Scholar]
- 31. Riley SPD, Boydston EE, Crooks KR, Lyren LM. 2010. Bobcats (Lynx rufus). In Gehert SD, Riley SPD, Cypher BL. (ed), Urban carnivores: ecology, conflict, and conservation. The Johns Hopkins University Press, Baltimore, MD [Google Scholar]
- 32. Riley SPD, Foley J, Chomel B. 2004. Exposure to feline and canine pathogens in bobcats and gray foxes in urban and rural zones of a national park in California. J. Wildl. Dis. 40:11–22 [DOI] [PubMed] [Google Scholar]
- 33. Salant H, Markovics A, Spira DT, Hamburger J. 2007. The development of a molecular approach for coprodiagnosis of Toxoplasma gondii. Vet. Parasitol. 146:214–220 [DOI] [PubMed] [Google Scholar]
- 34. Samuel WM, Pybus MJ, Kocan AA. 2001. Parasitic diseases of wild mammals, 2nd ed Iowa State University Press, Ames, IA [Google Scholar]
- 35. Scallan E, et al. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 17:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Scorza AV, Ballweber LR, Tangtrongsum SP, Lappin CMR. 2012. Comparisons of mammalian Giardia duodenalis assemblages based on the β-giardin, glutamate dehydrogenase and triose phosphate isomerase genes. Vet. Parasitol. doi:10.1016/j.vetpar.2012.04.032 [DOI] [PubMed] [Google Scholar]
- 37. Scorza AV, Brewer MM, Lappin MR. 2003. Polymerase chain reaction for the detection of Cryptosporidium spp. in cat feces. J. Parasitol. 89:423–426 [DOI] [PubMed] [Google Scholar]
- 38. Siembieda JL, et al. 2011. Zoonotic pathogens isolated from wild animals and environmental samples at two California wildlife hospitals. J. Am. Vet. Med. Assoc. 238:773–783 [DOI] [PubMed] [Google Scholar]
- 39. Thiry E, et al. 2007. Highly pathogenic avian influenza H5N1 virus in cats and other carnivores. Vet. Microbiol. 122:25–31 [DOI] [PubMed] [Google Scholar]
- 40. Thompson RCA, Kutz SJ, Smith A. 2009. Parasite zoonoses and wildlife: emerging issues. Int. J. Environ. Res. Public Health 6:678–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thompson RCA, Smith A. 2011. Zoonotic enteric protozoa. Vet. Parasitol. 182:70–78 [DOI] [PubMed] [Google Scholar]
- 42. Vollaire MR, Radecki SV, Lappin MR. 2005. Seroprevalence of Toxoplasma gondii antibodies in clinically ill cats in the United States. Am. J. Vet. Res. 66:874–877 [DOI] [PubMed] [Google Scholar]
- 43. Watson TG, Nettles VF, Davidson WR. 1981. Endoparasites and selected infectious agents in bobcats (Felis rufus) from West Virginia and Georgia. J. Wildl. Dis. 17:547–554 [DOI] [PubMed] [Google Scholar]