Abstract
Microscopic diagnosis and species identification of Plasmodium in areas of nonendemicity provide a robust method for malaria diagnosis but are technically challenging. A prospective study was conducted to measure the performance of BinaxNOW compared to microscopy (the gold standard) in a U.S. teaching hospital. Overall, BinaxNOW was 84.2% sensitive and 99.8% specific. Excluding patients on antimalarial therapy, the sensitivity was 92.9%. Importantly, BinaxNOW initially misclassified a case of Plasmodium falciparum malaria as non-falciparum. These results support the judicious use of BinaxNOW in screening of individuals suspected of having malaria in areas of nonendemicity.
INTRODUCTION
The accurate diagnosis of malaria by microscopy in the United States is complicated by a number of factors, including lack of experienced technologists, variable methodology and quality of smears, and partial morphological overlap between Plasmodium falciparum and other Plasmodium species (12, 14). Although the incidence of malaria in returned febrile travelers is approximately 21%, only 1,300 cases were reported in the United States in 2008, in comparison to 243 million cases worldwide during the same period (15, 19, 20). The development of rapid diagnostic tests (RDTs) has aided diagnosis of malaria in resource-poor settings, but these tests have limited capability for species identification, as they were originally designed to distinguish P. falciparum from other Plasmodium species that cause disease in humans. Numerous studies have demonstrated the utility of RDTs, which have sensitivity and specificity comparable to those of microscopy while offering rapid diagnosis, in Africa and Asia (1, 16). The performance of RDTs in areas of low malaria prevalence is less well investigated. Studies in France have demonstrated 96% sensitivity, 99% specificity, and high negative predictive values (NPV) with certain RDTs but conclude that microscopy is necessary for definitive confirmation (3, 4). A similar study in the United States demonstrated 99% overall sensitivity and 99.6% NPV for the diagnosis of malaria with the BinaxNOW Malaria test; sensitivity for P. falciparum was 100% (17). BinaxNOW Malaria, the only U.S. Food and Drug Administration-approved RDT for malaria, qualitatively detects both the histidine-rich protein 2 (HRP-2), specific to P. falciparum, and aldolase, a panmalarial antigen found in all Plasmodium species (11).
Here, we report on the performance of the BinaxNOW in a major U.S. academic medical center, describe a unique case of misidentification of P. falciparum by BinaxNOW, and discuss the advantages and disadvantages of using BinaxNOW as a screening tool in the United States.
MATERIALS AND METHODS
From July 2008 to March 2012, 484 BinaxNOW Malaria tests, on 407 unique patients, were performed concurrently with thin and thick blood smears in the Stanford Hospital Clinical Hematology Laboratory on consecutive blood samples from patients with suspected malaria. For each sample, one thick and two thin smears were prepared from venous EDTA whole blood and examined by two licensed clinical laboratory scientists who specialize in clinical hematology. Thin smears were prepared with long feathered edges using DIFF-SAFE blood dispensers (Alpha Scientific Corp., Malvern, PA) or microcapillary tubes. For thick smears, a hemolysate was prepared by mixing whole blood, 22% bovine albumin, and 1% Saponin in 0.9% saline in a ratio of 40:4:30. The hemolysate was incubated for 1 min and centrifuged at 2,350 × g for 1 min. The supernatant was decanted and centrifuged at 2,350 × g for 10 min. After the second spin, the supernatant was discarded, and the sediment was resuspended with a Pasteur pipette and thickly smeared on a glass slide. All slides were air dried for 10 min and stained with Coulter TruColor Wright Giemsa (Beckman Coulter Inc., Brea, CA). BinaxNOW testing was performed using the same whole-blood specimen according to the manufacturer's instructions (9). Positive and negative controls were tested with each new lot. The BinaxNOW results were available prior to microscopy and were not withheld from the technologists reading the smears. Discordant results between the blood smears and BinaxNOW were resolved by real-time PCR performed at ARUP Laboratories (Salt Lake City, UT) using a Rotor-Gene Q instrument (Qiagen Inc., Valencia, CA). ARUP was blinded to the findings of the BinaxNOW test and microscopy.
RESULTS
Of 484 blood samples tested, 465 were negative for Plasmodium by microscopy. Nineteen microscopy-positive results from 14 unique patients were identified to the genus level by blood smear (Tables 1 and 2). The parasitemias for these individuals ranged from less than 0.1% (5,000/μl) to 3.5% (175,000/μl). The interquartile range was <0.1% to 0.65% (<5,000/μl to 32,500/μl). For BinaxNOW, the sensitivity, specificity, positive predictive value, and negative predictive value of Plasmodium diagnosis to the genus level were 84.2% (16/19) (95% confidence interval [CI], 59.5 to 95.8), 99.8% (464/465) (95% CI, 98.6 to 100), 94.1% (95% CI, 69.2 to 99.7), and 99.4% (95% CI, 98.0 to 99.8), respectively. The three specimens with false-negative BinaxNOW results included one case of P. ovale and two unique cases of P. vivax infection. The two false-negative results involving P. vivax occurred in two different patients, each of whom had received 3 days of antimalarial therapy, one with atovaquone-proguanil and the other with artemether-lumefantrine. The parasitemia levels in all three cases were less than 0.1% (<5,000/μl). Excluding the duplicate tests ordered on previously microscopy-positive patients, the sensitivity of BinaxNOW increased to 92.9% (13/14) (95% CI, 64.2 to 99.6). Among cases that had recorded parasitemia, the sensitivity of BinaxNOW was 100% (2/2) at high parasitemia (>1.0%, or >50,000/μl), 100% (12/12) at medium parasitemia (0.1 to1.0%, or 5,000 to 50,000/μl), and 40% (2/5) at low parasitemia (<0.1%, or <5,000/μl). One sample with a false-positive BinaxNOW result was from a patient with a history of P. falciparum undergoing treatment with chloroquine.
Table 1.
Microscopy-positive malaria cases, July 2008 to March 2012
| Case no. | Patient no. | Microscopy result | Parasite count (%) | BinaxNOW result | PCR result | Treatment at time of testing |
|---|---|---|---|---|---|---|
| 1 | 1 | P. falciparum | 0.5 | P. falciparum | ||
| 2 | 2 | P. vivax | 1.7 | Non-falciparum | ||
| 3 | 3 | P. ovale | <0.1 | Negative | ||
| 4 | 4 | P. falciparum | 0.3 | P. falciparum | ||
| 5 | 5 | P. falciparum | <1.0 | P. falciparum | ||
| 6 | 6 | P. falciparum | 3.5 | P. falciparum | ||
| 7 | 7 | P. vivax | <1.0 | Non-falciparum | ||
| 8 | 8 | P. falciparum | 1.0 | Non-falciparum | P. falciparum | |
| 9 | 8 | P. falciparum | 0.3 | P. falciparum | Atovaquone-proguanil | |
| 10 | 9 | P. falciparum | <0.1 | P. falciparum | ||
| 11 | 10 | P. vivax | 0.5 | Non-falciparum | ||
| 12 | 11 | P. vivax | 0.3 | Non-falciparum | ||
| 13 | 12 | P. falciparum | 0.8 | P. falciparum | ||
| 14 | 12 | P. falciparum | <0.1 | P. falciparum | ||
| 15 | 13 | P. vivax | <1.0 | Non-falciparum | ||
| 16 | 13 | P. vivax | <0.1 | Negative | Atovaquone-proguanil | |
| 17 | 14 | P. vivax | 0.4 | Non-falciparum | ||
| 18 | 14 | P. vivax | 0.4 | Non-falciparum | Artemether-lumafantrine | |
| 19 | 14 | P. vivax | <0.1 | Negative | Artemether-lumafantrine |
Table 2.
Comparison of BinaxNOW to conventional microscopy for detection of Plasmodium
| Result | No. microscopy positivea |
No. microscopy negative | ||
|---|---|---|---|---|
| P.f. | P.v. | P.o. | ||
| BinaxNOW positive | ||||
| P. falciparum | 8 | 1c | ||
| Non-falciparum | 1b | 7 | ||
| BinaxNOW negative | 2c | 1 | 464 | |
P.f., P. falciparum; P.v., P. vivax; P.o., P. ovale. (No cases of P. malariae were identified.)
Confirmed by PCR as P. falciparum.
Patients were undergoing malaria treatment at the time of testing.
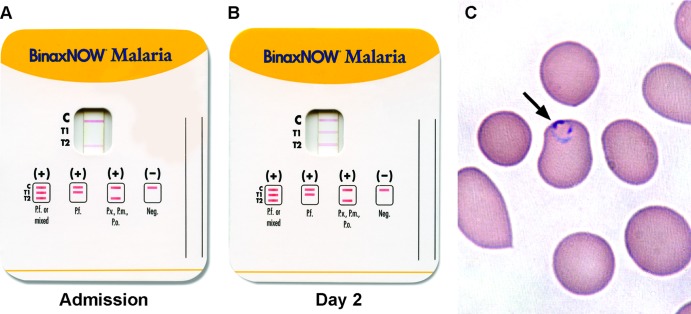
At the species level, 9 P. falciparum, 9 P. vivax, and 1 P. ovale isolate were identified by microscopy (Tables 1 and 2). No coinfections were identified. Among malaria cases detected by BinaxNOW, BinaxNOW correctly identified to the species level 88.9% (8/9) of P. falciparum and 100% (7/7) of non-falciparum cases. Significantly, BinaxNOW misclassified a case of P. falciparum infection as non-falciparum. The patient was a 67-year-old male who had recently returned from Côte d'Ivoire. BinaxNOW testing performed on the day of admission was reactive only in the aldolase (T2) window, suggestive of non-falciparum malaria (Fig. 1A). However, the morphology of trophozoites on thin smears was that of delicate ring forms, suggestive of P. falciparum, and parasitemia was 1% (50,000/μl) (Fig. 1C). BinaxNOW was repeated on the initial blood sample and showed the same result as the first test. The patient was started on therapy with atovaquone-proguanil. Two days following admission, BinaxNOW from the same lot was repeated on a new blood sample and demonstrated solid lines in both the HRP-2 (T1) and aldolase (T2) windows, supporting P. falciparum infection (Fig. 1B). Blood smear demonstrated 0.3% parasitemia on hospital day 2. A real-time PCR assay for detection and species identification of Plasmodium performed on the blood obtained at admission was positive for P. falciparum, with no other species detected.
Fig 1.
BinaxNOW test results for a patient with P. falciparum infection. (A) On admission, a faint line is present only in the aldolase (T2) and the control windows. (B) Two days after admission, both HRP-2 (T1) and aldolase reactivity are seen. (C) Wright-Giemsa-stained peripheral blood smear made from the admission specimen, demonstrating a classic thin, delicate ring trophozoite, supporting P. falciparum.
DISCUSSION
We report a nearly 4-year experience with BinaxNOW Malaria in a major U.S. academic medical center. While a point of care test for malaria has the greatest utility in resource-poor areas, we demonstrate high sensitivity (92.9%) and excellent specificity (99.8%) in a region where malaria is observed primarily in travelers returning from countries where malaria is endemic. A limitation of this study was the low number of positive cases; still, BinaxNOW was able to detect 100% (13/13) of non-Plasmodium ovale cases in untreated patients. BinaxNOW is known to have a lower sensitivity for P. ovale at lower parasitemia (5, 9). The current findings are similar to previous reports from the United States, Canada, and France, with 94 to 97% sensitivity for P. falciparum and 67 to 86% sensitivity for non- falciparum malaria using BinaxNOW (4, 5, 17). As would be expected with qualitative antigen testing, low parasitemia can cause false-negative results, as observed here and in other studies, including one from Canada that showed 75% sensitivity for P. falciparum at a parasitemia of 1 to 100/μl (<0.002%), and 96.2% sensitivity at 101 to 1,000/μl (<0.02%) (5). The same study showed 50% sensitivity for P. vivax at a parasitemia of 1 to 100/μl of blood (<0.002%) and 55% sensitivity at 101 to 1,000/μl (<0.02%) (5).
In this study, we had a single false-positive BinaxNOW result in a patient who was being treated for P. falciparum. This observation is not unusual, because despite successful treatment, the HRP-2 protein may persist in the blood for up to 28 days, and the persistence of antigenemia correlates positively with the level of parasitemia (8). The finding of a positive result in a patient with a documented history of recent infection and successful treatment is not clinically significant. However, a positive result in a traveler returning from a region where malaria is endemic 1 month after the last documentation of a positive result should prompt further investigation. The persistence of a positive result raises concern for treatment failure, drug resistance, and new infection. Also, the possibility of false-positive results due to rheumatoid factor should also be investigated (10).
Given the lower sensitivity of BinaxNOW in patients with low parasitemia, RDTs cannot be used in place of microscopy for diagnosis of malaria in low-prevalence areas. In fact, the package insert for BinaxNOW recommends the test be used in conjunction with other laboratory and clinical findings, with all negative results to be confirmed by thick/thin smear microscopy (9). It does not make recommendations for confirmation of positive results. However, in some practice settings such as outpatient clinics or small hospitals with minimal staffing during off hours, BinaxNOW could be useful as a screening test for triage of patients, with subsequent confirmation of all results by microscopy. As reading blood smears for malaria is technically challenging and time-intensive, use of BinaxNOW as an adjunct test may assist technologists and free resources when results are positive. BinaxNOW would be expected to detect the great majority of cases in therapy-naïve patients, as these patients are less likely to present with very low-level parasitemia, which is associated with prior antimalarial therapy. The patients at greatest risk for a false-negative BinaxNOW result are those with low-level parasitemia, and these individuals arguably pose a low risk for severe complications in the short term. Cases with very high parasitemia may also produce a false-negative BinaxNOW test as a consequence of antigen excess (prozone effect). Previous studies have demonstrated a prozone effect with parasitemia loads greater than 4% as a result of an excess of HRP-2 antigen, which interferes with binding of the antibody, leading to false-negative results (7). Moreover, as antigenemia may persist in the absence of parasitemia, BinaxNOW is not appropriate for following the response to therapy (9, 16). However, cases with parasitemia greater than 4% (200,000/μl) would be rare in regions of nonendemicity, as infected individuals would likely present for treatment of symptoms prior to achieving such a massive parasite burden, and preinfection rates of anemia are less common in individuals living in regions of nonendemicity.
The misidentification of P. falciparum as non-falciparum by BinaxNOW in one patient in this study is unusual and is, to our knowledge and according to the manufacturer of BinaxNOW, the first PCR-confirmed report of such a case. Reactivity with the aldolase antibody would not be unexpected with P. falciparum, as aldolase is a panmalarial antigen; however, the absence of HRP-2 reactivity is puzzling. A recent report described a positive association between P. falciparum parasitemia and coreactivity of aldolase and HRP-2 in the BinaxNOW test (18). Given that the parasitemia was not high, a prozone effect is unlikely to explain this phenomenon. A defective BinaxNOW test is also unlikely, because the result was reproducible on repetition, and a BinaxNOW test from the same lot was able to detect P. falciparum HRP-2 in a blood sample collected 2 days later. Additionally, mixed infection was ruled out by PCR. Genetic diversity of HRP-2 may also affect RDT results. One study has demonstrated variable sensitivities of RDTs to P. falciparum strains with differing amino acid repeats in HRP-2; however, this is more likely to be observed at low levels of parasitemia (<0.02%, or <1,000/μl) (2). Another group has shown that deletion of HRP-2, as detected by PCR, is a risk for false-negative results (13). However, the false-negative case described here is unlikely to be explained by either of these phenomena.
While the manufacturer of BinaxNOW does not recommend confirmation of positive results, the findings of this study argue for this practice. Potential consequences of misidentification include inappropriate and excessive therapy, as P. falciparum is resistant to many of the antimalarial agents and does not require therapy with the liver schizonticides used to prevent the relapse of dormant liver stages of P. ovale and P. vivax (6).
In summary, our findings support the use of BinaxNOW as a screening aid for malaria in therapy-naïve patients in areas of nonendemicity. Importantly, our findings demonstrate the possibility of incorrect species identification with this assay, and we therefore recommend definitive identification by either microscopy or PCR.
Footnotes
Published ahead of print 20 June 2012
REFERENCES
- 1. Abba K, et al. 2011. Rapid diagnostic tests for diagnosing uncomplicated P. falciparum malaria in endemic countries. Cochrane Database Syst. Rev. 7:CD008122 doi:10.1002/14651858.CD008122.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baker J, et al. 2005. Genetic diversity of Plasmodium falciparum histidine-rich protein 2 (PfHRP2) and its effect on the performance of PfHRP2-based rapid diagnostic tests. J. Infect. Dis. 192:870–877 [DOI] [PubMed] [Google Scholar]
- 3. De Monbrison F, et al. 2004. Comparative diagnostic performance of two commercial rapid tests for malaria in a non-endemic area. Eur. J. Clin. Microbiol. Infect. Dis. 23:784–786 [DOI] [PubMed] [Google Scholar]
- 4. Durand F, et al. 2005. Performance of the Now Malaria rapid diagnostic test with returned travellers: a 2-year retrospective study in a French teaching hospital. Clin. Microbiol. Infect. 11:903–907 [DOI] [PubMed] [Google Scholar]
- 5. Farcas GA, Zhong KJ, Lovegrove FE, Graham CM, Kain KC. 2003. Evaluation of the BinaxNOW ICT test versus polymerase chain reaction and microscopy for the detection of malaria in returned travelers. Am. J. Trop. Med. Hyg. 69:589–592 [PubMed] [Google Scholar]
- 6. Galappaththy GN, Omari AA, Tharyan P. 2007. Primaquine for preventing relapses in people with Plasmodium vivax malaria. Cochrane Database Syst. Rev. 1:CD004389 doi:10.1002/14651858.CD004389.pub2 [DOI] [PubMed] [Google Scholar]
- 7. Gillet P, et al. 2011. Prozone in malaria rapid diagnostics tests: how many cases are missed? Malar. J. 10:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Humar A, Ohrt C, Harrington MA, Pillai D, Kain KC. 1997. Parasight F test compared with the polymerase chain reaction and microscopy for the diagnosis of Plasmodium falciparum malaria in travelers. Am. J. Trop. Med. Hyg. 56:44–48 [DOI] [PubMed] [Google Scholar]
- 9. Inverness Medical Binax, Inc 2007. BinaxNOW Malaria package insert, p 1–10 Inverness Medical Binax, Inc., Scarborough, ME [Google Scholar]
- 10. Jelinek T, Grobusch MP, Nothdurft HD. 2000. Use of dipstick tests for the rapid diagnosis of malaria in nonimmune travelers. J. Travel Med. 7:175–179 [DOI] [PubMed] [Google Scholar]
- 11. Khairnar K, Martin D, Lau R, Ralevski F, Pillai DR. 2009. Multiplex real-time quantitative PCR, microscopy and rapid diagnostic immuno-chromatographic tests for the detection of Plasmodium spp: performance, limit of detection analysis and quality assurance. Malar. J. 8:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kilian AH, et al. 2000. Reliability of malaria microscopy in epidemiological studies: results of quality control. Trop. Med. Int. Health 5:3–8 [DOI] [PubMed] [Google Scholar]
- 13. Koita OA, et al. 2012. False-negative rapid diagnostic tests for malaria and deletion of the histidine-rich repeat region of the hrp2 gene. Am. J. Trop. Med. Hyg. 86:194–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maguire JD, et al. 2006. Production and validation of durable, high quality standardized malaria microscopy slides for teaching, testing and quality assurance during an era of declining diagnostic proficiency. Malar. J. 5:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mali S, Steele S, Slutsker L, Arguin PM, Centers for Disease Control and Prevention 2010. Malaria surveillance—United States, 2008. MMWR Surveill Summ. 59:1–15 [PubMed] [Google Scholar]
- 16. Murray CK, Gasser RA, Jr, Magill AJ, Miller RS. 2008. Update on rapid diagnostic testing for malaria. Clin. Microbiol. Rev. 21:97–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stauffer WM, et al. 2009. Diagnostic performance of rapid diagnostic tests versus blood smears for malaria in US clinical practice. Clin. Infect. Dis. 49:908–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Gool T, et al. 2011. A simple and fast method to exclude high Plasmodium falciparum parasitaemia in travellers with imported malaria. Malar. J. 10:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wilson ME, et al. 2007. Fever in returned travelers: results from the GeoSentinel Surveillance Network. Clin. Infect. Dis. 44:1560–1568 [DOI] [PubMed] [Google Scholar]
- 20. World Health Organization 2009. World malaria report 2009, p 27 World Health Organization, Geneva, Switzerland [Google Scholar]