Abstract
The serum (1→3)-β-d-glucan assay has emerged as an important diagnostic test for invasive fungal disease. The utility of this assay in coccidioidomycosis has not been previously studied. Using a cutoff value of ≥80 pg/ml, we found the sensitivity (43.9%), specificity (91.1%), positive predictive value (81.8%), and negative predictive value (64.1%) to be similar to those of the assay in diagnosing other invasive mycoses.
TEXT
The incidence of invasive fungal disease (IFDs) has increased in recent years, primarily due to the expanding immunosuppressed population (8, 15). IFDs are associated with significant morbidity and mortality and are often not readily diagnosed, leading to delays in treatment. Blood cultures are frequently unhelpful in the diagnosis of IFDs and histopathologic diagnosis is not always feasible in those at highest risk. For these reasons, interest in noninvasive diagnostic testing has increased. Among the newer diagnostic techniques is the assay measuring serum levels of (1→3)-β-d-glucan (BG), which is derived from fungal cell walls. This assay has exhibited a high specificity and positive predictive value (PPV) in studies evaluating its use in the diagnosis of invasive candidiasis and aspergillosis (9, 11–13, 17); however, its utility in the diagnosis of coccidioidomycosis has not been previously examined. We evaluated the performance characteristics of BG testing in a diverse cohort of patients with coccidioidomycosis.
(This work was presented, in part, at the 51st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL.)
Subjects evaluated for serologic evidence of coccidioidomycosis by the University of California Davis Coccidioidomycosis Serology Laboratory were included in this analysis. Patient samples and medical information arrived from requesting physicians across California and Arizona. Samples were included if sufficient clinical information was available for chart abstraction between September and December of 2010 and subsequently deidentified. Patients with hematologic malignancy, receiving dialysis, receiving current care within an intensive care unit, or receiving medications known to cause false-positive BG values were excluded, as these conditions were more likely to cause false-positive BG testing or placed the patient at significantly higher risk for an alternative IFD (10).
All samples were tested for coccidioidal antibodies by both immunodiffusion and complement fixation at the University of California—Davis Coccidioidomycosis Serology Laboratory using previously described methods (14). BG testing was performed in a blinded fashion by Beacon Diagnostics Laboratory using the Fungitell (Associates of Cape Cod) assay (13). All serum aliquots were kept frozen (−80°C) and shipped in bulk for testing. This study was approved by the UC—Davis Medical Center Institutional Review Board.
Two hundred twenty-eight patients met the criteria for inclusion in this study. Of these, 40 patients were excluded because of underlying diagnoses as outlined above. The remaining 188 patients included 47 with acute coccidioidomycosis (positive coccidioidal precipitin [IgM] antibody and pulmonary symptoms), 52 with past coccidioidal infection (positive coccidioidal complement fixation [CF] [IgG] antibody and no symptoms of ongoing infection; no antifungals for 1 year), 45 with confirmed meningeal or disseminated coccidioidomycosis (positive cerebrospinal fluid [CSF] coccidioidal antibody titer or recovery of Coccidioides spp. from extrapulmonary site) who were receiving triazole antifungal therapy, and 44 uninfected controls (no evidence of coccidioidomycosis clinically or serologically, and the patient was given an alternative diagnosis by the treating physician).
Of the 47 patients with acute coccidioidomycosis, 25 (53.2%) had BG values of ≥31 pg/ml (median, 31; interquartile range, 61). Three patients had a positive BG test prior to detectable IgM antibody (detected on subsequent samples). Nine patients (19.0%) with acute coccidioidomycosis had BG values of ≥80 pg/ml (Table 1 and Fig. 1). In the group with past coccidioidomycosis, 26 of 52 (50%) exhibited BG values greater than 31 pg/ml (median, <31; interquartile range, 47 pg/ml). Of note, 7 of 52 (13.5%) exhibited values exceeding 80 pg/ml. All seven patients with BG values of ≥80 pg/ml were positive by both complement fixation and immunodiffusion testing, whereas patients with past infection and undetectable BG were more commonly positive only by immunodiffusion (18/26).
Table 1.
Performance of the β-glucan assay in coccidioidomycosis patients using >80 pg/ml as the cutoff for a positive result
| Variable | Value in patients: |
|
|---|---|---|
| With acute coccidioidomycosis | Hospitalized with coccidioidomycosis | |
| Sensitivity (%) | 19.1 | 43.9 |
| Specificity (%) | 91.1 | 91.1 |
| Positive predictive value (%) | 69.2 | 81.8 |
| Negative predictive value (%) | 51.9 | 64.1 |
| Positive likelihood ratio | 2.15 | 6.03 |
| Negative likelihood ratio | 0.89 | 0.62 |
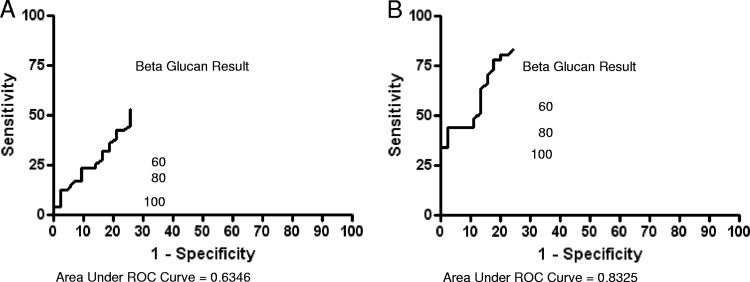
Fig 1.
Receiver operator characteristic (ROC) curves for β-d-glucan as a diagnostic test in acute coccidioidomycosis (A) and as a diagnostic test for coccidioidomycosis in hospitalized patients (B).
In the group with disseminated or meningeal coccidioidomycosis, 34 of 41 (83%) had BG values of >31 pg/ml (median, 85; interquartile range, 175). BG values were also significantly higher in the group with disseminated coccidioidomycosis than in the other three groups (P < 0.001 by Kruskal-Wallis and Dunn's multiple comparison test). However, BG values correlated poorly with serum coccidioidal CF (IgG) antibody titers (R2 = 0.096). Among uninfected controls, only 8 of 44 (18.2%) had BG values exceeding 31 pg/ml (median, <31; interquartile range, <31), while only four patients had values of ≥80 pg/ml.
A pooled analysis of these four groups consisting solely of hospitalized patients was performed to determine the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of BG testing for coccidioidomycosis in this setting (86 patients) (Table 1 and Fig. 1). This group was examined to determine the utility of BG testing in patients with disease severity warranting hospitalization and thus potentially benefiting from earlier diagnosis and antifungal therapy. Using the higher cutoff value of ≥80 pg/ml, these values were 43.9, 91.1, 81.8, and 64.1, respectively (Table 1). The receiver operating characteristic curves (ROC) for BG in the evaluation of patients with acute coccidioidomycosis and hospitalized with coccidioidomycosis are included in Fig. 1A and B.
The enzyme responsible for the production of (1→3)-β-d-glucan, (1→3)-β-glucan synthase, has been found to be essential in Coccidioides spp. (6). Yet prior reports of BG testing in endemic mycoses have been limited. Serum BG positivity has been previously detected in patients with histoplasmosis (4, 16) and in a limited number of patients with blastomycosis (5); however, analysis of BG in Coccidioides-infected patients has not previously been performed. In fact, only two prior reports have described a positive BG level, suggesting coccidioidomycosis as a possible diagnosis (2, 9). In the first report, Baden et al. described a 60-year-old renal transplant patient cared for in Massachusetts, where the disease is not endemic, and the patient was ultimately diagnosed with disseminated coccidioidomycosis. This case underscores previous reports that up to 10% of all cases of coccidioidomycosis are seen outside the typical area of endemicity and that diagnostic and treatment delays undoubtedly occur under these circumstances (1). Furthermore, the report by Baden et al. shows the potential impact that BG “screening” of patients may have—pointing to a possible fungal etiology of their illness while awaiting more specific diagnostic testing. The second report, by Koo et al., provides evidence of BG positivity in a patient with coccidioidomycosis; however, no patient data or BG value was presented.
It is noteworthy that a positive BG assay was obtained for three patients prior to positive Coccidioides antibody testing. Coccidioidal precipitin (IgM) antibodies develop 1 to 3 weeks following exposure, suggesting a possible role for antigen testing for those with “hyperacute” infection during this period. Our results suggest that the sensitivity of BG testing in those with acute disease is disappointing, and receiver operating characteristic analysis fails to identify an appropriate cutoff value that may be useful in clinical care. These findings underscore the elusive nature of a sensitive and specific coccidioidal antigen test, and others have similarly noted poor sensitivity (range, 3.5 to 71.4%) and specificity (cross-reaction with other endemic mycoses) of coccidioidal antigen testing (3, 7).
The Coccidioides life cycle in the evaluation of BG is also important, as Coccidioides spherules are roughly 60% β-glucan by dry weight, while arthroconidia contain only 20% BG (19). Kellner et al. have shown decreasing levels of FKS1 gene expression (the gene encoding the glucan synthase enzyme) in mature spherules compared to immature spherules (6), and these stage-specific differences may play an important role in the performance of BG testing during different clinical forms of infection and have a significant impact on the kinetics of BG in patients with coccidioidomycosis.
Patients with disseminated or meningeal disease frequently exhibited positive BG values, likely from the high burden of infection. However, seven patients were negative for BG despite evidence of ongoing clinical infection. Compartmentalization of BG in patients with suspected central nervous system mycosis has been observed, with elevated CSF levels coincident with low or negative serum BG levels (M. Finkelman, unpublished data). All seven patients were on triazole antifungals at the time samples were obtained.
There are several important limitations to this study, including the lack of longitudinal follow-up for the included patients and the possibility that patients with a positive BG test may have been diagnosed with an alternative fungal infection at a later date. Additionally, the concurrent use of antifungal therapy may have altered the sensitivity and specificity of BG testing in those with disseminated disease, as has been shown in animal models (20). Antifungals are also known to exhibit indirect effects on secondary targets (18), and thus, the fungal expression of BG, in coccidioidomycosis, may have been altered, although this remains speculative in this study.
In conclusion, we have evaluated the characteristics of BG testing in a diverse group of coccidioidomycosis patients and controls. The findings suggest that an assay of BG levels in serum may be a useful diagnostic test in the initial evaluation of coccidioidomycosis when epidemiologic factors suggest the disease and more specific laboratory testing is not immediately available. The sensitivity, specificity, PPV, and NPV are comparable to those seen with other fungal infections, and BG testing may additionally be a useful marker in patients with hyperacute coccidioidomycosis. Clearly, further work on the kinetics of BG in coccidioidomycosis is needed, including an analysis of BG expression during the unique life cycle of the pathogen and the performance of the assay in the setting of antifungal therapy.
ACKNOWLEDGMENTS
Existing funds were used during the preparation of the manuscript.
G.R.T has served as a consultant for Basilea and has received research support from Pfizer. G.R.T. is the assistant director of the Coccidioidomycosis Serology Laboratory; D.P. is the director of the Coccidioidomycosis Serology Laboratory. M.A.F. is an employee of Associates of Cape Cod, Inc.
Footnotes
Published ahead of print 12 June 2012
REFERENCES
- 1. Baddley JW, et al. 2011. Geographic distribution of endemic fungal infections among older persons, United States. Emerg. Infect. Dis. 17:1664–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baden LR, Digumarthy SR, Guimaraes AS, Branda JA. 2009. Case records of the Massachusetts General Hospital. Case 35–2009. A 60-year-old male renal-transplant recipient with renal insufficiency, diabetic ketoacidosis, and mental-status changes. N. Engl. J. Med. 361:1980–1989 [DOI] [PubMed] [Google Scholar]
- 3. Durkin M, et al. 2009. Detection of coccidioides antigenemia following dissociation of immune complexes. Clin. Vaccine Immunol. 16:1453–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Egan L, et al. 2008. Histoplasmosis as a cause for a positive Fungitell (1→3)-beta-d-glucan test. Med. Mycol. 46:93–95 [DOI] [PubMed] [Google Scholar]
- 5. Girouard G, Lachance C, Pelletier R. 2007. Observations on (1–3)-beta-d-glucan detection as a diagnostic tool in endemic mycosis caused by Histoplasma or Blastomyces. J. Med. Microbiol. 56:1001–1002 [DOI] [PubMed] [Google Scholar]
- 6. Kellner EM, et al. 2005. Coccidioides posadasii contains a single 1,3-beta-glucan synthase gene that appears to be essential for growth. Eukaryot. Cell 4:111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kirsch EJ, et al. 2012. Evaluation of coccidioides antigen detection in dogs with coccidioidomycosis. Clin. Vaccine Immunol. 19:343–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kontoyiannis DP, et al. 2010. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin. Infect. Dis. 50:1091–1100 [DOI] [PubMed] [Google Scholar]
- 9. Koo S, Bryar JM, Page JH, Baden LR, Marty FM. 2009. Diagnostic performance of the (1→3)-beta-d-glucan assay for invasive fungal disease. Clin. Infect. Dis. 49:1650–1659 [DOI] [PubMed] [Google Scholar]
- 10. Mohr JF, et al. 2011. Prospective survey of (1→3)-beta-d-glucan and its relationship to invasive candidiasis in the surgical intensive care unit setting. J. Clin. Microbiol. 49:58–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Obayashi T, et al. 1995. Plasma (1→3)-beta-d-glucan measurement in diagnosis of invasive deep mycosis and fungal febrile episodes. Lancet 345:17–20 [DOI] [PubMed] [Google Scholar]
- 12. Odabasi Z, et al. 2004. Beta-d-glucan as a diagnostic adjunct for invasive fungal infections: validation, cutoff development, and performance in patients with acute myelogenous leukemia and myelodysplastic syndrome. Clin. Infect. Dis. 39:199–205 [DOI] [PubMed] [Google Scholar]
- 13. Ostrosky-Zeichner L, et al. 2005. Multicenter clinical evaluation of the (1→3) beta-d-glucan assay as an aid to diagnosis of fungal infections in humans. Clin. Infect. Dis. 41:654–659 [DOI] [PubMed] [Google Scholar]
- 14. Pappagianis D. 2001. Serologic studies in coccidioidomycosis. Semin. Respir. Infect. 16:242–250 [DOI] [PubMed] [Google Scholar]
- 15. Pappas PG, et al. 2010. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 50:1101–1111 [DOI] [PubMed] [Google Scholar]
- 16. Sax PE, et al. 2011. Blood (1→3)-beta-d-glucan as a diagnostic test for HIV-related Pneumocystis jirovecii pneumonia. Clin. Infect. Dis. 53:197–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Senn L, et al. 2008. 1,3-Beta-d-glucan antigenemia for early diagnosis of invasive fungal infections in neutropenic patients with acute leukemia. Clin. Infect. Dis. 46:878–885 [DOI] [PubMed] [Google Scholar]
- 18. Thompson GR, III, et al. 2011. Early treatment with fluconazole may abrogate the development of IgG antibodies in coccidioidomycosis. Clin. Infect. Dis. 53:e20–e24 [DOI] [PubMed] [Google Scholar]
- 19. Viriyakosol S, Fierer J, Brown GD, Kirkland TN. 2005. Innate immunity to the pathogenic fungus Coccidioides posadasii is dependent on Toll-like receptor 2 and Dectin-1. Infect. Immun. 73:1553–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wiederhold NP, et al. 2008. Assessment of serum (1→3)-beta-d-glucan concentration as a measure of disease burden in a murine model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 52:1176–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]