Abstract
The control of vancomycin-resistant enterococci (VRE) has become an increasing burden on health care resources since their discovery over 20 years ago. Current techniques employed for their detection include time-consuming and laborious phenotypic methods or molecular methods requiring costly equipment and consumables and highly trained staff. An accurate, rapid diagnostic test has the ability to greatly reduce the spread of this organism, which has the ability to colonize patients for long periods, potentially even lifelong. Matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) is a technology with the ability to identify organisms in seconds and has shown promise in the identification of other forms of antimicrobial resistance in other organisms. Here we show that MALDI-TOF MS is capable of rapidly and accurately identifying vanB-positive Enterococcus faecium VRE from susceptible isolates. Internal validation of the optimal model generated produced a sensitivity of 92.4% and a specificity of 85.2%. Prospective validation results, following incorporation into the routine laboratory work flow, demonstrated a greater sensitivity and specificity at 96.7% and 98.1%, respectively. In addition, the utilization of MALDI-TOF MS to determine the relatedness of isolates contributing to an outbreak is also demonstrated.
INTRODUCTION
Glycopeptide antibiotics, particularly vancomycin, have for many years been the mainstay of treatment for increasingly antibiotic-resistant Gram-positive organisms, including enterococci. Since their discovery in the late 1980s, vancomycin-resistant enterococci (VRE) have become one of the most problematic groups of multiresistant organisms, imposing a significant burden on the health care system, particularly in terms of controlling their dissemination. In contrast to many other resistant organisms, colonization is long term, and there is no effective method for decolonization. Thus, patients are likely to be committed to stringent infection control precautions, potentially for life.
VRE were first isolated in Australia from a liver transplant recipient in a Melbourne hospital in 1994 (12) and were first reported in Queensland in 1996 (11, 16). Early studies showed that the epidemiology of VRE in Australia was different from that experienced elsewhere, with VanB Enterococcus faecium predominating and highly polyclonal strains appearing in diverse locations independently (3). Since then, the incidence of vancomycin-resistant enterococci has escalated dramatically. A study of resistance in isolates of Enterococcus species causing clinical disease among in- and outpatients from 17 institutions around Australia showed that the prevalence of vancomycin resistance in E. faecium had more than doubled, from 7.2% in 2005 to 15.4% in 2007 (6, 7). The prevalence of VRE colonization in Queensland has risen, with from 0.3% of VRE screens in 2006 to 3.6% in 2011 reported to be positive (5). VRE infection rates paralleled those for colonization, with the incidence of enterococcal isolates from clinical specimens identified as VRE rising from 0.1% in 2006 to 3.3% in 2011 (5).
To further complicate the situation, studies suggest that the incidence of more unusual enterococcal species, such as E. durans, E. gallinarum, and E. casseliflavus, from clinical specimens has increased significantly (22). Although some are intrinsically resistant to vancomycin, they do not pose the same infection control concerns as E. faecium or E. faecalis VRE, as their resistance is chromosomal rather than plasmid mediated. Their resistance to vancomycin does, however, mean that they will grow on selective medium designed to screen for VRE. This therefore adds to the number of positive screens that need to be further identified. Further, some of these more unusual enterococcal species may not be as reliably identified by commercial identification systems (22). The ability to promptly identify these enterococcal species correctly would greatly reduce the number of false-positive VRE screens (growth of intrinsically resistant species on screening medium containing vancomycin) that require further identification.
Bacterial identification based on spectra obtained by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) was proposed over 30 years ago. Only recently, it has been used as a rapid, inexpensive, and accurate method for identifying isolates that belong to certain bacterial phyla (19). Its application is increasing rapidly, and it is now routinely used for bacterial identification in many clinical laboratories. Although the utilization of this technology for more defined applications, including identification of antibiotic resistance, is evolving rapidly, to our knowledge, it has not been reported for the identification of vancomycin resistance or VRE. Changes in mass spectra have been shown with strains of Staphylococcus aureus differing in their susceptibility to methicillin, even when they were indistinguishable by pulsed-field gel electrophoresis (PFGE) (14) (9). MALDI-TOF MS was also possibly able to detect resistance to the glycopeptide teicoplanin (14). MALDI-TOF MS has been used for analysis of genetically determined resistance of Streptococcus pneumoniae to fluoroquinolones, through detection of mutations in the fluoroquinolone target structures before they were able to be detected phenotypically (15). The methods by which MALDI-TOF MS has previously been able to identify antibiotic resistance have varied; however, the method for identification of vancomycin resistance in enterococci that we describe here has not been previously reported.
Strain typing to determine epidemiologic relatedness is central to outbreak management. Currently employed genotypic typing methods include PFGE and multilocus sequence typing (MLST). Although these have high discriminatory power, they are labor-intensive and costly with long turnaround times, which limit their clinical utility. The speed with which MALDI-TOF MS can analyze bacterial isolates not only for identification purposes but also potentially for relatedness makes it an attractive alternative to these genotypic methods. In addition to S. aureus (18, 24), some examples of the application of MALDI-TOF MS for this purpose include the typing of Listeria species (2), the differentiation of Streptococcus pneumoniae isolates associated with an outbreak of conjunctivitis (23), and the characterization and discrimination between closely related environmental strains of Escherichia coli, thereby enabling their classification according to source (20). To date, however, the use of MALDI-TOF MS for the investigation of relatedness of enterococci has not been reported.
The C-terminal d-Ala–d-Ala residues of bacterial cell wall precursors are the target for the glycopeptide antibiotics vancomycin and teicoplanin. Once bound, these antibiotics inhibit the transglycosylation and transpeptidation reactions of peptidoglycan assembly. Resistance to glycopeptides is mediated through six Van types, the acquired VanA, VanB, VanD, VanE, and VanG and also VanC, which is intrinsic to E. gallinarum, E casseliflavus, and E. flavescens. Organisms of the VanA phenotype are resistant to both vancomycin and teicoplanin, whereas vanB-positive organisms are resistant to vancomycin only. In Australia, VanB is the more common vancomycin-resistant phenotype. Resistance to vancomycin arises from alterations in the antibiotic binding site due to changes in the ligase gene. More specifically, the vanB gene cluster, as described in E. faecalis V583, consists of genes encoding polypeptides assigned to the regulation of glycopeptide resistance genes (vanRB and vanSB), synthesis of the depsipeptide d-alanyl–d-lactate (vanHB and vanB), and hydrolysis of precursors of normal d-Ala–d-Ala peptidoglycan (vanXB and vanYB) (10, 17). The fact that the upstream two-component regulatory system is induced by vancomycin but not teicoplanin explains the susceptibility to teicoplanin of VanB-type VRE (10).
It is postulated that any one or perhaps the combination of these polypeptides could sufficiently alter the spectra obtained by MALDI-TOF MS to enable the identification of vancomycin-resistant enterococci, thus superseding the laborious and time-consuming phenotypic and molecular methods currently employed. Our routine diagnostic microbiology laboratory is uniquely positioned in having a specialist proteomics division within the same pathology department. We aim to utilize specialized post-data-acquisition analysis methods available through this collaboration to demonstrate that MALDI-TOF MS is capable of differentiating vancomycin-sensitive from vancomycin-resistant (vanB-positive) E. faecium isolates in a much more rapid time frame than existing methods (minutes versus days). Once this is demonstrated, we aim to translate this information back into the routine diagnostic laboratory to enable the rapid identification of VRE from clinical specimens. In addition, given that the vast majority of acquisitions occur by nosocomial spread and are often associated with outbreaks, we investigate the use of MALDI-TOF MS to determine the epidemiologic relatedness of VRE isolates.
MATERIALS AND METHODS
Study design.
Consecutive unique Enterococcus faecium isolates that were phenotypically suspected of being and subsequently confirmed to be vanB positive by PCR and that were collected over a continuous 18-month period from January 2009 to June 2010 inclusive were included in the analysis. These isolates and controls were analyzed using a Bruker microflex apparatus (Bruker Daltonics GmbH, Bremen, Germany), and data were analyzed with the proprietary software program ClinProTools to determine the accuracy of identification using the MALDI-TOF MS technology, the relatedness of the clinical isolates, and whether MALDI-TOF MS can be used to identify resistance to vancomycin in the form of VanB VRE. Once MALDI-TOF MS was shown to be able to differentiate VanB-positive from VanB-negative enterococci, the final phase of this study entailed prospective validation of this approach by incorporating it into the standard work flow of the routine diagnostic microbiology laboratory.
Study venue.
The Division of Microbiology at Mater Misericordiae Health Services (South Brisbane, Australia) receives specimens from the Mater Health Services (1,060 beds), comprising Mater Adults Hospital, Mater Mothers Hospital (public and private), Mater Children's Hospital (public and private), and the two Mater private hospitals (South Brisbane and Redlands), as well as Holy Spirit Northside Private Hospital and a number of general practice surgeries in Brisbane and surrounding areas. The Mater Complex includes four intensive care units (adults [public and private], children's, and neonatal) and handles a large oncology patient population as well as a full range of adult/maternity/pediatric and neonatal services.
Isolates.
Consecutive VanB E. faecium isolates were recovered from clinical specimens and from screening swabs submitted to Mater Pathology. VRE were suspected if growth was observed on vancomycin-resistant enterococcal selective medium that contained bile esculin, sodium azide, and vancomycin (6 μg/ml) (BBL Enterococcosel agar; Becton, Dickinson and Company) or resistance to vancomycin was detected during routine susceptibility testing according to CLSI guidelines (8). Phenotypic identification was performed by conventional microbiological methods based on colony morphology and Gram stain, a negative catalase reaction, a positive pyrrolidonylaminopeptidase (PYR) reaction, a negative overnight methyl-α-d-glucopyranoside (MGP) reaction, and absence of motility (4). Identification and susceptibility testing by broth microdilution were performed from a subculture of the isolate using the Vitek 2 automated identification system (bioMérieux). MICs for vancomycin were obtained by Etest as per CLSI guidelines (8).
Given the requirement for repeated subculture and the time taken for the various components of the phenotypic identification, this method generally takes 3 to 4 days to report the positive identification of VRE. Although the total cost of identification of a VRE by this method is difficult to quantitate fully, particularly in terms of the scientists' labor required, the quantifiable cost of reagents and consumables for conventional identification by this phenotypic method was estimated to be approximately AU$25 to AU$30.
Detection of VanB.
Isolates phenotypically suspected of being vancomycin resistant were referred to Pathology Queensland for PCR (the “gold standard”). Pathology Queensland utilizes a multiplex assay based on specific detection of genes encoding d-alanine–d-alanine ligases (ddl) to confirm identification as E. faecium or E. faecalis and VanA or VanB as part of the National VRE Network (NaVREN) and on the primers developed by Bell and colleagues (4).
Controls.
Four control groups were utilized to identify peaks in the mass spectra that may be attributable to the presence of vanB in E. faecium. These controls were (i) eight E. faecium isolates from a variety of sources phenotypically suspected of being vancomycin resistant but found to lack vanB (and vanA) by PCR by Pathology Queensland (supplied by Pathology Queensland), (ii) non-VRE E. faecium reference strain ATCC 19434, (iii) a vanA-positive E. faecium reference strain (supplied by Jan Bell), and (iv) non-VRE E. faecalis reference strain ATCC 51299.
The eight isolates phenotypically suspected of being vancomycin resistant but PCR negative were the principle negative control utilized as they most closely control for variations attributable to components other than the presence of vanB, such as those that determine local strains. Six of these were suspected of being VRE on the basis of results from the Vitek 2 automated identification system (bioMérieux), and two had growth on screening medium containing vancomycin. In addition to the absence of both VanA and VanB determinants, the species was confirmed to be E. faecium with ddl PCR (4). E. faecium ATCC 19434 was included to enlarge the negative-control population, given the small numbers of available isolates phenotypically suspected of being VRE but PCR negative, as this is a relatively rare occurrence.
PFGE.
Four representative isolates obtained in the first 6 months of the time period of the study (isolates 4, 15, 17, and 19) were randomly selected for further analysis by PFGE (performed by Pathology Queensland) to enable correlation with MALDI-TOF MS relatedness analysis of the same isolates.
MALDI-TOF MS.
Two methods are commonly employed for the preparation of bacterial isolates for MALDI-TOF MS analysis: the direct colony method and the extraction method. The direct colony method involves spotting a small amount of biological material (often, an individual colony) directly onto the target plate (typically onto multiple spots) using a toothpick. Although this is the quickest and easiest method and results in a spectral resolution sufficient for the routine identification of most bacterial isolates, studies have shown that the physical disruption of peptidoglycan in the Gram-positive bacterial cell wall using this method may not be ideal to adequately prepare proteins for MALDI-TOF MS analysis (21). The direct transfer of a colony from an agar plate to the target may also reduce the resolution of the spectra obtained due to metabolites, pigments, and/or agar potentially interfering with the crystallization process (9). An extraction method involves the lysis of the bacterial cells, releasing proteins into an extract that can then be applied to the target plate for MALDI-TOF MS analysis, and overcomes some of the shortcomings of the direct colony method. Alatoom and colleagues compared the direct colony method and the extraction method for identification of Gram-positive cocci (including enterococci) and showed that the proprietary extraction method was superior for the identification of isolates to the genus and species levels (1). Given that the first aim of our study was to analyze enterococci, to detect what may be very subtle differences in the spectra obtained, and subsequently generate a reference spectral profile against which potential VRE isolates in our laboratory will be compared, during the initial phase of the study we utilized an extraction method to optimize the resolution of the resultant spectra.
Ethanol-formic acid extraction.
The proprietary extraction method described in the MALDI Biotyper instruction booklet (version 2.0) and the procedure for microorganism profiling with MALDI Biotyper preliminary version/090109 were used with slight modification.
All steps were performed at room temperature. Nuclease-free water (300 μl; Qiagen) was aliquoted into a plasticizer-free 1.5-ml Eppendorf tube, one loop of organisms from a single colony was added, and the water and organisms were thoroughly mixed by vortexing. To this suspension of organisms, 900 μl 100% ethanol was added, and again, the organisms and the suspension were thoroughly mixed by vortexing. To deposit the biological material, the tubes were centrifuged at 10,000 rpm for 2 min and the supernatant was decanted. To remove the residual ethanol, the tubes were centrifuged again and the ethanol was removed by careful pipetting. Twenty microliters of 70% formic acid (Fluka Analytical) was added to the pellet, and the formic acid and pellet were well mixed by vortexing, followed by the addition of 20 μl of pure acetonitrile (Sigma-Aldrich), which was carefully mixed with the other components by pipetting up and down. Finally, the tubes were centrifuged at 10,000 rpm for 2 min, resulting in a supernatant ready for analysis.
Target preparation.
One microliter of supernatant was carefully placed onto a single spot on a 96-spot polished steel target and allowed to dry. Once dry, this was overlaid with 1 μl matrix solution (a saturated solution of α-cyano-4-hydroxycinnamic acid [HCAA] matrix [Sigma-Aldrich] in 50% acetonitrile [Sigma-Aldrich] and 2.5% trifluoroacetic acid [Auspep]) and once again allowed to dry.
MS spectrum acquisition.
MALDI-TOF MS was performed on a Bruker Microflex LT benchtop instrument controlled by FlexControl software (version 2.0; Bruker Daltonics GmbH, Leipzig, Germany). Spectra were acquired as per the standard recommended proprietary method utilizing the Biotyper preprocessing standard method and the Biotyper MSP identification standard method (2,000 to 20,000 Da, linear positive method).
Data analysis.
Data acquired by MALDI-TOF MS were analyzed using ClinProTools (version 2.2; Bruker Daltonics GmbH, 2007) following the generation of relevant XML files using a ClinProtSpectraImport XML generator. Once the XML files were opened in ClinProTools, the data were prepared by recalibration, average peak list calculation, and peak calculation.
Models were generated using all four available algorithms (genetic algorithm [GA], support vector machine [SVM], supervised neural network [SNN], and QuickClassifier [QC]) and compared. For each model, the default settings were left unaltered; for example, with the support vector machine model, automatic detection was selected for peaks in model and the k-nearest-neighbor (KNN) classification was left at a number of neighbors equal to 3. With the genetic algorithm, the maximum number of peaks in the model was left at 10 and the maximum number of generations was left at 50. For each model, the recognition capability and cross validation were calculated to demonstrate the reliability and accuracy of the model.
Prospective validation.
Following the completion of the statistical analysis of the study isolates, the isolates proven to be vanB positive by PCR and the isolates clinically suspected of being VRE but vanB PCR negative were entered into the MALDI-TOF MS database and labeled “VRE positive” and “VRE negative,” respectively. Comparison of isolates analyzed by MALDI-TOF MS with these two reference spectra, in addition to the proprietary data set, allowed the identification of vancomycin resistance in enterococci (specifically, of the VanB type) during routine identification of isolates in the laboratory. Prospective validation of this method was conducted in the first 2 months of 2012. To accomplish this, all growth obtained on the VRE screening medium (vancomycin-resistant enterococcal selective medium containing bile esculin, sodium azide, and vancomycin [6 μg/ml] [BBL Enterococcosel agar; Becton, Dickinson and Company]) from screening swabs submitted to the laboratory was analyzed by MALDI-TOF MS in parallel with the conventional phenotypic methods presented above. In addition, in our laboratory, all blood culture isolates are analyzed by MALDI-TOF MS, so these and other clinical enterococcal isolates suspected of potentially being VRE were included in the analysis. As the spectral resolution necessary to perform the higher-level proteomic analysis and generate the reference spectra was not required when comparing clinical isolates to the reference spectra that had been added to the database, the extraction step was not performed. Given that the focus for routine use of MALDI-TOF MS is speed and ease of use and that the laboratory had already validated the direct colony method for the identification of clinical isolates, this method was chosen for the prospective validation. This involved touching a toothpick on the colony and spotting it directly in a circular motion onto two consecutive spots on the polished steel target plate. Once it was dry, the smear of organisms was overlaid with 2 μl matrix solution (a saturated solution of HCAA matrix [Sigma-Aldrich] in 50% acetonitrile [Sigma-Aldrich] and 2.5% trifluoroacetic acid [Auspep]) and allowed to dry. MALDI-TOF MS data acquisition was performed as previously outlined.
RESULTS
VRE results over the 18-month period.
Sixty-seven unique VRE isolates were identified utilizing the routine laboratory methods outlined above with both identification as E. faecium and the presence of VanB confirmed by PCR by the reference laboratory and therefore included in the analysis.
In addition to the 67 VanB E. faecium isolates which were obtained during the study period and utilized in the subsequent analysis, E. faecalis (as opposed to E. faecium) isolates that were vanB positive (representing 6% of all VREs isolated) were cultured from four patients. Given the relatively low incidence of E. faecalis VRE not only in this data set but also nationwide, these four isolates were not analyzed. No vanA-positive isolates were identified during the study period.
Six of the 67 isolates included in the study were obtained from clinical specimens, and the remainder were cultured from dedicated multiresistant organism (MRO) screens. Of the clinical specimens, two were sputum samples and four were urine samples. There were a total of 13 patients whose clinical specimens tested positive for VanB E. faecium during the study period. In addition to the six patients in which the first specimen that was VRE positive was a clinical sample, an additional seven patients had a positive result for VRE from a culture of a clinical specimen subsequent to them returning a positive MRO screen. Three of these patients had positive blood cultures, two had positive urine cultures, one had a positive sputum culture, and one had a positive wound culture. The distribution by month ranged from 0 in June 2009 to 13 in October 2009, with a mean of 3.7 cases per month (standard deviation, 3.0). During October 2009, a nosocomial outbreak was identified on the basis of evidence of in-hospital transmission.
Identification by MALDI-TOF MS. (i) Patient isolates.
Of the 67 VRE isolates analyzed, 66 (98.5%) were identified correctly (to the species level) by MALDI-TOF MS, according to the results of the gold standard PCR. The mean score obtained for the 66 isolates with a correct identification was 2.456 (range, 1.924 to 2.592), which, according to the proprietary key, is in the category “highly probable species identification.” Of the 66 correctly identified, the majority (48 or 73%) were identified as Enterococcus faecium 20218_1 CHB, with 18 identified as Enterococcus faecium 11037_CHB (27%). According to Bruker, these 2 database entries represent different E. faecium strains obtained from the Charité Berlin Culture Collection, so it is likely that they are similar.
The isolate that was not correctly identified by MALDI-TOF MS was reported to be Enterococcus gallinarum DSM 20717_DSM, with a score of 1.818, followed by Enterococcus casseliflavus DSM 20382 DSM, with a score of 1.784. This isolate was cultured during the month of peak incidence, October 2009; it was phenotypically identified as E. faecium in our laboratory, and the ddl PCR from the reference laboratory confirmed this. Given that the score was below what is considered an acceptable cutoff for probable species identification (2.0 to 2.299), this result should be considered incomplete rather than incorrect.
(ii) Controls.
Of the 8 PCR-negative controls run in duplicate, 11 were identified as Enterococcus faecium 11037_CHB (69%) and 5 were identified as Enterococcus faecium 20218_1 CHB (31%), with an average score of 2.35.
The other control isolates were analyzed each time that a VRE isolate was analyzed, which was a total of 11 times. The VRE-negative E. faecium control strain ATCC 19434 was identified as Enterococcus faecium 11037_CHB 10 out of 11 times analyzed (91%) and as Enterococcus faecium 20218_1 CHB once (9%), with an average score of 2.40.
The E. faecalis control strain ATCC 51299 was identified as Enterococcus faecalis 20247_4_CHB 8 out of 11 times analyzed (73%) and as Enterococcus faecalis ATCC 7080_THL 3 out of 11 (27%), with an average score of 2.42.
The vanA-positive E. faecium isolate was identified as Enterococcus faecium 20218_1 CHB 10 out of 11 times analyzed (91%) and as Enterococcus faecium 11037_CHB once (9%), with an average score of 2.42.
(iii) Overall.
Of the 67 patient isolates and the 49 spots generated from the controls (a total of 116 samples analyzed), only 1 sample was not correctly identified, giving an accuracy to the species level of 99.13%. It is clear from these results that the existing database gives no indication of the presence of vanB (or vanA) on the basis of the closest match in the database alone.
(iv) Data analysis on ClinProTools.
The models generated using all four of the standard algorithms, GA, SVM, SNN, and QC, yielded similar results.
SVM gave a higher recognition capability (effectively sensitivity) at 99.24%, compared to one of 95.12% for the GA. Cross validation (equivalent to specificity) was similar between the two models at 88.45% for SVM and 88.24% for GA. Use of the SNN algorithm resulted in a slightly lower recognition capability and cross validation (97.73% and 86.34%, respectively). Use of the QC resulted in the lowest scores of all, with a recognition capability of 91.75% and cross validation of 79.45%. Thus, although the differences were not striking, SVM was the most reliable model for differentiating VRE-positive from VRE-negative isolates.
Overall, the peaks or integration regions chosen for differentiation of VRE status by all four of the models were very similar. The important peaks not only from the SVM but also from all models were 2, 21, 23, 28, and 45; the peak statistics for these five peaks are shown in Table 1.
Table 1.
ClinProTools peak statistics for the five peaks of interesta
| Index peak | Mass | DAve | PTTA | PWKW | PAD | Avg1 | Avg2 | SD1 | SD2 | CV1 | CV2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 2,211.5 | 3.63 | < 0.000001 | < 0.000001 | 0.612 | 10.1 | 6.48 | 2.67 | 1.66 | 26.4 | 25.6 |
| 21 | 4,717 | 2.6 | 0.0000888 | 0.000656 | 0.000729 | 6.21 | 3.61 | 3.21 | 2 | 51.7 | 55.3 |
| 23 | 5,094.7 | 8.95 | < 0.000001 | 0.00359 | < 0.000001 | 11.3 | 2.39 | 8.68 | 1.64 | 76.5 | 68.6 |
| 28 | 5,945.7 | 2.97 | 0.00012 | 3.94E-06 | < 0.000001 | 3.09 | 6.06 | 1.29 | 2.94 | 41.9 | 48.5 |
| 45 | 8,327.9 | 1.52 | 8.47E-06 | 0.000038 | 0.175 | 4.07 | 2.56 | 1.26 | 1.12 | 30.9 | 43.9 |
Sort mode, delta average arithmetic; index, peak index or isolate number; mass, m/z value; DAve, difference between the maximal and the minimal average peak area/intensity of all classes; PTTA, P value of t test; PWKW, P value of Wilcoxon (preferable for nonnormally distributed data); PAD, P value of Anderson-Darling test, which gives information about normal distribution (range, 0 to 1; 0, nonnormally distributed; 1, normally distributed); Avg1 and Avg2, peak area/intensity average of class 1 (the VREs) and class 2 (the non-VREs), respectively; SD1 and SD2, standard deviations of the peak area/intensity average of class 1 (the VREs) and class 2 (the non-VREs), respectively; CV1 and CV2, coefficient of variation (in percent) of class 1 (the VREs) and class 2 (the non-VREs), respectively.
(v) Peak statistics.
The low P values for the Anderson-Darling test (PAD) are evidence of the nonnormal distribution of the data obtained. Therefore, the P value of the Wilcoxon test/Kruskal-Wallis test (PWKW) is preferred over the P value of the t test/ANOVA test (PTTA) (as this is preferable for normally distributed data). The low P values obtained from PWKW (all were <0.005) indicate that the observed intensity differences of the individual peaks are highly statistically significant and not based on coincidence (the lower that the P value is, the better that a respective peak signal is suited to be used to separate the two classes [13]). This confirms that they each can be used to differentiate VRE-positive from VRE-negative isolates. To demonstrate the differences visually, three representative peaks of interest are shown in Fig. 1 to 3, where the average spectra of the VRE-positive and VRE-negative isolates can be seen overlying the corresponding gel views.
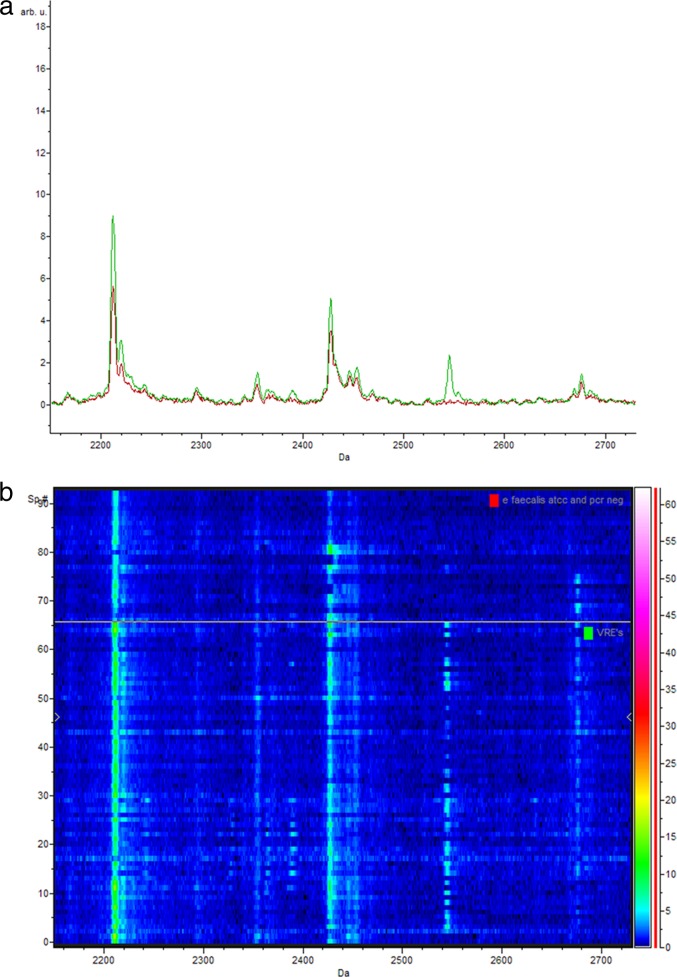
Fig 1.
(a) Representative comparison of the average spectra of the vanB-positive E. faecium isolates (green) and the vanB-negative E. faecium isolates (isolates phenotypically suspected of being VRE yet PCR negative and control strain ATCC 19434) (red) between 2,160 Da and 2,720 Da; (b) corresponding gel view representation (rainbow-scale color scheme) of the same region. The peak with a mass of 2,211 Da is peak 2 used in the classification model. arb. u., arbitrary units; Sp. #, spectrum number.
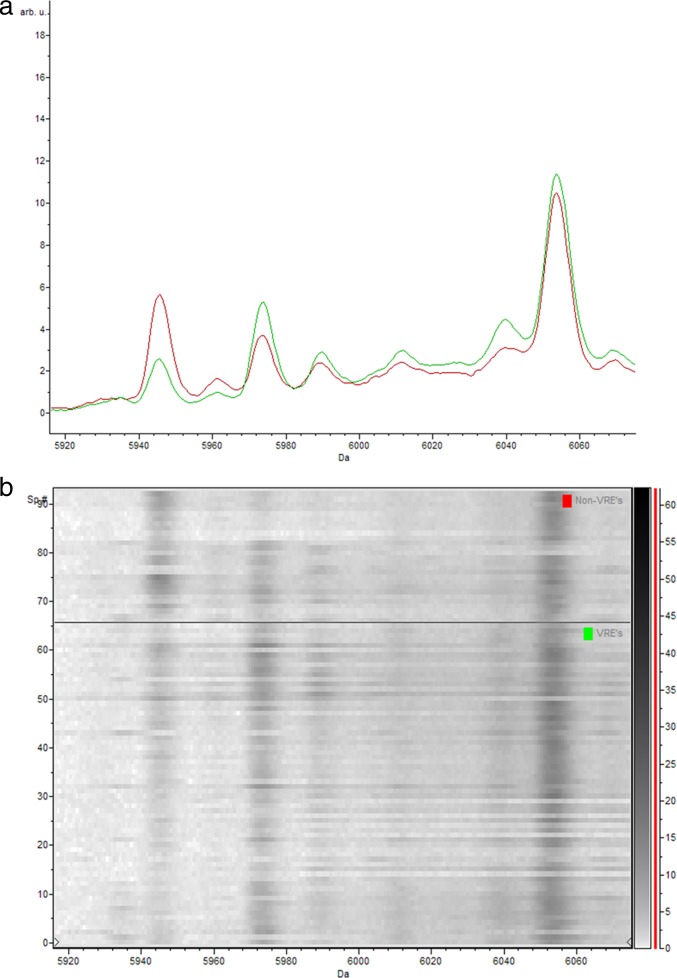
Fig 3.
(a) Representative comparison of the average spectra of the vanB-positive E. faecium isolates (green) and the vanB-negative E. faecium isolates (isolates phenotypically suspected of being VRE yet PCR negative and control strain ATCC 19434) (red) between 5,916 Da and 6072 Da; (b) corresponding gel view representation (gray-scale color scheme) of the same region. vanB-positive E. faecium isolates (bottom) and vanB-negative E. faecium isolates (isolates phenotypically suspected of being VRE yet PCR negative and control strain ATCC 19434) (top). The peak with a mass of 5,945 Da is peak 28 used in the classification model. arb. u., arbitrary units; Sp. #, spectrum number.
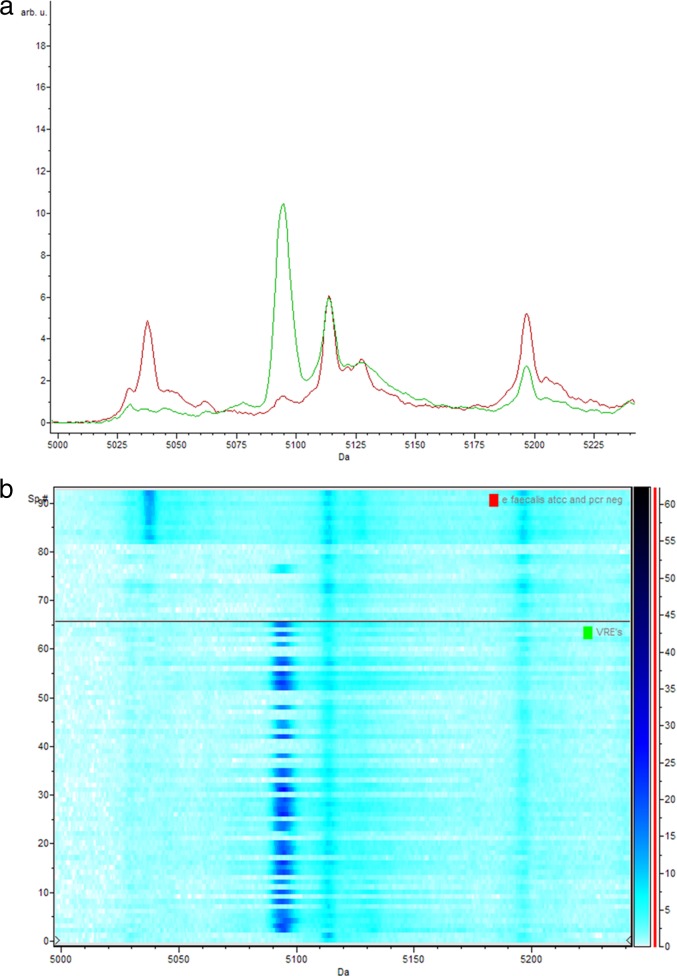
Fig 2.
(a) Representative comparison of the average spectra of the vanB-positive E. faecium isolates (green) and the vanB-negative E. faecium isolates (isolates phenotypically suspected of being VRE yet PCR negative and control strain ATCC 19434) (red) between 5,000 Da and 5,240 Da; (b) corresponding gel view representation (blue-scale color scheme) of the same region. vanB-positive E. faecium isolates (bottom) and vanB-negative E. faecium isolates (isolates phenotypically suspected of being VRE yet PCR negative and control strain ATCC 19434) (top). The peak with a mass of 5,095 Da is peak 23 used in the classification model and is essentially not present in the control samples. arb. u., arbitrary units; Sp. #, spectrum number.
(vi) Receiver operator curves.
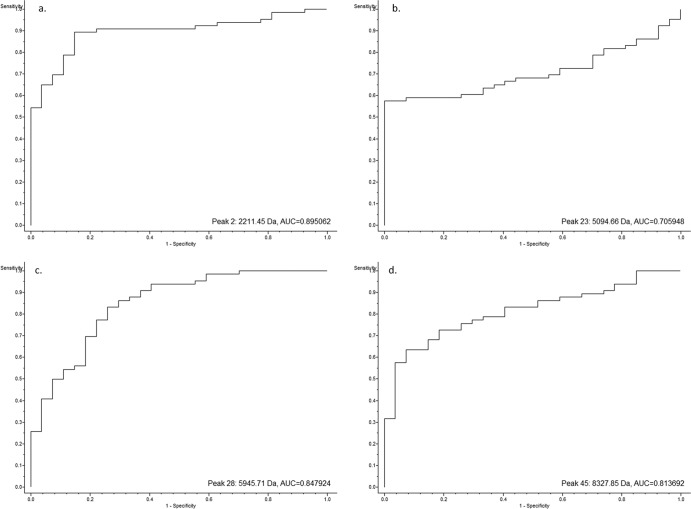
The receiver operating characteristic (ROC) curves for each of the peaks of interest generated by the SVM model were also obtained from the ClinProTools software (Fig. 4). These curves give a graphical overview of the specificity and sensitivity of a test or, within ClinProTools, an evaluation of the discrimination quality of a peak.
Fig 4.
ROC curves of four of the peaks of interest generated from the SVM model showing the discrimination quality of the individual peaks.
A perfect test with a sensitivity and a specificity of 1 would show a ROC curve that starts at the origin (0, 0), ascends vertically up the y axis to (0, 1), and then extends horizontally across to (1, 1). In contrast, a test with no diagnostic capability would produce a diagonal line from the origin to (1, 1). All four of the ROC curves shown ascend vertically from the origin for some time, before gradually curving toward (1, 1), and are well above the diagonal connecting (0, 0) and (1, 1) at all times. This is therefore another representation of the ability of each peak to differentiate the vanB-positive isolates from the controls. The area under the curve (AUC) also demonstrates the diagnostic utility of each peak, with an AUC of 0.5 representing pure chance and an AUC of 1 indicating a perfect test (sensitivity and specificity, 100%). The AUCs of the ROCs for peaks 2, 23, 28, and 45 are 0.895, 0.706, 0.848, and 0.814, respectively (Fig. 4).
(vii) Internal validation.
To internally validate the SVM model generated by ClinProTools, two-thirds of both the VRE-positive and VRE-negative isolates were randomly allocated to form a discovery set, to enable the remaining third of isolates from both cohorts to function as a validation set. To that end, 44 of the PCR-confirmed vanB isolates were randomly allocated into the discovery set with 18 PCR-negative isolates. The validation set therefore comprised the remaining 22 positive isolates with the remaining 9 controls. After the calculation of the SVM model, the validation was performed using the validation function in ClinProTools. This procedure was performed three times. From the three internal validations, the average sensitivity was 92.4% and the average specificity was 85.2%.
(viii) Differentiating VanA from VanB.
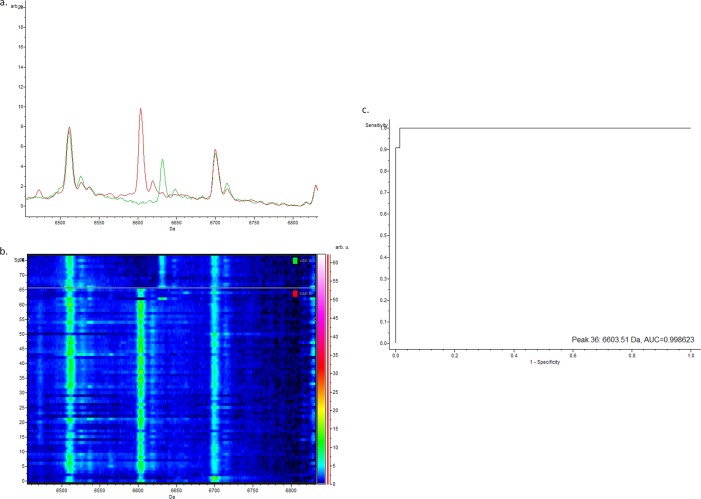
Comparisons between the vanA- and vanB-positive isolates using the SVM model produced a cross validation (essentially, specificity) of 95.04% and a recognition capability (basically, sensitivity) of 100%. The peak with the highest weighting was peak 36, with a mass of 6,603 Da; the average spectra, gel view, and ROC can be seen in Fig. 5. It was clear that vanA-positive E. faecium and vanB-positive E. faecium isolates are readily distinguishable by MALDI-TOF MS analysis.
Fig 5.
(a) Representative comparison of the average spectra of the vanB-positive E. faecium isolates (red) and the vanA-positive E. faecium controls (green) between 6,460 Da and 6,830 Da; (b) corresponding gel view representation (rainbow-scale color scheme) of the same region. vanB-positive E. faecium isolates (bottom) and vanA-positive E. faecium controls (top); the peak with a mass of 6,603 Da is peak 36 used in the classification model; (c) corresponding ROC curve for peak 36. arb. u., arbitrary units; Sp. #, spectrum number.
(ix) Prospective validation.
A total of 281 spots from 129 samples were successfully analyzed as part of the prospective validation of the use of MALDI-TOF MS to detect vancomycin resistance in enterococci (specifically, of the VanB type) during January and February 2012. The majority (271 or 96%) were from screening swabs, with 5 clinical specimens analyzed (in duplicate). Of the 281 spots analyzed, 274 (97.5%) were correctly identified by MALDI-TOF MS compared to the results of conventional phenotypic methods. Only 7 (2.5%) were incorrectly identified, with 4 (1.4%) false negatives and 3 (1.1%) false positives, resulting in a sensitivity of 96.7% and a specificity of 98.1%.
Strain relatedness.
The study period included an outbreak during which time it was clinically relevant to investigate whether all VRE isolates were identical or whether new strains were introduced and contributed to the outbreak. To that end, four isolates were sent to the reference laboratory for PFGE analysis. All four VRE strains analyzed by PFGE were indistinguishable from each other and from the reference outbreak strain, strain A1, from the reference laboratory.
(i) MALDI-TOF MS to investigate relatedness.
To investigate whether MALDI-TOF MS in conjunction with ClinProTools could establish relatedness, hierarchical cluster analysis was performed in ClinProTools on all 66 isolates positive for vancomycin resistance; the dendrograms produced are shown in Fig. 6 and 7.
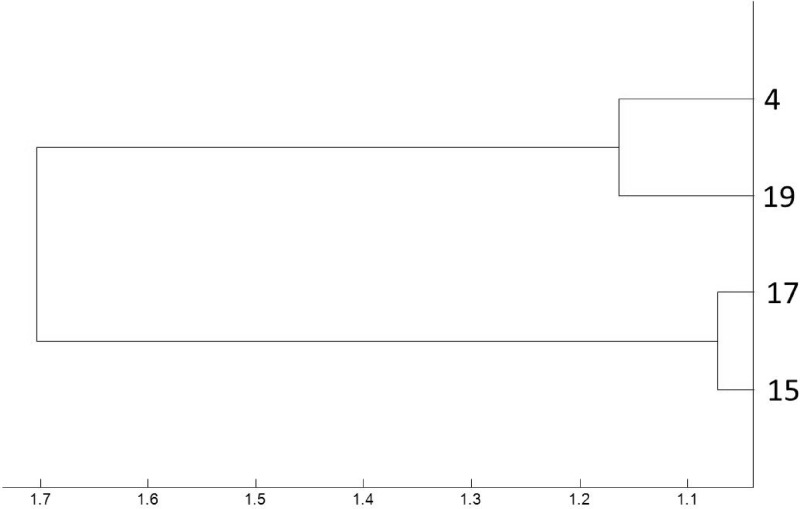
Fig 6.
Magnified dendrogram (representation of hierarchical cluster analysis) of the four vanB-positive E. faecium isolates from our laboratory shown to be identical by PFGE and their representative numbers. All four isolates are well within the arbitrary relatedness cutoff of 2.5.
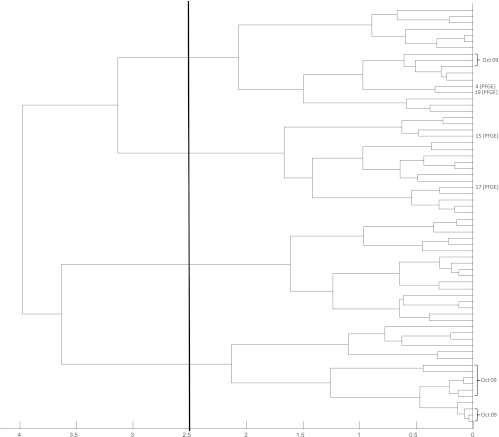
Fig 7.
Dendrogram (representation of hierarchical cluster analysis) of all 66 vanB PCR-positive patient isolates. The four vanB-positive E. faecium isolates from our laboratory shown to be identical by PFGE are shown by their representative number. The 12 vanB-positive isolates from the month with peak incidence (October 2009) are also shown. The vertical line indicates the similarity cutoff of 2.5 arbitrarily chosen for MALDI-TOF MS group definition.
The four isolates shown to be identical by PFGE and to reference outbreak strain A1 were demonstrated to be related by MALDI-TOF MS hierarchical cluster analysis when analyzed in isolation, with a maximum separation of 1.7 (Fig. 6). Although all were within the similarity cutoff of 2.5, the magnified dendrogram suggests that isolates 4 and 19 and isolates 17 and 15 are more closely related to each other than to the isolates in the other pair.
Hierarchical cluster analysis of the 66 vanB PCR-positive VRE isolates correctly identified by MALDI-TOF MS is shown in Fig. 7. Given that the month of October 2009 contributed the peak number of VRE isolates to the cohort analyzed, the 12 isolates from this month correctly identified as E. faecium (the incorrectly identified isolate was also cultured during this month) are also indicated. This dendrogram suggests, on the basis of the arbitrary similarity cutoff of 2.5, that there may have been four strains or related clusters that contributed cases during the study period. Close examination of the isolates cultured during the peak month of October 2009 shows that the majority (n = 9) cluster closely together at the bottom of the dendrogram, with a second cohort comprising three isolates appearing in the top cluster. This suggests that a second strain may have been introduced or contributed cases to the outbreak that occurred in October 2009.
DISCUSSION
These results indicate that MALDI-TOF MS can easily and reliably distinguish between E. faecium isolates that are vanB positive and, hence, vancomycin resistant and those that are not. It also shows that this technology is likely to be able to indicate the relatedness of strains of this organism to a degree similar to the existing gold standard for analyzing relatedness, PFGE.
Of the clinical isolates analyzed in this study, the majority were obtained from urine specimens, with the rate of bacteremia in colonized patients being 4.5%. This is comparable to the findings of other studies, including data from the local statewide public hospital laboratory information system (the Centre for Healthcare Related Infection Surveillance and Prevention) that demonstrated that the majority of the increase in VRE infections in Queensland was of the urinary tract (5). On the basis of annually adjusted data from the first 7 months of 2011, they found a higher bacteremia rate of 11%.
The significant increase in cases identified in our facility in October 2009 not only is a manifestation of the increased transmission due to the outbreak at that time but also is likely due to increased detection resulting from increased screening implemented as part of outbreak management. In this facility, routine screening does not occur in all clinical areas, and it is likely that the true number of cases at other times may have been slightly higher than that reported. During nonoutbreak times, routine screening occurred only in high-risk units and on patients transferred from other hospitals.
The low score of 1.818 obtained from the isolate incorrectly identified as Enterococcus gallinarum DSM 20717_DSM suggests that there may have been a problem with the extraction process or the preparation of the spot of the extract on the target plate. As the aim of this study was to establish the ability of MALDI-TOF MS to correctly identify VRE E. faecium, this was not repeated, as repeating it would have compromised the validity of the results. In routine application, however, a sample with a result such as this should be retested.
There is already a VRE in the proprietary database, Enterococcus faecium VRE (PX) 16086218_MLD. This was not closely matched to any of the isolates analyzed in this study, and it is not known whether this is a VanA or a VanB VRE strain (Bruker Daltonics GmbH, personal communication). Therefore, had we relied on the ability of the MALDI-TOF MS with the proprietary software to identify vancomycin resistance in the form of VRE, none of the isolates would have been correctly identified.
The support vector machines model was shown to be the most reliable for differentiating the VRE-positive isolates from the negative controls utilizing ClinProTools. This is in keeping with the method utilized by this model. Support vector machines is an algorithm for the determination of optimal separating planes between different data classes using formal approaches from optimization theory to separate the given data sets. The fact that different models chose to utilize essentially the same peaks adds strength to the argument that the peaks chosen are able to differentiate between the two cohorts analyzed.
The receiver operator curves, including the AUCs, indicate that each of peaks 2, 23, 28, and 45 would be satisfactory diagnostic tests for separating the vanB-positive isolates from the controls. It is important to point out that the diagnostic capability of MALDI-TOF MS is actually a combination of not only these peaks displayed but a profile that includes these in addition to many other peaks analyzed simultaneously.
Despite the internal validation showing that the sensitivity and specificity of the model for differentiating the isolates with or without the VRE phenotype were high (92.4% and 85.2%, respectively), the power of this calculation is reduced by nature of the way in which it is calculated. Internal validation results in an underrepresentation of the power of the model as a whole, due to the reduction in sample size required to generate a discovery set that leaves isolates to be allocated to the validation set. If the same isolates were consistently incorrectly classified in each of the three validations, then this would represent a flaw in the data (and indicate that those isolates were likely misidentified). This was not the case, however, as the VRE-positive isolates and the controls that were incorrectly classified were different in each of the three validations. This is therefore likely to be simply a reflection of the reduced power of the internal validation.
Once the ability of MALDI-TOF MS to distinguish the vancomycin-resistant vanB-positive E. faecium isolates from the PCR-negative controls was established from the study isolates, a prospective validation of the use of MALDI-TOF MS for this purpose in the routine clinical microbiology laboratory was successfully completed. The finding that in routine use, with the modifications to the database, MALDI-TOF MS has a sensitivity of 96.7% and a specificity of 98.1% has a number of significant implications. First, it enhances the relevance of our findings to the routine diagnostic laboratory, whereby a number of operators were able to utilize this technology to obtain results that were comparable to those obtained by the more labor-intensive, time-consuming, and expensive method currently utilized. This demonstrates that the use of MALDI-TOF MS is not restricted to just a few highly specialized operators, as is the case with most molecular methods. It also significantly increased the sample size to which our method was applied to enhance the validity of our findings. The results from the prospective validation show that there were only 3 false-positive spots of the 281 analyzed. The false-positive spots were from two patient specimens, with one of these having multiple other samples positive by both conventional means and MALDI-TOF MS, so this is more likely to be a false-negative result by conventional methods than a false-positive result by MALDI-TOF MS. In addition, there were only four false-negative results during the entire prospective validation period. It can be seen that although it was used for generating the reference spectra and the initial higher-level proteomic analysis, the more laborious extraction method is not required for the successful identification of vancomycin resistance in clinical specimens. This greatly enhances the relevance of our findings to the routine diagnostic laboratory, whereby the extraction method would negate the benefits of MALDI-TOF MS, as it adds significant time and cost to each isolate analyzed. These results show that for routine use the direct colony method produces results that are comparable to those produced by phenotypic methods, and it has successfully passed a prospective validation in comparison to currently laboratory practice that is beyond the level required for local regulatory bodies. Hence, it has been incorporated into routine laboratory practice as the method of choice.
Currently, growth on screening medium with chromosomal van gene-positive species of enterococci (such as in E. gallinarum) occurs reasonably frequently; this technology is shown here to be able to very easily distinguish these chromosomal van gene-positive species of enterococci from E. faecium isolates by correctly identifying the organisms to the species level over 99% of the time and far more rapidly and cost-effectively than the current phenotypic methods employed. There were 44 intrinsically vancomycin-resistant enterococcal species analyzed as part of the prospective validation, and not 1 tested falsely positive for VRE utilizing this methodology.
The implications of this study are significant. Without the ability to detect vanB-positive E. faecium isolates using molecular methods, our laboratory (and many others) currently relies on a lengthy and costly phenotypic method to identify likely VRE isolates. The requirement for repeated subculturing adds significant time to this process; a positive result can often be reported only on day 3 or 4 after receipt of the specimen. With the procedure described here, it is feasible that VRE isolates can be determined as soon as growth is evident on the screening medium, which often occurs less than 24 h after the specimen is received. In addition to the cost savings in reduced laboratory scientist time and the consumables required (including multiple agar plates, Etests, Vitek cards, etc.), the ability to more rapidly implement appropriate infection control precautions has significant implications for control of nosocomial infections. Although not addressed specifically by this study, it seems reasonable to hypothesize that a rapid diagnostic test for VRE has the potential to reduce spread to the staff, hospital environment, and other patients that the positive case would have come into contact with during the period that current procedures would have taken to confirm the presence of VRE. The converse is also likely, that saving on very costly infection control precautions by more rapidly declaring a patient negative could result in a significant saving. Rapid diagnosis thus has the potential to turn a disease that is currently an increasing burden into something that may be more controllable.
This work also shows the potential for MALDI-TOF MS to demonstrate the relatedness of isolates using hierarchical cluster analysis, a function that can be useful in an outbreak situation to ascertain whether cases are a result of transmission of local strains or possibly contributed to by new imported strains. Hierarchical cluster analysis utilizes the cluster algorithm to determine the relatedness between individual measurements, in this case, the MS spectrum, of each isolate. Spectra are merged in successive rounds of analysis until only one measurement remains. The merging patterns of spectra are then represented as a dendrogram or tree structure. Since spectra are merged by relatedness, the distance of branches on the tree or dendrogram relates directly to the similarity of spectra and, hence, the similarity of bacterial isolates.
It can be difficult to confirm or refute relatedness in an organism that is so polyclonal; however, this work suggests that MALDI-TOF MS performs comparably to the current gold standard, PFGE.
The four isolates shown to be identical by PFGE were also shown to be closely related by MALDI-TOF MS, particularly when analyzed in isolation (Fig. 6).
Figures 6 and 7 show that, despite appearing to be identical by PFGE, isolates 15 and 17 and isolates 4 and 19 appear to be more closely related to each other than the other pair. This could be a manifestation of the resolution of MALDI-TOF MS exceeding that of PFGE in terms of differentiating isogenic strains, showing subtle differences that are not evident on PFGE, which has previously been shown to be the case (14). There were no processing or technical reasons that could otherwise explain this relationship. In order to produce this dendrogram (Fig. 6), these four isolates were analyzed on the same target on the same run. If relevant to the outcome of this study, this apparent relationship between isolates 4 and 19 and isolates 17 and 15 could possibly be more firmly established utilizing a leave-one-out method, whereby each one of the four isolates is excluded and the hierarchical cluster analysis is performed repeatedly, ensuring that the same relationship is apparent in a reproducible fashion. Given that this has no bearing on the outcome of the study, this was not performed.
Hierarchical cluster analysis of all of the VanB-positive isolates (Fig. 7) suggested that there may have been four strains or related clusters that contributed isolates during the study period. It is not surprising that there appear to be multiple strains or clusters, as VRE in Australia have generally been shown to be highly polyclonal and appear in diverse locations independently (3). This dendrogram also suggests that a second cluster may have contributed cases to the outbreak that occurred in October 2009. This finding is clinically relevant, because if the analysis had been performed in real time, it could have significantly altered the infection control response. The four isolates shown to be identical by PFGE are actually spread across two clusters, where the division actually exceeds three on this dendrogram. It is likely that this is simply a reflection of the hierarchical cluster analysis of such a uniform population. The relationship between isolates 4 and 19 and isolates 15 and 17 still holds true, as both of these pairs are clustered together well within the similarity cutoff of 2.5. Thus, although PFGE was not performed on all isolates, it may be that the differences between seemingly unrelated isolates by MALDI-TOF MS in this population may still be similar by PFGE.
A larger sample size of isolates subjected to an alternative typing method would allow more inferences to be made about the ability of MALDI-TOF MS to determine relatedness compared to the ability of other methods. The limitations of the alternative methods, such as PFGE, including cost, requirement for specialized staff, long turnaround times, and, as such, limited availability, meant that this was not possible for this study.
Ideally, when comparing the relationship between isolates using hierarchical cluster analysis, all isolates would be analyzed at the same time following identical processing, including identical incubation times on the same medium and simultaneous extraction. Given the date range and number of isolates, this was not feasible. This does not seem to have affected the data presented, however, as the isolates have not grouped according to when they were analyzed.
One limitation of this study is the separation in time and location of the molecular gold standard assay and our proteomics-based assay. An issue with comparison of proteomic methods to genomic methods is that the presence of the genotype does not always correlate linearly with the phenotype. Ideally, the same isolate would have been subjected to both MALDI-TOF MS and PCR analysis without any subculture and from the same medium under the same conditions; however, this was not possible. It is possible that during transport or subculture the expression of the genes of interest could be up- or downregulated or even lost. It appears from these results that this has not significantly affected the outcome.
The relatively small sample size of the PCR-negative control cohort is another limitation of our study. Unfortunately, given that this negative-control group was comprised of isolates phenotypically suspected of being VanB positive yet being VanB negative by PCR by the reference laboratory, which is a relatively rare event, the number of isolates available was very limited. To address this, other VRE-negative organisms were also analyzed (and analyzed repeatedly, a total of 11 times each), and the PCR-negative isolates were analyzed in duplicate. Although it is not statistically valid to combine the results of the initial phase of the investigation with the prospective validation results, when looking at the results of the two phases of the study together, the number of true positives (n = 186; 67 PCR-positive isolates and 119 phenotypically confirmed isolates) and true negatives (n = 204; 16 from the PCR-negative but phenotypically suspected group, 33 from the other negative controls utilized in the initial phase of the study, and 155 phenotypically confirmed to be VRE negative in the prospective validation) shows that sufficient analysis has been performed to be confident of the findings of this study.
Further characterization of the proteins accounting for the peaks used to identify VRE in this study, using more sophisticated proteomic techniques, is possible. Given that it does not contribute to the ability of MALDI-TOF MS to identify VRE, which was the primary aim of this study, this is not presented here.
This work adds to the mounting number of applications of MALDI-TOF MS in a way that has enormous implications for the diagnosis and, therefore, control of the significant nosocomial multiresistant organism VRE. The results of this study show that as well as being much more rapid (minutes as opposed to days) and far cheaper (approximately AU$0.12 as opposed to at least AU$25 in consumable cost per isolate), MALDI-TOF MS is a highly sensitive and specific method for identifying resistance to vancomycin in enterococci compared to conventional phenotypic methods. Collaboration with proteomics scientists allowed extension of this technology beyond the capabilities of the routine diagnostic laboratory to perform a sophisticated analysis that has not previously been described in a form such as this. Subsequently, the knowledge gained was able to be translated back into the diagnostic lab, where it has had a tremendous impact. The robustness of the identification of vancomycin resistance in enterococci described in this study was due in part to our access to proteomics expertise and informatics tools. It is our hope that now that we have demonstrated the validity of this approach, other laboratories can follow to create their own unique resistance signatures, possibly even without the need for specialized assistance. Even though this study focused on the particular VRE problematic in our country, the principles presented herein are able to be replicated to readily address any VRE (E. faecium or E. faecalis and VanA or VanB) and could potentially even be extrapolated for use for the rapid identification of other multiresistant organisms with similar resistance mechanisms.
ACKNOWLEDGMENTS
We thank all of the microbiology staff from Mater Pathology (Mater Health Services, South Brisbane) for their assistance during this project, Bruker Daltonics GmbH for the provision of the Bruker Microflex LT benchtop instrument and its ongoing support, Mater Pathology for the provision of all reagents and consumables, Pathology Queensland for providing the phenotypically suspected yet PCR-negative E. faecium controls and for assisting with the PFGE results, Jan Bell (National Antimicrobial Resistance Surveillance Program, Department of Microbiology and Infectious Diseases, Women's and Children's Hospital, Adelaide, South Australia) for providing the vanA-positive controls, and James McCarthy for critical appraisal of the manuscript.
We have no conflicts of interest to declare.
Footnotes
Published ahead of print 27 June 2012
REFERENCES
- 1. Alatoom AA, Cunningham SA, Ihde SM, Mandrekar J, Patel R. 2011. Comparison of direct colony method versus extraction method for identification of gram-positive cocci by use of Bruker Biotyper matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 49:2868–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barbuddhe SB, et al. 2008. Rapid identification and typing of Listeria species by matrix-assisted laser desorption ionization–time of flight mass spectrometry. Appl. Environ. Microbiol. 74:5402–5407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bell J, Turnidge J, Coombs G, O'Brien F. 1998. Emergence and epidemiology of vancomycin-resistant enterococci in Australia. Commun. Dis. Intell. 22:249–252 [DOI] [PubMed] [Google Scholar]
- 4. Bell JM, Paton JC, Turnidge J. 1998. Emergence of vancomycin-resistant enterococci in Australia: phenotypic and genotypic characteristics of isolates. J. Clin. Microbiol. 36:2187–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centre for Healthcare Related Infection Surveillance and Prevention 2011. Vancomycin resistant enterococci (VRE) discussion paper. Centre for Healthcare Related Infection Surveillance and Prevention, Queensland Health, Brisbane, Australia [Google Scholar]
- 6. Christiansen K, et al. 2007. Antimicrobial susceptibility report of enterococcus isolates. Australian Group on Antimicrobial Resistance (AGAR) 2007 surveillance report. Australian Group on Antimicrobial Resistance, Victoria, Australia [Google Scholar]
- 7. Christiansen KJ, Turnidge JD, Bell JM, George NM, Pearson JC. 2007. Prevalence of antimicrobial resistance in Enterococcus isolates in Australia, 2005: report from the Australian Group on Antimicrobial Resistance. Commun. Dis. Intell. 31:392–397 [DOI] [PubMed] [Google Scholar]
- 8. Clinical and Laboratory Standards Institute 2011. Performance standards for antimicrobial susceptibility testing; 21st informational supplement. M45-A2. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 9. Du Z, Yang R, Guo Z, Song Y, Wang J. 2002. Identification of Staphylococcus aureus and determination of its methicillin resistance by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Anal. Chem. 74:5487–5491 [DOI] [PubMed] [Google Scholar]
- 10. Evers S, Courvalin P. 1996. Regulation of VanB-type vancomycin resistance gene expression by the VanS(B)-VanR(B) two-component regulatory system in Enterococcus faecalis V583. J. Bacteriol. 178:1302–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Faoagali J, Bodman J, Geary A. 1996. Isolation of vancomycin-resistant enterococci in Queensland, case 2. Commun. Dis. Intell. 1996:402–403 [Google Scholar]
- 12. Kamarulzaman A, Tosolini FS, Boquest AL, Geddes JE, Richards MJ. 1995. Vancomycin-resistant Enterococcus faecium in a liver transplant recipient. Aust. N. Z. J. Med. 25:560 [Google Scholar]
- 13. Ketterlinus R, Hsieh SY, Teng SH, Lee H, Pusch W. 2005. Fishing for biomarkers: analyzing mass spectrometry data with the new ClinProTools software. Biotechniques 38(Suppl):S37–S40 [DOI] [PubMed] [Google Scholar]
- 14. Majcherczyk PA, McKenna T, Moreillon P, Vaudaux P. 2006. The discriminatory power of MALDI-TOF mass spectrometry to differentiate between isogenic teicoplanin-susceptible and teicoplanin-resistant strains of methicillin-resistant Staphylococcus aureus. FEMS Microbiol. Lett. 255:233–239 [DOI] [PubMed] [Google Scholar]
- 15. Malakhova MV, et al. 2007. MALDI-ToF mass-spectrometry in analysis of genetically determined resistance of Streptococcus pneumoniae to fluoroquinolone. Antibiot. Khimioter. 52:10–17 (In Russian.) [PubMed] [Google Scholar]
- 16. Paterson D, Jennings A, Allen A, Sherlock K, Whitby M. 1996. Isolation of vancomycin-resistant enterococci in Queensland, case 1. Commun. Dis. Intell. 1996:400–401 [Google Scholar]
- 17. Reynolds PE, Depardieu F, Dutka-Malen S, Arthur M, Courvalin P. 1994. Glycopeptide resistance mediated by enterococcal transposon Tn1546 requires production of VanX for hydrolysis of d-alanyl–d-alanine. Mol. Microbiol. 13:1065–1070 [DOI] [PubMed] [Google Scholar]
- 18. Schlebusch S, et al. 2010. First outbreak of PVL-positive nonmultiresistant MRSA in a neonatal ICU in Australia: comparison of MALDI-TOF and SNP-plus-binary gene typing. Eur. J. Clin. Microbiol. Infect. Dis. 29:1311–1314 [DOI] [PubMed] [Google Scholar]
- 19. Seng P, et al. 2009. Ongoing revolution in bacteriology: routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin. Infect. Dis. 49:543–551 [DOI] [PubMed] [Google Scholar]
- 20. Siegrist TJ, et al. 2007. Discrimination and characterization of environmental strains of Escherichia coli by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS). J. Microbiol. Methods 68:554–562 [DOI] [PubMed] [Google Scholar]
- 21. Smole SC, King LA, Leopold PE, Arbeit RD. 2002. Sample preparation of Gram-positive bacteria for identification by matrix assisted laser desorption/ionization time-of-flight. J. Microbiol. Methods 48:107–115 [DOI] [PubMed] [Google Scholar]
- 22. Willey BM, et al. 1999. Practical approach to the identification of clinically relevant Enterococcus species. Diagn. Microbiol. Infect. Dis. 34:165–171 [DOI] [PubMed] [Google Scholar]
- 23. Williamson YM, et al. 2008. Differentiation of Streptococcus pneumoniae conjunctivitis outbreak isolates by matrix-assisted laser desorption ionization-time of flight mass spectrometry. Appl. Environ. Microbiol. 74:5891–5897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wolters M, et al. 2011. MALDI-TOF MS fingerprinting allows for discrimination of major methicillin-resistant Staphylococcus aureus lineages. Int. J. Med. Microbiol. 301:64–68 [DOI] [PubMed] [Google Scholar]