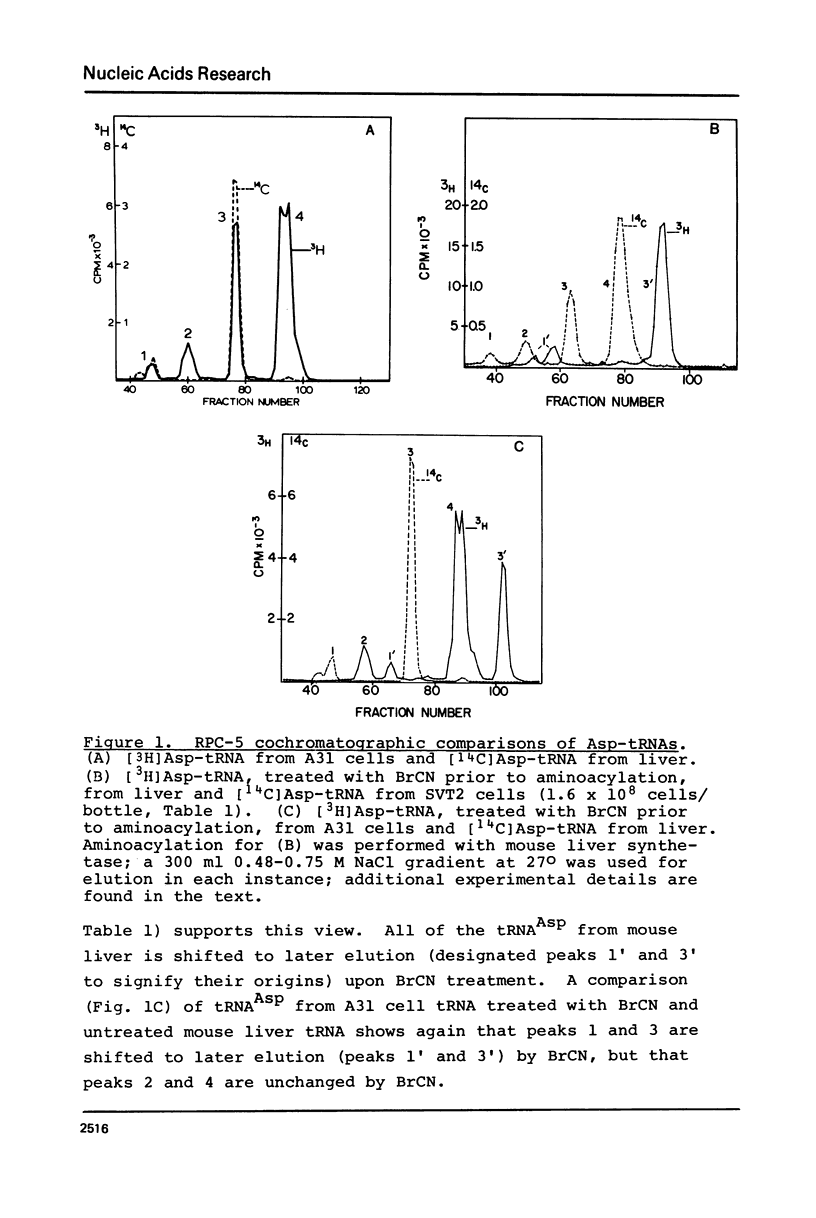
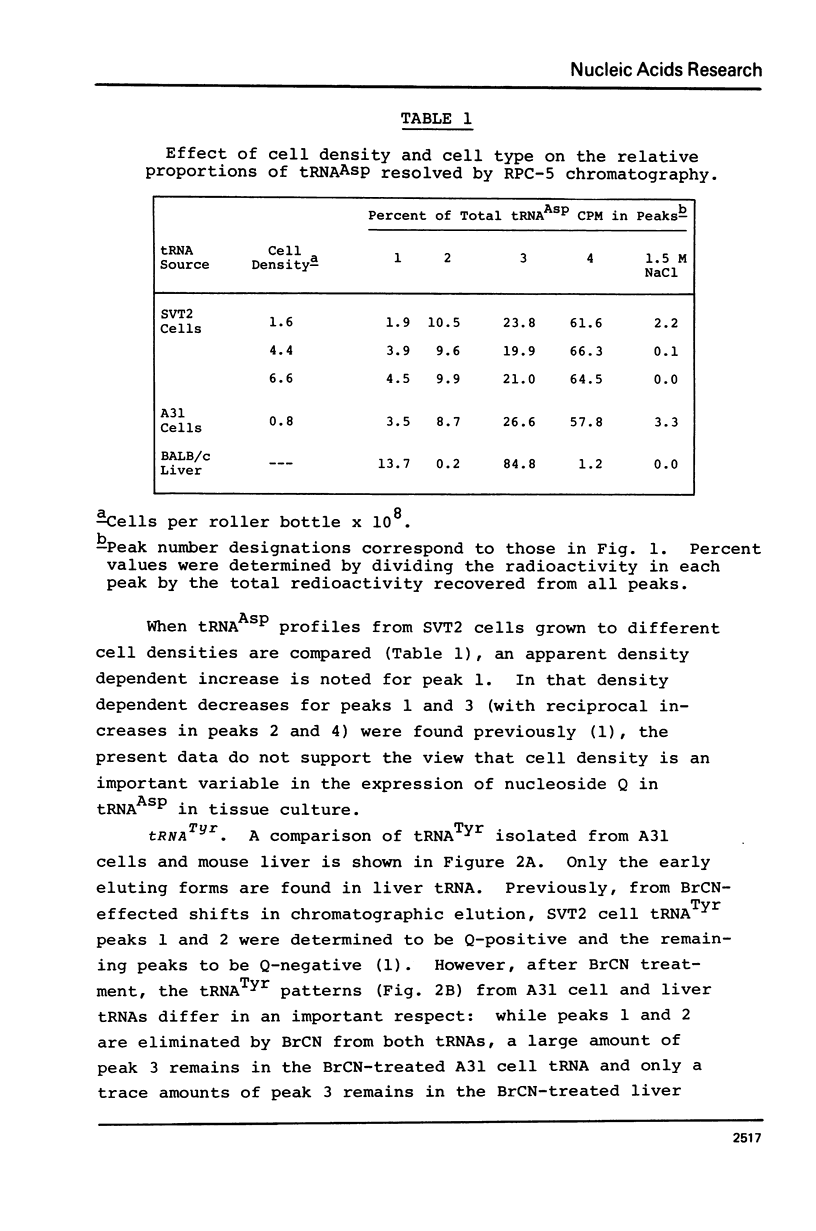
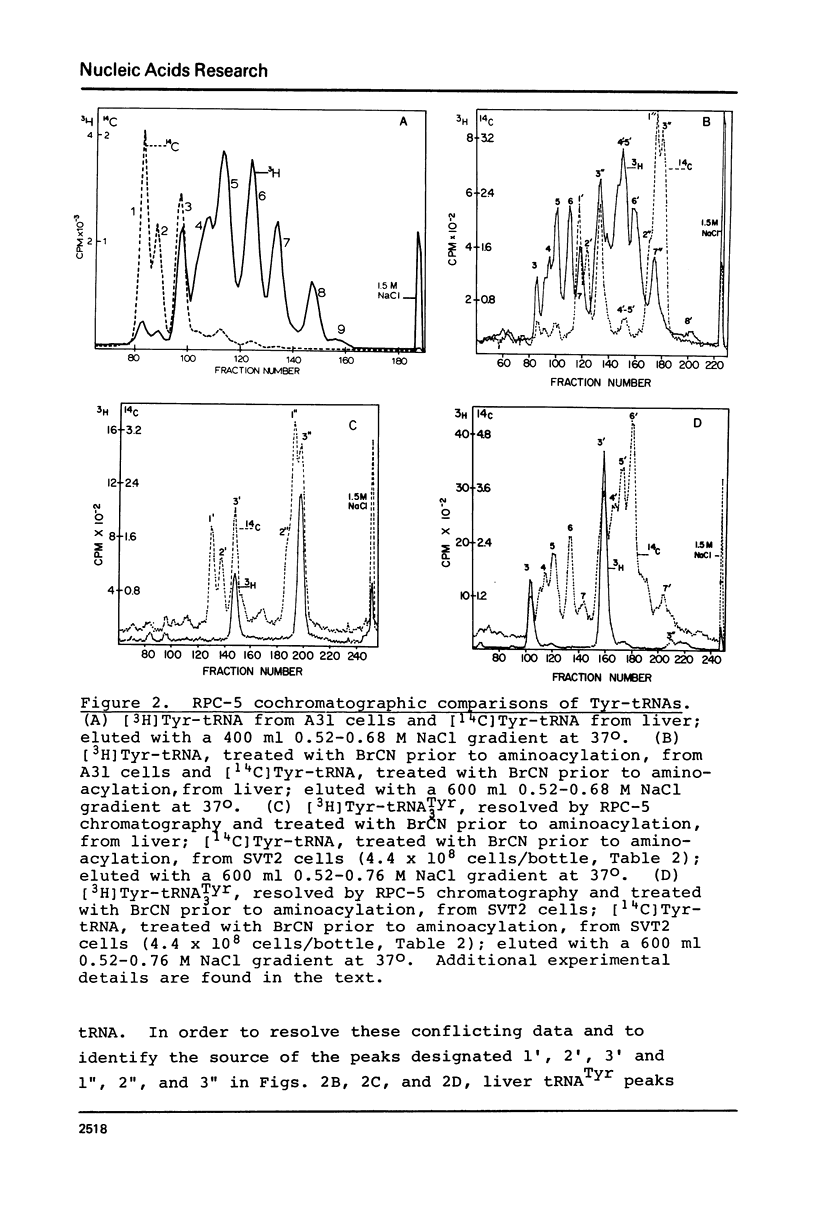
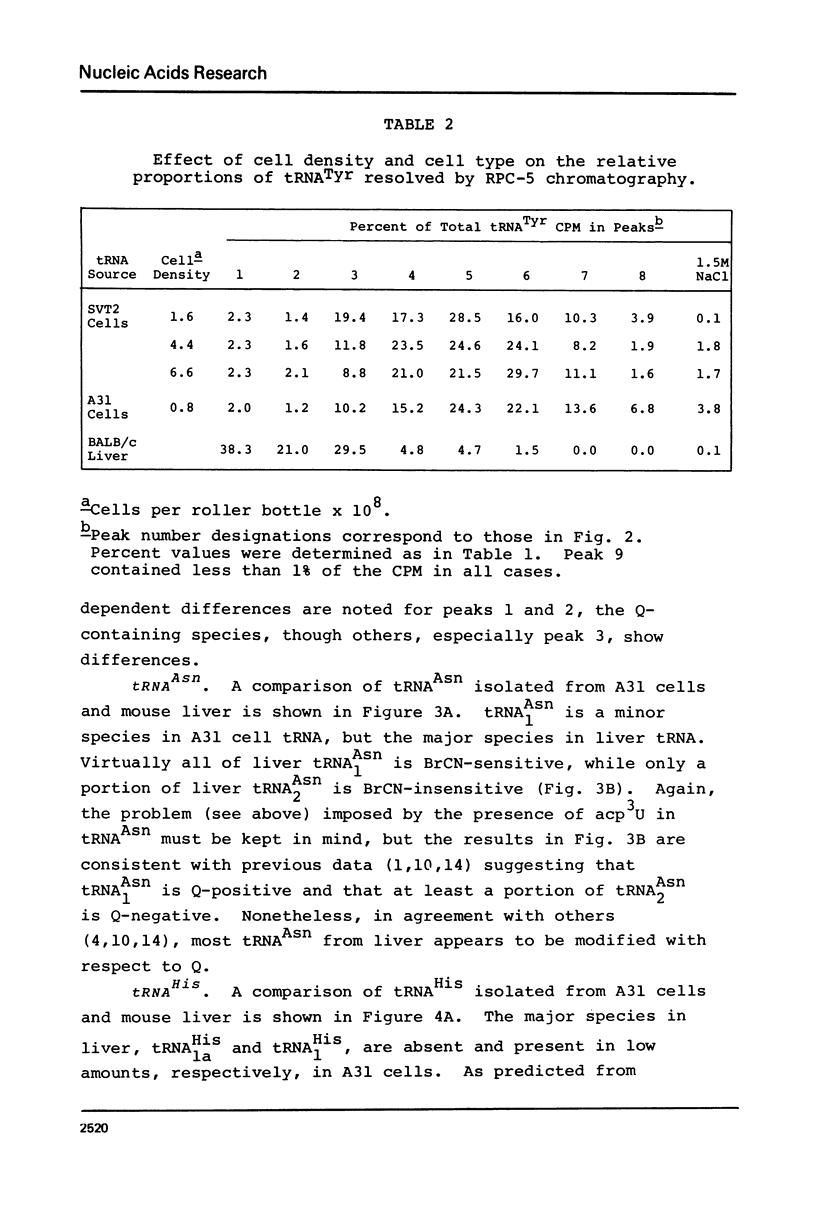
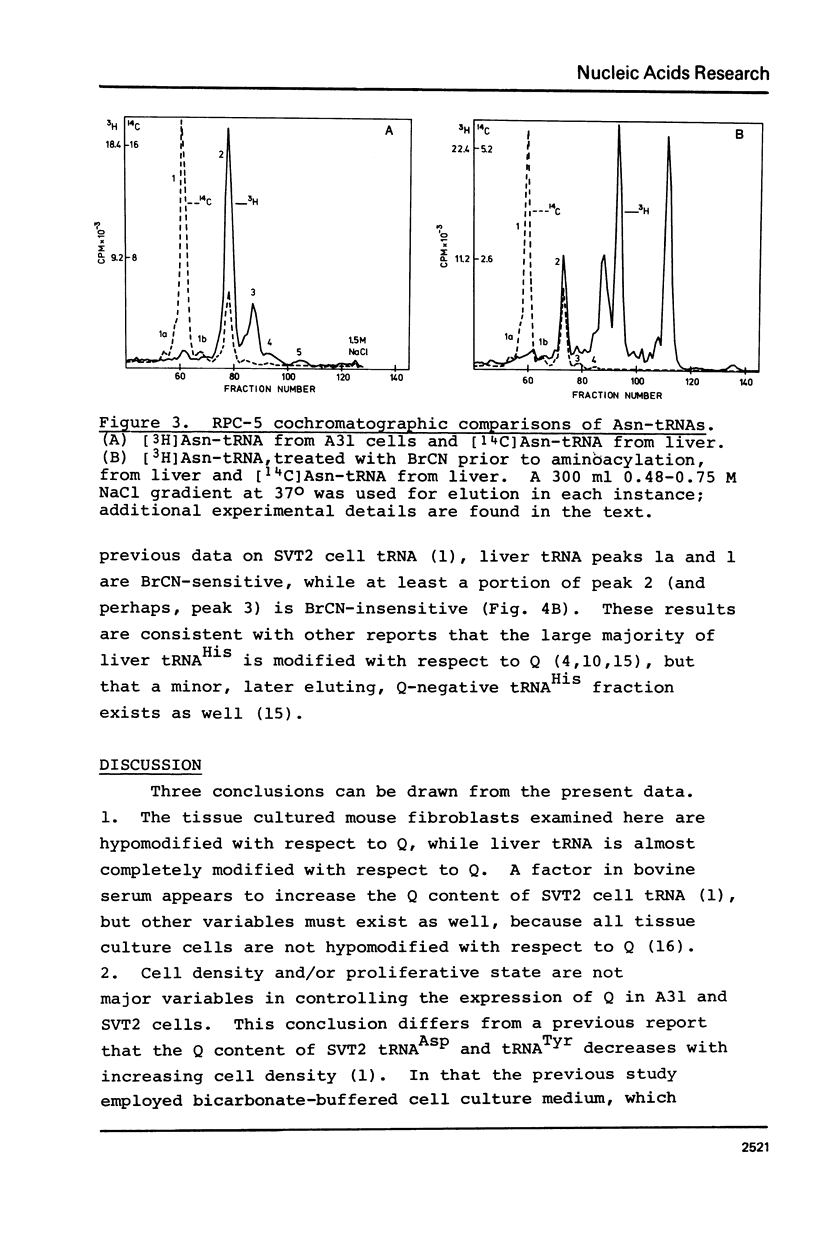
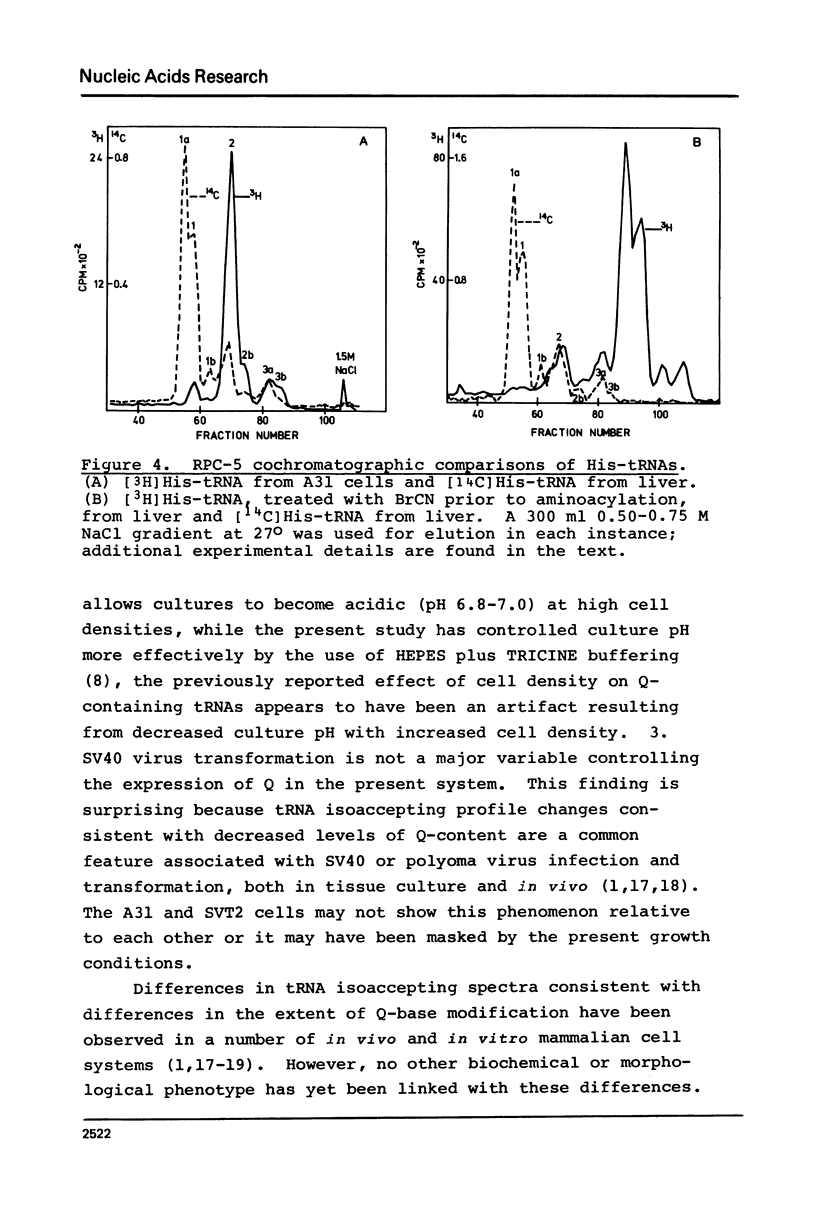
Abstract
An examination, using reversed-phase chromatography and cyanogen bromide treatment, of tRNATyr, tRNAHis, tRNAAsn, and tRNAAsp from SV40-transformed mouse fibroblasts grown to different cell densities, untransformed cells grown to confluence, and mouse liver indicates that: (1) The tissue cultured mouse fibroblasts examined here are hypomodified with respect to nucleoside Q, while liver tRNA is almost completely modified with respect to Q. (2) Cell density and/or proliferative state do not present as major variables in controlling the expression of Q in the present system. (3) SV40 virus transformation is not a major variable controlling the expression of Q in the present system. The present results support previous use of cyanogen bromide effected shifts in chromatographic elution as an assay for nucleoside Q.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J. Development of 3T3-like lines from Balb-c mouse embryo cultures: transformation susceptibility to SV40. J Cell Physiol. 1968 Oct;72(2):141–148. doi: 10.1002/jcp.1040720208. [DOI] [PubMed] [Google Scholar]
- Brambilla R., Rogg H., Staehelin M. Unexpected occurrence of an aminoacylated nucleoside in mammalian tRNATyr. Nature. 1976 Sep 9;263(5573):167–169. doi: 10.1038/263167a0. [DOI] [PubMed] [Google Scholar]
- Briscoe W. T., Griffin A. C., McBride C., Bowen J. M. The distribution and properties of aspartyl transfer RNA in human and animal tumors. Cancer Res. 1975 Sep;35(9):2586–2593. [PubMed] [Google Scholar]
- Briscoe W. T., Syrewicz J. J., Marshall M. V., Griffin A. C. Regulation of an aspartyl-tRNA species in BHK cells in culture and in solid tumor form. Biochim Biophys Acta. 1975 Apr 2;383(4):441–445. doi: 10.1016/0005-2787(75)90314-7. [DOI] [PubMed] [Google Scholar]
- Ceccarini C., Eagle H. pH as a determinant of cellular growth and contact inhibition. Proc Natl Acad Sci U S A. 1971 Jan;68(1):229–233. doi: 10.1073/pnas.68.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrul E. F., Farkas W. R. Partial purification and properties of the reticulocyte guanylating enzyme. Biochim Biophys Acta. 1976 Sep 6;442(3):379–390. doi: 10.1016/0005-2787(76)90312-9. [DOI] [PubMed] [Google Scholar]
- Farkas W. R., Chernoff D. Identification of the minor guanylated tRNA of rabbit reticulocytes. Nucleic Acids Res. 1976 Oct;3(10):2521–2528. doi: 10.1093/nar/3.10.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S., Li H. J., Nakanishi K., Van Lear G. 3-(3-amino-3-carboxy-n-propyl)uridine. The structure of the nucleoside in Escherichia coli transfer ribonucleic acid that reacts with phenoxyacetoxysuccinimide. Biochemistry. 1974 Jul 2;13(14):2932–2937. doi: 10.1021/bi00711a024. [DOI] [PubMed] [Google Scholar]
- Gallagher R. E., Ting R. C., Gallo R. C. A common change of aspartyl-tRNA in polyoma- and SV40 -transformed cells. Biochim Biophys Acta. 1972 Jul 31;272(4):568–582. doi: 10.1016/0005-2787(72)90512-6. [DOI] [PubMed] [Google Scholar]
- Kasai H., Nakanishi K., Macfarlane R. D., Torgerson D. F., Ohashi Z., McCloskey J. A., Gross H. J., Nishimura S. Letter: The structure of Q* nucleoside isolated from rabbit liver transfer ribonucleic acid. J Am Chem Soc. 1976 Aug 4;98(16):5044–5046. doi: 10.1021/ja00432a071. [DOI] [PubMed] [Google Scholar]
- Katze J. R. Alterations in SVT2 cell transfer RNAs in response to cell density and serum type. Biochim Biophys Acta. 1975 Mar 10;383(2):131–139. doi: 10.1016/0005-2787(75)90254-3. [DOI] [PubMed] [Google Scholar]
- Katze J. R., Mason K. H. Comparison of the acceptance activity of the ribosome-bound and the total cellular transfer ribonucleic acids from SV40-transformed mouse fibroblasts. Biochim Biophys Acta. 1973 Dec 21;331(3):369–381. doi: 10.1016/0005-2787(73)90023-3. [DOI] [PubMed] [Google Scholar]
- Katze J. R. Relation of cell type and cell density to the degree of post-transcriptional modification of tRNALys and tRNAPhe. Biochim Biophys Acta. 1975 Nov 4;407(4):392–398. doi: 10.1016/0005-2787(75)90291-9. [DOI] [PubMed] [Google Scholar]
- Okada N., Shindo-Okada N., Nishimura S. Isolation of mammalian tRNAAsp and tRNATyr by lectin-Sepharose affinity column chromatography. Nucleic Acids Res. 1977 Feb;4(2):415–423. doi: 10.1093/nar/4.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter K. R., Todaro G. J., Fonte V. A scanning electron microscope study of surface features of viral and spontaneous transformants of mouse Balb-3T3 cells. J Cell Biol. 1973 Dec;59(3):633–642. doi: 10.1083/jcb.59.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe B. A., Stankiewicz A. F., Chen C. Y. Chromatographic behavior of several mammalian tRNAs on acylated dihydroxyl-borate cellulose and Aminex A-28. Nucleic Acids Res. 1977 Jul;4(7):2191–2204. doi: 10.1093/nar/4.7.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. N. Chromatographic changes in specific tRNAs after reaction with cyanogen bromide and sodium periodate. Biochim Biophys Acta. 1974 Jul 11;353(3):283–291. doi: 10.1016/0005-2787(74)90021-5. [DOI] [PubMed] [Google Scholar]



