Abstract
We assessed the effect of increasing manganese concentrations in test media (0.001 to 1,024 mg/liter) on MICs of tigecycline. For both broth microdilution (BMD) and Etests, this effect was negligible for physiological concentrations, but MICs increased when concentrations exceeded 8 mg/liter. Susceptibility testing should be performed on media with standardized low manganese content.
TEXT
Tigecycline is one of the few antimicrobial drugs that can be used to treat infections with highly resistant organisms (7, 8). Several studies reported that in vitro bacterial susceptibility to tigecycline varies by test medium and condition (3, 4, 10, 13, 14), and a recent study showed that addition of manganese (Mn) to the test medium led to increased MICs of tigecycline, as determined by an Etest for Enterobacteriaceae and staphylococci. (5) Variability in Mn concentrations in standard media may thus result in falsely elevated MICs for tigecycline. A concentration-effect relationship was not established, however, and the threshold concentration of Mn below which MICs are unaffected remains unknown. Because such a threshold may depend on the test method used, we evaluated the effect of increasing manganese concentrations in the test medium on MICs, as determined by broth microdilution, Etest, and disk diffusion.
The study included strains of five species of Enterobacteriaceae (Escherichia coli, Klebsiella pneumoniae, Citrobacter freundii, Enterobacter cloacae, and Proteus mirabilis) and Acinetobacter baumannii, which were collected and stored at −80 degrees in a prospective cohort study in 18 Dutch hospitals as described previously (15). An ATCC control strain (Table 1) was included for each species.
Table 1.
MICs for the ATCC control strains with each medium manganese concentrationa
| Strain | MIC (μg/ml) with each added concn of Mn (mg/liter) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1,024 | |
| Escherichia coli ATCC 29522 | 0.09 | 0.19 | 0.19 | 0.25 | 0.25 | 0.38 | 0.5 | 0.5 | 0.5 | 1 | 4 |
| Klebsiella pneumonia ATCC 700603 | 3 | 4 | 4 | 4 | 6 | 6 | 6 | 8 | 8 | 16 | 48 |
| Enterobacter cloacae ATCC 23355 | 0.75 | 0.75 | 0.75 | 1 | 1 | 1 | 1.5 | 1.5 | 2 | 4 | 12 |
| Citrobacter freundii ATCC 8090 | 0.38 | 0.38 | 0.38 | 0.38 | 0.38 | 0.38 | 0.5 | 0.75 | 1 | 2 | 4 |
| Acinetobacter baumannii ATCC 19606 | 1 | 0.75 | 0.75 | 1 | 1 | 1.5 | 3 | 4 | 4 | 8 | 8 |
| Proteus mirabilis ATCC 43071 | 2 | 1.5 | 2 | 2 | 2 | 2 | 3 | 2 | 3 | 6 | 16 |
MICs were tested on Iso-Sensitest agar with a standard manganese concentration of 2 mg/liter. Data are from Etests only.
In the first experiment, we determined the effect of increasing medium Mn concentrations on MICs, as measured by Etest (bioMérieux), and on zone diameters, as measured by disk (15 μg tigecycline; Oxoid, United Kingdom) diffusion. Strains were thawed and inoculated on Columbia agar plates with 5% sheep blood and incubated for 18 to 24 h at 35°C. Subsequently, a suspension of a 0.5 McFarland standard (in 0.9% saline) was used to inoculate freshly prepared Iso-Sensitest agar plates (Oxoid, United Kingdom). The Iso-Sensitest medium has been developed specifically to overcome the variability in mineral concentrations observed in Mueller-Hinton medium and has a stable mineral content with an MnCl concentration of 2 mg/liter (2). Media were freshly prepared to avoid effects of oxygenation (1, 9) and supplemented with MnCl2 (VWR, The Netherlands) in 2-fold serial dilutions to achieve Mn concentrations ranging between 2 mg/liter and 1,024 mg/liter. Plates were incubated for 18 to 24 h at 35°C before values were read.
Because reference values for Mn concentrations in human serum (0.2 to 1.4 μg/liter) (12) are substantially lower than concentrations found in standard susceptibility test media (≥2 mg/liter), a second experiment in which the tested Mn concentration range also included physiological concentrations was performed. MICs were measured using broth microdilution (BMD), and MnCl2 was added to a manganese-free medium (synthetic amino acid medium [SAAM-B]; U.S. Biological, MA; catalog number S9270-02) in a series of 21 2-fold dilutions to achieve concentrations in the test wells ranging between 1,024 and 0.001 mg/liter. Tests were performed according to guidelines from the International Organization for Standardization (ISO; guideline 20776) (6). Tigecycline was provided by Pfizer. MICs were read visually as the lowest concentrations that completely inhibited growth.
A simple linear regression model (SPSS v15.0 for Windows; SPSS, Chicago, IL) was used to model the relationship between manganese concentrations in the test medium and MIC values. MIC values were log2 transformed so that a 1-unit increment in log values corresponded to a doubling of the MIC.
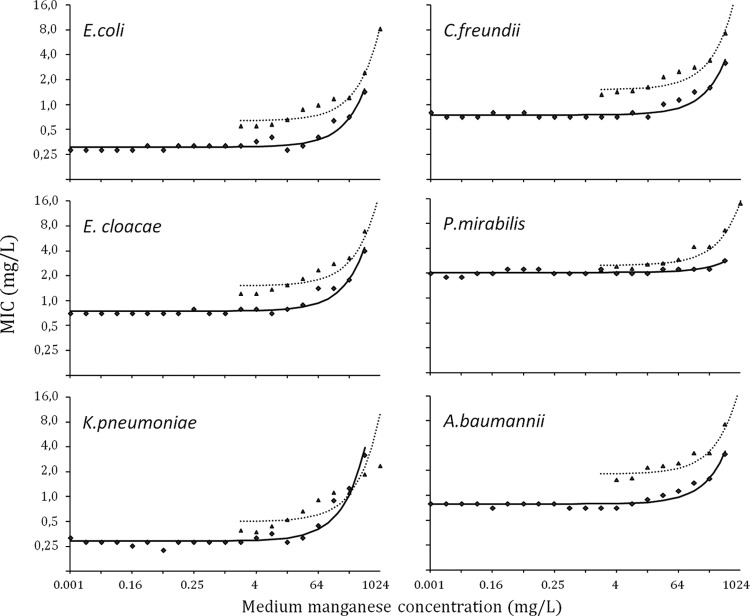
Figure 1 shows the MICs for tigecycline for each species, determined by broth microdilution (solid line) or Etest (dotted line), as a function of the concentration of manganese in the medium. For all species tested, the effect of manganese on geometric mean MICs was negligible for medium concentrations of less than 8 mg/liter. MIC values increased substantially, however, when medium manganese concentrations exceeded 64 mg/liter. The threshold whereby this increase occurred did not differ substantially between species except for with P. mirabilis, for which the effect seemed less pronounced. Compared to values measured on a Mn-free medium, MICs doubled at medium Mn concentrations of 220, 240, 200, 230, and 136 mg/liter for E. coli, K. pneumoniae, C. freundii, E. cloacae, and A. baumannii, respectively.
Fig 1.
Effect of increasing medium manganese concentrations on MIC (geometric mean) by bacterial species. Medium manganese concentrations are presented on a log-transformed x axis. The regression line models the relationship between medium manganese concentrations and MICs for broth microdilution (solid line) and Etest (dotted line).
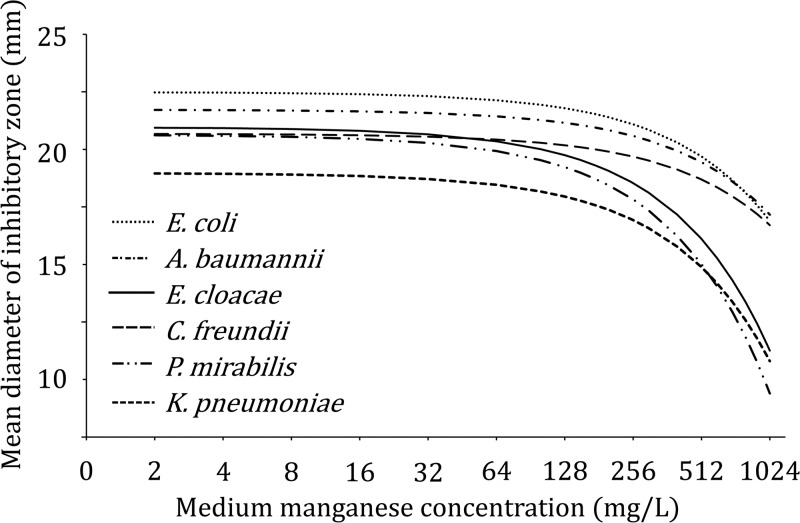
The effect of medium manganese concentrations on MIC values did not differ between BMD and Etests (P = 0.20). Independent of medium manganese concentrations, MIC values determined by Etest were consistently higher than those determined by broth microdilution (geometric mean difference, 0.8 doubling dilutions; 95% confidence interval [CI], 0.7 to 0.9). Similar patterns were observed for the influence of medium manganese concentrations on inhibitory zone diameters (Fig. 2).
Fig 2.
Effect of increasing medium manganese concentrations on average zone diameters, measured by disk diffusion.
These data show that in vitro bacterial susceptibility to tigecycline is influenced by manganese concentrations in the test medium, whereby the effect of variations in manganese concentrations appears negligible when concentrations in the test medium are below 8 mg/liter. This suggests that the activity of tigecycline will be unaffected in human serum, which has a considerably lower concentration of manganese. Our findings emphasize the need to use test media with standardized low-Mn concentrations for tigecycline susceptibility testing. Due to poor reproducibility of its mineral content, the Mueller-Hinton medium may be unsuitable for this purpose. Whereas most commonly available test media probably have concentrations below the critical range, considerable variability in the concentration of divalent cations in Mueller-Hinton broth or agar from different manufacturers has been reported (11), and manganese concentrations in Mueller-Hinton agar that are well above 500 mg/liter have been found (5).
The mechanisms by which manganese influences in vitro activity of tigecycline require further investigation. These may involve the induction of bacterial resistance by manganese or the inactivation of tigecycline due to the formation of complexes between manganese and tigecycline (5). Other divalent cations may have similar effects on susceptibility test results, and because we did not use the same medium for the Etests and for the BMD, it is possible that differences in the concentrations of minerals other than manganese may partly explain the observed differences in MICs between these 2 methods. Further studies are needed to identify causal factors involved. Meanwhile, results of tigecycline susceptibility testing by Etest should be interpreted with caution.
Lastly, because Mn concentrations in human serum are substantially lower than those found in standard susceptibility test media, Mn is unlikely to interfere with tigecycline antibacterial activity under physiological conditions.
ACKNOWLEDGMENTS
We thank A. Antonissen and L. Mook (Laboratory for Microbiology and Infection Control, Amphia Hospital, Breda, The Netherlands) for their assistance in performing the experiments.
This study was sponsored by Pfizer Inc.
J. A. J. W. Kluytmans is a consultant for Pfizer and has received honorary payments for lectures from Pfizer.
Footnotes
Published ahead of print 20 June 2012
REFERENCES
- 1. Bradford PA, et al. 2005. Tigecycline MIC testing by broth dilution requires use of fresh medium or addition of the biocatalytic oxygen-reducing reagent oxyrase to standardize the test method. Antimicrob. Agents Chemother. 49:3903–3909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bridson EY. (ed). 2006. The Oxoid manual, 9th ed Oxoid Limited, Hampshire, United Kingdom [Google Scholar]
- 3. Casal M, et al. 2009. Influence of testing methodology on the tigecycline activity profile against presumably tigecycline-non-susceptible Acinetobacter spp. J. Antimicrob. Chemother. 64:69–72 [DOI] [PubMed] [Google Scholar]
- 4. Cohen Stuart J, et al. 2010. Evaluation of Etest to determine tigecycline MICs of Enterobacter species. Antimicrob. Agents Chemother. 54:2746–2747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fernandez-Mazarrasa C, et al. 2009. High concentrations of manganese in Mueller-Hinton agar increase MICs of tigecycline determined by Etest. J. Clin. Microbiol. 47:827–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. International Organization for Standardization 2006. Clinical laboratory testing and in vitro diagnostic test systems. Susceptibility testing of infectious agents and evaluation of performance of antimicrobial susceptibility test devices. Part 1. Reference method for testing the in vitro activity of antimicrobial agents against rapidly growing aerobic bacteria involved in infectious diseases. International standard 20776-1. International Organization for Standardization, Geneva, Switzerland [Google Scholar]
- 7. Kelesidis T, Karageorgopoulos DE, Kelesidis I, Falagas ME. 2008. Tigecycline for the treatment of multidrug-resistant Enterobacteriaceae: a systematic review of the evidence from microbiological and clinical studies. J. Antimicrob. Chemother. 62:895–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Livermore D. 2005. Tigecycline, what is it, and where should it be used? J. Antimicrob. Chemother. 56:611–614 [DOI] [PubMed] [Google Scholar]
- 9. Petersen PJ, Bradford PA. 2005. Effect of medium age and supplementation with the biocatalytic oxygen-reducing reagent oxyrase on in vitro activities of tigecycline against recent clinical isolates. Antimicrob. Agents Chemother. 49:3910–3918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pillar CM, Draghi DC, Dowzicky MJ, Sahm DF. 2008. In vitro activity of tigecycline against gram-positive and gram-negative pathogens as evaluated by broth microdilution and Etest. J. Clin. Microbiol. 46:2862–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reller BL, Schoenknecht FD, Kenny MA, Sherris DC. 1974. Antibiotic susceptibility testing of Pseudomonas aeruginosa: selection of a control strain and criteria for magnesium and calcium content in media. J. Infect. Dis. 130:454–463 [DOI] [PubMed] [Google Scholar]
- 12. Rukgaueri M, Klein J, Kruse Jarres JD. 1997. Reference values for the trace elements copper, manganese, selenium, and zinc in the serum/plasma of children, adolescents, and adults. J. Trace Elem. Med. Biol. 11:92–98 [DOI] [PubMed] [Google Scholar]
- 13. Torrico M, et al. 2010. Influence of media and testing methodology on susceptibility to tigecycline of Enterobacteriaceae with reported high tigecycline MIC. J. Clin. Microbiol. 48:2243–2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Verkade EJM, Verhulst CJMM, Huijsdens XW, Kluymans JAJW. 2010. In vitro activity of tigecycline against methicillin-resistant Staphylococcus aureus, including livestock-associated strains. Eur. J. Clin. Microbiol. Infect. Dis. 29:503–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Willemsen I, et al. 2011. Highly resistant microorganisms in a teaching hospital: the role of horizontal spread in a setting of endemicity. Infect. Control Hosp. Epidemiol. 32(4):333–341 [DOI] [PubMed] [Google Scholar]