Abstract
Noroviruses (NoVs) have emerged as the leading cause of acute viral gastroenteritis (GE) in humans. Although diagnostic facilities have greatly improved, significant underdiagnosis of NoV in hospitals may still occur, thereby increasing clinical burden and nosocomial spread. We evaluated the underdiagnosis of sporadic NoV infections in a tertiary care hospital and estimated its clinical impact. From December 2008 until July 2009, fecal samples specifically referred for bacterial but not viral examination were retrospectively tested for NoV by real-time PCR. The clinical and virological data from patients with undiagnosed NoV infection (missed patients) were evaluated and compared with those from patients with recognized NoV. During the study period, 45 patients with undiagnosed NoV were detected, whereas 50 patients were regularly diagnosed. The missed NoV cases were more frequently adults than children (80% versus 46%; P < 0.001). The viral load levels did not differ between the diagnosed and missed patients, but missed patients more frequently presented without diarrhea (20% versus 4%; P < 0.07). The newly admitted missed NoV cases with GE underwent more diagnostic imaging (24% versus 4%; P < 0.01) and tended to be hospitalized longer. When missed-NoV patients were included, the number of nosocomial clusters doubled and missed patients were index cases in 5 of the 6 clusters. These data indicate that NoV infections are frequently missed despite routine laboratory testing and demonstrate that underdiagnosis of NoV patients is associated with costly abdominal imaging and nosocomial clustering. Awareness of NoV infection in adult patients and education about the importance of viral GE should be increased.
INTRODUCTION
Noroviruses (NoVs) have emerged as the one of the most important pathogens causing acute gastroenteritis (GE) in children and adults (8, 12). Nursing homes and hospitals are widely confronted with NoV outbreaks. Additionally, isolated (sporadic) cases of NoV frequently occur, but their incidence and clinical impact in hospitals have not been studied systematically (2, 17). Sporadic cases of NoV may result both from community-acquired infections in newly admitted patients and from nosocomial transmissions between patients, personnel, or visitors (9). Although sensitive commercial and homemade diagnostic assays for NoV have become widely available, sporadic NoV infections in hospitalized patients remain underdiagnosed, increasing the clinical burden and potential for nosocomial spread (1, 3, 21). Underrecognition of NoV may result in the individual patient undergoing more diagnostic procedures and may increase the influx of infectious patients into hospital wards, where they may trigger outbreaks (5, 14). Underdiagnosis of NoV may result from a referral bias, as well as from suboptimal laboratory facilities and inadequate specimen collection. This bias may occur when physicians selectively refer GE patients for bacteriological or parasitological testing but not virological testing. In the present study, we prospectively evaluated the underrecognition of NoV patients in a tertiary referral center with separate testing for viral and bacterial pathogens. For this purpose, the aliquots of fecal samples referred for bacteriologic testing were stored and retrospectively examined for NoV during a 6.5-month period, which included the NoV seasonal peak. The characteristics of missed NoV patients and the clinical impact on diagnosis, duration of hospitalization, and infection prevention were evaluated.
MATERIALS AND METHODS
Patients.
The Erasmus Medical Center (EMC) and its affiliated hospitals comprise a 1,100-bed university hospital, a 269-bed children's hospital, a 137-bed oncology center, and a 160-bed nonacademic general hospital. From 16 December 2008 until 1 July 2009, patients from the EMC and affiliated hospitals were included in the study. Physicians can refer patients with gastroenteritis (GE) for either bacteriological, virological, or parasitological testing or any combination of these options. During the study period, patient samples referred for virological testing were routinely tested for NoV, whereas samples referred for bacteriological testing were aliquoted and stored at −80°C for NoV testing at a future time. For each patient, an authorized member of the medical staff accessed the following information: age, sex, date of hospitalization, results of bacteriological stool cultures, and clinical information from the virology and bacteriology database. The presence or absence of diarrhea was recorded in the laboratory. If stool samples showed no watery diarrhea, data from the medical records were reviewed to confirm the presence or absence of diarrhea. The data were anonymized with a unique code and entered into a separate database for use by the study team. The NoV RNA-positive samples were stored for genotyping.
Detection of bacterial pathogens.
Collected stool samples were inoculated onto MacConkey (MC) agar (Difco, BBL), Salmonella-Shigella (SS) agar (Difco, BBL), taurocholate-tellurite-gelatin agar (TTGA), and Brucella agar (Difco, BBL) supplemented with 5% sheep's blood and five antibiotics (amphotericin B, cephalothin, polymyxin B, trimethoprim, vancomycin) for the isolation of Salmonella, Shigella, Vibrio, and Campylobacter spp., respectively. All the plates were incubated at 37°C for 18 to 24 h; Brucella agar was incubated at 42°C in an anaerobic jar with a CampyGen pack (product no. CN0025; Oxoid Ltd., United Kingdom) for 48 h. Along with being streaked directly, each sample was enriched in selenite broth (Difco, BBL) and bile peptone broth at 37°C for 18 to 24 h to enhance the isolation of Salmonella spp. and Vibrio spp., respectively. The enrichment broth for Salmonella was subcultured onto SS agar, and the enrichment broth for Vibrio was subcultured onto TTGA; both were incubated at 37°C for 18 to 24 h. Bacterial enteric pathogens were identified by colony characteristics and by biochemical tests using conventional and API 20 biochemical profiles (bioMérieux, France) when necessary. Isolates were further confirmed serologically using commercially available specific antisera (Denka Seiken, Japan). Campylobacter species isolates were differentiated as C. jejuni and C. coli by the hippurate hydrolysis test. Cefsulodin-triclosan-novobiocin agar was planted for isolation of Yersinia enterocolitica. Feces were evaluated for Clostridium difficile toxin with ImmunoCard toxins A and B (Meridian Bioscience).
Detection of norovirus by real-time PCR.
Two hundred micrograms of feces (200 μl if liquid) were suspended in 600 μl of stool transport and recovery (STAR) buffer that had been preheated in a 37°C water bath. Each tube was vortexed briefly, and 80 μl of chloroform was added. After being vortexed, samples were clarified by centrifugation for 1 min at maximum speed (Eppendorf model 4515 R). A 190-μl aliquot of supernatant and 10 μl of an internal control were transferred to a MagNA Pure LC isolation plate for reverse transcriptase PCR (RT-PCR) (the program was total nucleic acid extraction according to the manufacturer's instructions; MagNA Pure LC) with an elution volume of 50 μl (Roche Diagnostics GmbH). For detection, 20-μl RNA extractions were reverse transcribed to cDNA with random hexamers using the MultiScribe reverse transcriptase kit (Applied Biosystems) according to the manufacturer's instructions. Subsequently, the cDNA was used in a real-time NoV PCR assay for qualitative analysis (2, 11).
Molecular analysis of norovirus.
cDNA was amplified by a seminested PCR, and subsequently, region A of the polymerase gene was sequenced using the ABI-Prism BigDye Terminator v3.0 ready reaction cycle kit (ABI Prism 7700 sequence detection system; Applied Biosystems, Foster City, CA) as described previously (20). Sequences were assembled in BioNumerics (Applied Maths, Sint-Martens-Latem, Belgium, software package 6.6.4), evaluated manually for their quality by looking for the number of ambiguities, errors, mismatches, and deletions, and genotyped with an NoV-genotyping tool (http://www.rivm.nl/mpf/norovirus/typingtool) (13). Phylogenetic analysis (unweighted-pair group method using average linkages [UPGMA], multiple alignment) was done to identify links between strains for each genotype (variant) separately.
Nosocomial transmission.
Given that the incubation period for NoV is 1 to 3 days, community-acquired NoV infection was assumed if stool had been sampled in relation to GE complaints and within 1 day of admission. Nosocomial transmission of NoV was assumed if patients had been sampled for the first time more than 5 days after admission, as described previously (2). From the patients sampled on days 2 to 5 after admission, clinical information about the presence of GE symptoms at the first day of admission was used to differentiate between nosocomial and community-acquired NoV infection. Clustering of NoV infections was defined as the presence of two or more patients with NoV in one ward within 5 days after the onset of disease, with at least one nosocomially infected patient (2). In addition, the patients had to be part of a molecular cluster. A molecular cluster was assumed if strain sequences were identical (for a 200-bp region A pol gene fragment) or had maximally 1 mismatch over a 600-bp fragment of the polymerase gene. This approach was validated by comparison with strain sequences entered into the NoroNet database around the same period of time from other parts of the country, as well as internationally. Only the admitted patients were included in the cluster analyses.
Statistical analyses.
The clinical and laboratory data from patients initially suspected of and diagnosed with an NoV infection (recognized NoV patients) and those of patients suspected of having bacterial GE with an undiagnosed NoV infection (missed patients) were compared using the SPSS statistical software package (version 15; SPSS, Chicago, IL) and SAS (version 9.2 for Windows; SAS Institute Inc.). The Mann-Whitney U test (two-tailed) was used to compare the average lengths of stay in the hospital for recognized NoV patients and missed patients. P values of <0.05 were considered statistically significant. Logistic regression analysis was performed to determine which of 19 variables (Table 1) could be identified as univariate predictors of a missed NoV infection. Those variables with a P value of less than 0.20 in the univariate analysis were included in the multivariate model. The variables remained in the multivariate model if the P value was less than 0.10, whereas the backward selection procedure was used. The missing values were classified as unknowns so that the maximum number of cases was included in the multivariate logistic regression model. The analyzed variables were included as continuous variables where possible or categorized based on 50th percentiles in the group of recognized NoV cases.
Table 1.
Characteristics and clinical parameters of recognized and missed NoV patients in a tertiary care hospital from 16 December 2008 to 30 June 2009
| Parametera | Value for undiagnosed patients (n = 45) | Value for diagnosed patients (n = 50) | Univariate analysisc |
|
|---|---|---|---|---|
| OR | 95% CI | |||
| % male gender (no./total) | 58 (26/45) | 40 (20/50) | NS | |
| Mean age (yr) | 42 | 31 | ||
| % ≥18 yr (no./total) | 80 (36/45) | 46 (23/50) | 4.7 | 1.87–11.8 |
| % outpatients (no./total) | 21 (10/45) | 10 (6/50) | NS | 0.69–6.33 |
| % admitted with comm.-acq. GE (no./total) | 40 (18/45) | 40 (20/50) | NS | |
| % admitted without GE symptomsb (no./total) | 38 (17/45) | 49 (24/50) | NS | 0.29–1.49 |
| % with preexisting disease(s) (no./total) | 53 (24/45) | 63 (30/46) | NS | |
| % immunocompromised (no./total) | 31 (14/45) | 43 (21/50) | NS | |
| % with chronic NoV (no./total) | 4 (2/45) | 2 (1/50) | NS | |
| % with vomiting (no./total) | 42 (13/31) | 50 (19/38) | NS | |
| % with diarrhea (no./total) | 80 (28/35) | 95 (39/41) | 0.21 | 0.04–1.06 |
| % subjected to abdominal imaging (no./total) | 24 (11/45) | 4 (2/50) | 7.76 | 1.62–37.3 |
| Mean CT value (no. of cycles) | 24.4 | 24.6 | ||
| Illness duration at diagnosis (days) (no. of patients) | 4.7 (28) | 3.8 (39) | NS* | |
| Avg hospital duration (days) for all patients | 16.3 (33) | 18.1 (45) | NS* | |
| Hospital duration of patients with comm.-acq. NoV (days) (no. affected) | 6.2 (17) | 4.7 (20) | NS* | |
| No. of clusters (no. of patients in each cluster) | 3 (2, 2, 2) | 3 (3, 2, 2) | ||
| % who had died at 1 mo (no./total) | 2 (1/45) | 2 (1/50) | NS | |
| % who had died at 1 yr (no./total) | 9 (4/45) | 6 (3/50) | NS | |
Comm.-acq., community-acquired.
Nosocomially infected and/or asymptomatic patients.
Values in boldface are statistically significant. 95% CI, 95% confidence interval; NS, nonsignificant; *, Mann-Whitney U test (two-tailed).
RESULTS
Samples and patients.
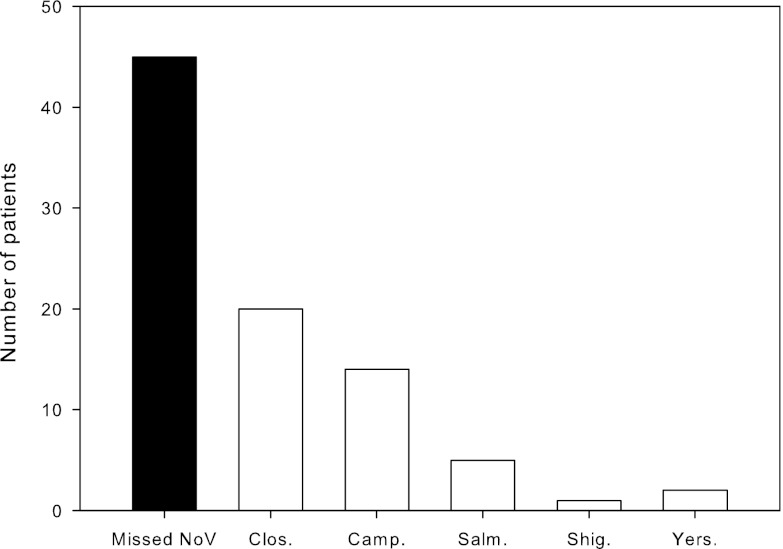
From December 2008 until July 2009, 1,809 patients were tested in the departments of virology (606 patients) and bacteriology (1,203 patients) of the EMC. In the virology department, 50 patients (8%) tested positive for NoV (recognized patients). Among the patients submitted for bacteriological testing, our retrospective analysis revealed 45 (4%) additional NoV patients who had not been diagnosed in the virology department (missed patients) (Fig. 1). For all patients combined, the diagnostic yield was 5.3% (95/1,809).
Fig 1.
Missed norovirus infections in relation to bacterial infections in stool samples of patients (n = 1,203) sent for bacteriological culture from December 2008 to July 2009. Clos., Clostridium difficile; Camp., Campylobacter spp.; Salm., Salmonella spp.; Shig., Shigella spp.; Yers., Yersinia spp.
Characteristics and clinical symptoms of recognized and missed patients.
The characteristics of the recognized (n = 50) and missed (n = 45) NoV patients are shown in Table 1. Compared to the recognized patients, missed patients frequently were adult (age ≥18) (80% versus 46% <18; P < 0.01), male (58% versus 40% female; P = 0.1), or outpatient (21% versus 10% inpatient; P = 0.27). A substantial number of NoV patients in both groups suffered from underlying diseases (51% versus 63% with no underlying disease) or were immunocompromised (31% versus 42% not immunocompromised), but these differences were not significant. Missed patients with NoV infection were more frequently from the affiliated nonuniversity hospital than from elsewhere (24% versus 6%; P < 0.02) but not more frequently related to a specific ward or department. Most of the clinical and virological characteristics were similar for recognized and missed NoV cases. These characteristics included viral load levels in fecal samples, reflected by a mean cycle threshold (CT) of 24 for both groups, and the presence of vomiting (42% versus 50% with no vomiting). However, diarrhea was less commonly reported for the missed patients (80% versus 95% of recognized patients; P = 0.07). Most patients not reporting diarrhea (n = 9) were adults with complex underlying conditions, such as cancer with liquid feeding or end-stage diseases. From two other patients, stool samples had been taken in the context of a bacteriological screening protocol. For both missed and recognized patients, the patients without diarrhea had high CT values (>25) significantly more often than the patients with diarrhea (6 missed patients and 2 recognized patients with diarrhea versus 24 missed patients and 43 recognized patients without diarrhea; odds ratio [OR], 5.4; P < 0.05).
Factors associated with missed NoV cases.
In logistic regression analysis, 9 of the 19 investigated factors were associated with being a missed NoV case (Table 2). In multivariate analysis, the following factors were independently associated with being a missed case: abdominal examination and admission to the affiliated general (nonacademic) hospital. The factors identified as independent indicators of recognized cases were admission at the children's hospital, symptoms of diarrhea, and higher age (in years). The effect of risk of higher age is no longer present after correcting for hospital departments.
Table 2.
Risk ratios and 95% confidence intervals for independent associations between different variables and missed norovirus casesa
| Variable | Category | No. of patients | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |||
| Gender | Female | 49 | Ref | Ref | ||
| Male | 46 | 2.1 | 0.9–4.7 | NS | ||
| Age (yr) by category | All | 95 | 1.01 | 1.00–1.03 | 0.95 | 0.91–0.98 |
| <18 | 36 | Ref | ||||
| ≥18 | 59 | 4.7 | 1.9–11.8 | |||
| Hospital visit due to acute GE (5 not diagnosed) | No | 45 | Ref | Ref | ||
| Yes | 45 | 0.4 | 0.1–1.5 | NS | ||
| Admission duration (days) (1 not diagnosed) | 0 (outpatients) | 16 | Ref | Ref | ||
| 1–9 | 38 | 0.5 | 0.1–1.7 | NS | ||
| >9 | 40 | 0.3 | 0.1–1.0 | NS | ||
| Group | University adults | 45 | Ref | Ref | ||
| University children | 36 | 0.3 | 0.1–0.7 | 0 | 0.0–0.2 | |
| Other | 14 | 2.9 | 0.7–12.0 | 5.2 | 1.0–27.7 | |
| Symptoms of acute GE (18 missing) | No | 7 | Ref | Ref | ||
| Yes | 70 | 0.3 | 0.1–1.7 | NS | ||
| Diarrhea (19 not diagnosed) | No | 9 | Ref | Ref | ||
| Yes | 67 | 0.2 | 0.0–1.1 | 0.1 | 0.0–0.8 | |
| Abdominal examination | No | 82 | Ref | Ref | ||
| Yes | 13 | 4.5 | 1.1–17.5 | 11.7 | 2.3–58.0 | |
| Kidney failure | No | 82 | Ref | Ref | ||
| Yes | 13 | 0.3 | 0.1–1.1 | NS | ||
Patients were in a hospital population from 16 December 2008 to 30 June 2009. Values are from univariate and multivariate logistic regression analyses. Boldface indicates a statistically significant difference. Ref, reference category; NS, not significant.
Hospitalization and diagnostic imaging.
Twenty-eight missed patients (10 outpatients and 18 newly admitted patients) had been infected outside the hospital (community-acquired NoV). The presumptive diagnoses for these patients at presentation are shown in Table 3. For 25 (86%) of the patients, the presumptive clinical diagnosis was not confirmed, and NoV infection was the likely explanation for the clinical symptoms in all of these cases. In two patients, the presumptive diagnosis was confirmed. However, NoV infection was clinically relevant in one of these patients.
Table 3.
Presumptive diagnoses and abdominal imaging results for missed NoV patients presenting at the emergency room with community-acquired NoV infectiona
| Presumptive diagnosis | Abdominal imaging performed | Diagnosis confirmedb | NoV relevantc |
|---|---|---|---|
| Cholecystitis | + | − | |
| Subacute bacterial peritonitis | Sigmoidoscopy + echo | + | + |
| AIDS-related pneumonia | − | + | |
| Inflammatory bowel disease | Echo + colonoscopyd | − | + |
| C. difficile infection, exacerbation, CU | X ray + sigmoidoscopy | − | + |
| Protein-losing diarrhea | X ray + scoping (twice) + CT | − | + |
| Addison crisis | − | + | |
| Food poisoning | X ray | − | + |
| Renal dysfunction (n = 3) | − | + | |
| Thymoma, Giardia infection, CMV | Endoscopy + CT | − | + |
| Graft vs host disease | − | + | |
| Celiac disease, CU, Crohn's disease | Echo | − | + |
| Diverticulitis | Sigmoidoscopy | − | + |
| AIDS-presenting symptom | − | + | |
| Ileus | X ray | − | + |
| Tropical infection (n = 2) | − | + | |
| Bacterial infection (n = 10) | − | + |
Twenty-eight NoV patients presented at the emergency room with community-acquired NoV infection. CU, colitis ulcerosa; GE, gastroenteritis; CT, computed tomography; CMV, cytomegalovirus; echo, abdominal echogram.
+, the presumptive diagnosis was confirmed.
+, NoV infection retrospectively explained the clinical symptoms.
Routinely tested.
A total of 11 missed patients underwent abdominal imaging (including abdominal echogram, X ray, computed tomography [CT] scan, or duodenal scoping/colonoscopy) during their diagnostic workup for acute GE. Abdominal imaging occurred significantly more often with the missed patients than with the recognized patients (24% versus 4%; P < 0.01) (Tables 2 and 3). Most (8 out of 11) of the missed patients with diagnostic imaging had underlying diseases. The imaging in these patients was usually performed to exclude exacerbation, complications, or progression of these underlying diseases, although in one patient the imaging was part of a routine control. In all three patients without underlying disease, the diagnostic imaging was performed to explain abdominal complaints that potentially might have been due to NoV infection. Finally, durations of hospitalization were compared between the newly admitted recognized (n = 17) and missed (n = 20) patients with NoV. Overall, the durations of hospital stay for those with the community-acquired NoV tended to be longer for the missed patients than for the recognized patients (6.2 days versus 4.7 days), but the difference did not reach statistical significance (Mann-Whitney U test, P = 0.48).
Nosocomial spread and clustering.
We evaluated nosocomial clustering in the recognized and missed hospitalized NoV patients. For this analysis, we excluded the outpatients. In addition to considering clustering in time and place, genotyping was performed to link the cases within the hospital as described in Materials and Methods. When only the recognized patients were considered, three clusters consisting of 2 patients each were present. When missed NoV patients were included, three more clusters of 3, 2, and 2 patients were recognized. Furthermore, with missed NoV patients included, two of the previous clusters would have increased by 3 patients and 1 patient, and one cluster would have been identified 4 days earlier. Based on the onset of disease symptoms, missed patients were designated index cases in 5 of the 6 clusters.
DISCUSSION
The present study was initiated to assess the potential underdetection of NoV as a cause of illness in patients admitted to the hospital or during a hospital stay by retrospectively analyzing stool samples that were sent to the laboratory to exclude bacterial causes of intestinal complaints. We found that this approach approximately doubled the number of recognized NoV shedders and that the missed NoV patients underwent significantly more diagnostic imaging for GE, including colonoscopies, computed tomography, and X-ray examinations. This underrecognition of NoV originated mainly from inadequate referrals to the laboratory by clinicians and therefore occurred regardless of the availability of a routine diagnostic NoV RT-PCR offered on a daily basis. To our knowledge, this routine is not atypical, and therefore, similar rates of underdiagnosis may occur in many hospitals (10, 21). We demonstrated that patients with unrecognized NoV infection not only had significantly more costly additional nonlaboratory procedures but also were the most likely sources for nosocomial infection in 5 instances during the relatively short period of time evaluated. Therefore, the results are relevant not only for individual patients but also for hospital infection control and for tracing NoV transmission chains.
Underdiagnosis of NoV occurred significantly more frequently in adults (60%) than in children (26%). However, this difference was no longer present after correcting for hospital departments, which suggests that the increased risk for underdiagnosis in adults likely relates to a low awareness of viral GE among physicians in adult wards rather than the patient's age. Alternatively, the general awareness of rotavirus in children might contribute to the effective recognition of viral GE, including NoV infections, in children.
Several recent studies underscore the fact that NoV infection affects people of all ages and can cause severe disease in elderly and immunosuppressed individuals (3, 4, 10, 15, 18, 19). In our study, the relevance of NoV infections in adults was emphasized by the finding that NoV infections in adults largely exceeded the number of bacterial GE infections based on the currently used methods for detection (59 adults versus 36 children).
The clinical characteristics and mean viral loads of the missed NoV patients were comparable to those of the recognized patients. This indicates that missed patients were not predominantly patients with a mild or late stage of disease or with a low viral load. The only exception to this was the finding that significantly more missed NoV patients than diagnosed patients reported an absence of diarrhea (14% missed versus 5% recognized patients). However, the absolute number of missed NoV patients not reporting diarrhea was low, and most of these patients had complex underlying diseases for which diarrhea may not have been reported explicitly.
Comparing the clinical parameters between missed and recognized NoV patients, our study highlights the clinical impact of missing NoV infections within a hospital setting. First, the results demonstrate that missed NoV patients were involved in 5 out of 6 nosocomial clusters that occurred during the study period. If the missed NoV patients are excluded, only three such clusters would have been recognized, one of which would have been at a later point in time. In five clusters, the index was found to be a missed patient, which suggests that diagnosing these missed patients could effectively improve the timely institution of infection-preventive measures. Friesema et al. recently reported a beneficial effect of the early institution of preventive measures for NoV (7). Second, we found that missed NoV patients underwent significantly more abdominal imaging than recognized patients, including colonoscopy, computed tomography, and abdominal X-ray examination. These investigations often were requested in relation to abdominal complaints (3 patients) but also to exclude exacerbations and complications of preexisting conditions (8 patients). Our findings that most (9 out of 11) imaging remained negative and that the recognized patients had similar underlying diseases but significantly less imaging support the view that physicians may request less diagnostic imaging when norovirus is diagnosed. In this context, it should be stressed that the mere presence of norovirus should not always exclude other possible causes of GE, since the infection can be asymptomatic in patients, especially when the viral load is low. Third, a subgroup analysis of the newly admitted patients with community-acquired GE showed that hospitalization tended to be longer for the missed NoV patients than for the recognized patients. Although not statistically significant, this difference may indicate that the laboratory diagnosis of NoV enables a more rapid discharge of newly admitted patients with GE.
Although we assumed that hospitalized patients with symptomatic GE were routinely sampled to eliminate an infectious cause, it is possible that in a small number of patients, notably those patients with only mild or no symptoms, no sampling was performed. Consequently, the underascertainment of NoV patients might be even higher than we report here. Furthermore, we did not address undiagnosed NoV infections among hospital personnel, although recent studies have indicated that infected personnel can play an important role in the NoV transmission chain (16). Hence, appropriate collection and testing in both patients and personnel will be required for developing new evidence-based strategies to prevent the introduction and spread of NoV (20).
The presented data demonstrate that a substantial level of underdiagnosis of NoV may occur in hospital settings and stress the need for education about the importance of viral GE to physicians in these settings. Since our results confirm that missed NoV patients are associated with increased clinical burden and nosocomial clustering, routine testing for NoV in adult patients with GE during the NoV seasonal peak likely will be cost-effective (6).
ACKNOWLEDGMENTS
We do not have a commercial or other association that might pose a conflict of interest.
This study was supported in part by ZonMW, The Netherlands (grant 125010002).
Footnotes
Published ahead of print 11 July 2012
REFERENCES
- 1. Amar CF, et al. 2007. Detection by PCR of eight groups of enteric pathogens in 4,627 fecal samples: re-examination of the English case-control Infectious Intestinal Disease Study (1993–1996). Eur. J. Clin. Microbiol. Infect. Dis. 26:311–323 [DOI] [PubMed] [Google Scholar]
- 2. Beersma MF, et al. 2009. Norovirus in a Dutch tertiary care hospital (2002–2007): frequent nosocomial transmission and dominance of GIIb strains in young children. J. Hosp. Infect. 71:199–205 [DOI] [PubMed] [Google Scholar]
- 3. Bobo LD, Dubberke ER. 2010. Recognition and prevention of hospital-associated enteric infections in the intensive care unit. Crit. Care Med. 38(Suppl 8):S324–S334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bresee J, et al. 2012. The etiology of severe acute gastroenteritis among adults visiting emergency departments in the United States. J. Infect. Dis. 205:1374–1381 [DOI] [PubMed] [Google Scholar]
- 5. Cheng VC, et al. 2011. Prevention of nosocomial transmission of norovirus by strategic infection control measures. Infect. Control Hosp. Epidemiol. 32:229–237 [DOI] [PubMed] [Google Scholar]
- 6. Friesema IH, et al. 7 January 2012. Costs of gastroenteritis in the Netherlands, with special attention for severe cases. Eur. J. Clin. Microbiol. Infect. Dis. [Epub ahead of print.] doi:10.1007/s10096-011-1518-1 [DOI] [PubMed] [Google Scholar]
- 7. Friesema IH, et al. 2009. Norovirus outbreaks in nursing homes: the evaluation of infection control measures. Epidemiol. Infect. 137:1722–1733 [DOI] [PubMed] [Google Scholar]
- 8. Hall AJ, et al. 2011. Incidence of acute gastroenteritis and role of norovirus, Georgia, U.S.A. 2004–2005. Emerg. Infect. Dis. 17:1381–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haustein T, Harris JP, Pebody R, Lopman BA. 2009. Hospital admissions due to norovirus in adult and elderly patients in England. Clin. Infect. Dis. 49:1890–1892 [DOI] [PubMed] [Google Scholar]
- 10. Henke-Gendo C, et al. 2009. New real-time PCR detects prolonged norovirus excretion in highly immunosuppressed patients and children. J. Clin. Microbiol. 47:2855–2862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kageyama T, et al. 2003. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J. Clin. Microbiol. 41:1548–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Koopmans M. 2009. Noroviruses in healthcare settings: a challenging problem. J. Hosp. Infect. 73:331–337 [DOI] [PubMed] [Google Scholar]
- 13. Kroneman A, et al. 2011. An automated genotyping tool for enteroviruses and noroviruses. J. Clin. Virol. 51:121–125 [DOI] [PubMed] [Google Scholar]
- 14. Lopman BA, Reacher MH, Vipond IB, Sarangi J, Brown DW. 2004. Clinical manifestation of norovirus gastroenteritis in health care settings. Clin. Infect. Dis. 39:318–324 [DOI] [PubMed] [Google Scholar]
- 15. Mattner F, et al. 2006. Risk groups for clinical complications of norovirus infections: an outbreak investigation. Clin. Microbiol. Infect. 12:69–74 [DOI] [PubMed] [Google Scholar]
- 16. Morter S, et al. 2011. Norovirus in the hospital setting: virus introduction and spread within the hospital environment. J. Hosp. Infect. 77:106–112 [DOI] [PubMed] [Google Scholar]
- 17. Patel MM, et al. 2008. Systematic literature review of role of noroviruses in sporadic GE. Emerg. Infect. Dis. 14:1224–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Podkolzin AT, et al. 2009. Hospital-based surveillance of rotavirus and other viral agents of diarrhea in children and adults in Russia, 2005–2007. J. Infect. Dis. 200(Suppl 1):S228–S233 [DOI] [PubMed] [Google Scholar]
- 19. Rondy M, et al. 2011. Norovirus disease associated with excess mortality and use of statins: a retrospective cohort study of an outbreak following a pilgrimage to Lourdes. Epidemiol. Infect. 139:453–463 [DOI] [PubMed] [Google Scholar]
- 20. Sukhrie FH, et al. 2011. Using molecular epidemiology to trace transmission of nosocomial norovirus infection. J. Clin. Microbiol. 49:602–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wolffs PF, Bruggeman CA, van Well GT, van Loo IH. 2011. Replacing traditional diagnostics of fecal viral pathogens by a comprehensive panel of real-time PCRs. J. Clin. Microbiol. 49:1926–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]