Abstract
Background
Neuropathic pain is common and difficult to treat. Recently a technique was developed to selectively inhibit nociceptive inputs by simultaneously applying two drugs: capsaicin, a transient receptor potential vanilloid receptor 1 channel activator and QX-314, a lidocaine derivative that intracellularly blocks sodium channels. We used this technique to investigate whether transient receptor potential vanilloid receptor 1-expressing nociceptors contribute to neuropathic pain.
Methods
The rat chronic constriction injury model was used to induce neuropathic pain in order to test the analgesic effects of both peripheral (perisciatic) and central (intrathecal) administration of the QX-314/capsaicin combination. The Hargreaves and von Frey tests were used to monitor evoked pain-like behaviors and visual observations were used to rank spontaneous pain-like behaviors.
Results
Perisciatic injections of the QX-314/capsaicin combination transiently increased the withdrawal thresholds by ~3 fold for mechanical and thermal stimuli in rats (n = 6/group) with nerve injuries suggesting that peripheral transient receptor potential vanilloid receptor 1-expressing nociceptors contribute to neuropathic pain. In contrast, intrathecal administration of the QX-314/capsaicin combination did not alleviate pain-like behaviors (n = 5/group). Surprisingly, intrathecal QX-314 alone (n = 9) or in combination with capsaicin (n = 8) evoked spontaneous pain-like behaviors.
Conclusions
Data from the perisciatic injections suggested that a component of neuropathic pain was mediated by peripheral nociceptive inputs. The role of central nociceptive terminals could not be determined because of the severe side effects of the intrathecal drug combination. We concluded that only peripheral blockade of transient receptor potential vanilloid receptor 1-expressing nociceptive afferents by the QX-314/capsaicin combination was effective at reducing neuropathic allodynia and hyperalgesia.
Introduction
It is estimated that neuropathic pain affects ~18% of the general population in America, drastically diminishing their quality of life.1 It often leads to considerable individual suffering and a financial burden to society.2,3 Neuropathic pain, caused by a primary lesion, dysfunction, or transitory perturbation of the peripheral or central nervous system, is clinically characterized by spontaneous pain and/or amplified pain responses to noxious or nonnoxious stimuli. Despite extensive research, neuropathic pain remains one of the most difficult challenges for physicians. Typically, a pharmacological approach is employed to manage neuropathic pain4, however, the medications are often ineffective.5–10 Clearly there is a pressing need for new and effective strategies for managing neuropathic pain.11,12
A novel approach was recently described to provide analgesia using local anesthetics without producing the detrimental side-effects commonly associated with these drugs. The technique selectively inhibits a subpopulation of nociceptive neurons with a combination of two drugs: capsaicin, the pungent substance found in chili peppers, and QX-314, a positively charged lidocaine derivative.13 In vitro studies show that the QX-314/capsaicin combination selectively inhibits the activity of transient receptor potential vanilloid receptor 1 (TRPV1)-expressing dorsal root ganglion neurons. Behavioral experiments indicate that peripheral application of this drug combination reduces acute pain in normal rats, increasing the thresholds to mechanical and thermal stimulation without producing the motor deficits that are common for local anesthetics.13–16
The potential of this new approach to treat neuropathic pain is extremely attractive. We thus extended these studies by testing whether TRPV1-expressing nociceptors contributed to neuropathic pain and if the QX-314/capsaicin combination alleviated chronic neuropathic pain. The analgesic effects of the drug combination were examined in a rat model for neuropathic pain that exhibits long-lasting disorders in pain sensation similar to those observed in humans, the sciatic nerve chronic constriction injury model. We tested both the peripheral (perisciatic) and central (intrathecal) effects of the drugs because several studies have shown that both peripheral and central neurons become more excitable after nerve injury.6,17,18 In addition, the expression of the TRPV1 protein increases in injured peripheral nerves, the superficial laminae of the dorsal horn containing the synaptic terminals of the nociceptors, and the spinal interneurons that process nociceptive information.19–23 We hypothesized that the peripheral or central application of the QX-314/capsaicin combination would target QX-314 to the TRPV1-expressing nociceptors, suppress their excitability, and alleviate neuropathic pain without producing the detrimental side effects of local anesthetics that are currently used in clinical practice.
Materials and Methods
The experimental protocols were approved by the Animal Care and Use Committee of Cleveland Clinic. Experiments were performed on ~90 male Sprague-Dawley rats (250–300 g) that were purchased from Harlan Laboratories (Indianapolis, IN). In general, the animals were housed in group cages (two or three rats per cage), allowed free access to food and water, and maintained on a 12/12 h light/dark cycle. Rats that exhibited excessive circling or aggressive behaviors were removed from the group cages and housed separately.
Chronic constriction injury
The chronic constriction injury (CCI) surgery was a modification of the method described by Bennett and Xie.24 Briefly, rats were anesthetized with an intraperitoneal injection of pentobarbital (40–60 mg/kg body weight) prior to surgery. The common right sciatic nerve was exposed at the mid-thigh level and isolated from surrounding tissue by blunt dissection. Proximal to the sciatic trifurcation, chromic gut (4-0) was used to tie two to four loose ligatures around the exposed nerve at 1 mm intervals. The incision was closed in layers with 4-0 silk sutures.
The drug combination
We used the capsaicin/QX-314 combination to selectively inhibit a sub-population of nociceptive inputs.13 The specificity of this drug combination is based on the protein expression profile of nociceptors and the chemical properties of QX-314. First, capsaicin activates ion channels (TRPV1) that are expressed predominantly by nociceptors.19,20,25,26 These TRPV1 ion channels are unusual in that they undergo pore dilation and allow the permeation of large cations such as the styryl dye FM1-43, gentamicin, and QX-314 through the open pore.13,27,28 Next, QX-314 is a positively charged derivative of lidocaine in physiological saline that is not likely to cross cell membranes. Since local anesthetics need access to intracellular domains of the voltage-gated sodium channels in order to inhibit their activity,29,30 QX-314 is a poor inhibitor of sodium channels when applied extracellularly. However, QX-314 is a potent sodium channel inhibitor after intracellular application.31 In theory, when the QX-314/capsaicin combination is applied, capsaicin should open the pore of the TRPV1 channel, facilitating the selective passage of QX-314 into the nociceptive neurons. QX-314 should subsequently inhibit the generation of action potentials blocking the transmission of pain signals from peripheral nociceptors to the central nervous system.13 Indeed, the peripheral application of this drug combination reduces acute pain in normal rats, increasing the thresholds to mechanical and thermal stimulation without producing motor deficits.13–16
Peripheral nociceptive blockade
The ability of QX-314, lidocaine, capsaicin, or the combination of QX-314 and capsaicin to selectively block nociceptive pathways was tested on the same rats before and after nerve ligation. Initially, naive rats were placed into plastic restraint cones to facilitate drug injection. Drugs were injected near the sciatic nerve at the mid-thigh level in a volume of 0.1 ml with a tuberculin syringe and a 27-gauge needle. Since the restraint and injections could be stressful to the rats, a second experiment was performed in which the rats were briefly anesthetized with isoflurane before injections. Qualitatively similar results were obtained for both procedures. The isoflurane data was presented in the figures and text. The drugs tested were 0.2% QX-314, 2% lidocaine, 0.2% lidocaine, 0.5 mg/ml capsaicin, and a combination of QX-314 and capsaicin. For the coadministration of QX-314 and capsaicin, QX-314 was normally injected 10 min before capsaicin. Capsaicin, QX-314, and lidocaine hydrochloride were purchased from Sigma Chemical Co. (St. Louis, MO). Stock solutions of lidocaine and QX-314 were prepared in phosphate buffered saline (PBS: 10 mM NaH2PO4 and 137 mM NaCl, pH 7.4). The capsaicin stock was prepared in dimethyl sulfoxide (DMSO, Sigma). All of the drugs were diluted and filter sterilized before administration. The final injection solution (vehicle) contained 12.5 % DMSO in PBS.
The rats were handled for ten days before sciatic nerve injections and behavioral tests in order to familiarize them with the environment and to minimize stress. Rats were randomly assigned to the mechanical or thermal sensitivity test groups. Behavioral tests began before the drug injection in order to determine the baseline and resumed 15 min after the injection. Testing continued at regular intervals for 6 h. Behavioral tests were performed every other day and the side of injection was alternated to allow the animals to recover from the injections. Several different drugs or drug combinations were tested each day, but individual rats received only one drug or combination of drugs per day.
When the experiments on the nonligated rats were completed, the right sciatic nerves were ligated to study the effects of peripheral nociceptive blockade on a neuropathic pain model. The mechanical and thermal sensitivity were regularly tested until the rats exhibited hyperalgesia. After detecting hyperalgesia, the drug injections and behavioral tests were performed as described for the naÔve rats except that only the leg with the nerve ligation was tested. (We did not alternate the side that was injected.) The interval between experiments was increased to 3–5 days to allow the animals to recover.
Implantation of intrathecal catheters
Rats were anesthetized with an intraperitoneal injection of pentobarbital (40–60 mg/kg) prior to surgery. The rat was placed in a stereotaxic head holder with the head tilted forward and a midline incision was made above the occipital crest. The cisterna magna was exposed by a blunt dissection of the surrounding musculature. A 20-gauge needle was used to open the membrane and the Alzet catheter (Durect Corporation, Cupertino, CA) was inserted into the intrathecal space. The tip of the catheter was slowly advanced until it reached the lumbar enlargement. The musculature was closed with 4-0 silk sutures holding the catheter in position. A second incision was made in the middle of the back ~1 cm to the left of the spinal column to create a subcutaneous pocket for an ALZET pump (Model 2002, Durect Corporation). The free end of the catheter was tunneled to the pocket and attached to the pump. The pump was inserted and both incisions were closed with 4-0 silk sutures. Initially, the pump was filled with 5 % heparin in PBS to prevent clots from forming in the catheter. Rats were allowed to recover from the surgery for 1 week. After the recovery period, the rats were briefly anesthetized with pentobarbital, an incision was made in the skin near the pump, and the old pump was replaced with a new one containing the drugs or vehicle. In some experiments the nerve ligation surgery and the intrathecal catheter implantation were done at the same time.
Central nociceptive blockade
Mechanical and thermal sensitivity were tested every other day after the sciatic nerve ligation. When hyperalgesia and allodynia reliably developed, usually 7 days after the surgery, new pumps were inserted containing the drugs or vehicle. The drugs administered via intrathecal catheters to test their antihyperalgesic and antiallodynic effects were 0.2% QX-314, 0.5 mg/ml capsaicin, or the combination of QX-314 and capsaicin. All of the drugs were dissolved in a vehicle containing 5% heparin and 12.5% DMSO in PBS (pH 7.4). The intrathecal concentration of DMSO is well below neurotoxic levels after dilution in the cerebrospinal fluid.32 Control animals were implanted with pumps that contained only the vehicle without any drugs. Drug effects were observed over a period of 14 days.
Behavioral tests
Rats were handled before behavioral testing and surgical procedures to familiarize them with the environment and to minimize stress. The behavioral measurements began prior to surgery to determine the baseline behavioral responses. These experiments were done in a quiet room by a trained observer that was blinded to the drug treatment.
Mechanical sensitivity
The nociceptive threshold to mechanical stimulation was determined using a Semmes-Weinstein von Frey Touch Test Sensory Evaluator (North Coast Medical, Inc., Morgan Hill, CA), as we have described before.12 Animals were loaded into plexiglass chambers resting on a 6 mm wire grid. After a 15 min acclimation period, the plantar surface of both footpads was stimulated with calibrated filaments. Filaments were applied to the paw surface in ascending order of force (0.4 – 60 grams) until they bent and held there for ~3 s or until the rat withdrew its foot. After a foot withdrawal, the filaments were applied in descending order, beginning with at the next lower filament until there was no response. The threshold was the lowest filament to evoke a withdrawal. The procedure was repeated three times at 5-min intervals to avoid sensitization and withdrawal thresholds were averaged and recorded.
Thermal sensitivity
The nociceptive threshold to thermal stimulation was determined using the procedure described by Hargreaves et al.33 The Basile Plantar test apparatus used to perform these experiments was purchased from Stoelting Company (Wood Dale, IL). Animals were loaded into Perspex enclosures resting on a pane of glass. The animal was unrestrained within the enclosure. After a 15-min acclimation period, a movable infrared generator was positioned below the plantar surface of the hind paw and activated. When the animal withdrew its paw, the infrared generator and a timer measuring the duration of the stimulus was automatically stopped. The withdrawal latency was the interval between the activation of the heat source and the paw withdrawal. The intensity of the heat stimulus was adjusted to evoke a withdrawal response of 10–20 s in normal animals. A cut-off of 30 s was used to prevent potential tissue damage. The procedure was repeated three times at 5-min intervals to avoid sensitization and withdrawal latencies were averaged and recorded.
Behavioral observations
Rats were placed into a plexiglass chamber and allowed to acclimate for 15 min before behavioral observations began. The behavior was monitored for a period of 10 min. The behavioral responses were ranked using the following scores: 0, no sign of excitation; 1, restlessness, scratching and biting of the flank or tail, tail flicks, mild circling; 2, mild vocalization with restlessness, scratching and biting of the flank or tail, tail flicks, mild circling; 3, vocalization with spontaneous running and vigorous circling; 4, vigorous vocalization with running, circling, rolling and jumping. The scores were recorded for every minute of observation and summed at the end of the experiment. The observer was blinded to the experimental treatments.
Histology
After the experiment, the rats were sacrificed using carbon dioxide. A laminectomy was performed to determine the location of catheter tip in all of the animals. The catheter tip was found to be located on or near the dorsum of the lumbar enlargement. The spinal cord segments near the tip of the catheter were removed and fixed with 4 % paraformaldehyde in 0.1 M phosphate buffer (7.4) overnight at 4 °C. Tissue samples were dehydrated through graded alcohols and a xylene substitute, infiltrated and embedded in paraffin, and sectioned at 5 µm using a Leica RM2125 rotary microtome (Leica Microsystems, Wetzlar, Germany). Sections were stained with Harris hematoxylin and eosin on a Leica ST 5020 autostainer (Leica Microsystems), dehydrated, cleared, and coverslipped. Spinal cords were visualized using bright field illumination at 20× magnification on a Leica DMR microscope (Leica Microsystems). Images were captured using a CCD video camera and QCapture Pro imaging software from QImaging (Surrey, British Columbia, Canada).
Immunohistochemical staining of astrocytes and microglia
Sections of the spinal cord were further used for immunohistochemical staining of astrocytes and microglia. Tissue samples were dehydrated through graded alcohols and a xylene substitute, infiltrated and embedded in paraffin, and sectioned at 5 µm†using a Leica RM2125 rotary microtome (Leica Microsystems). Sections were stained for astrocytes with anti-glial fibrillary acidic protein antibody produced in rabbit (G9269; Sigma-Aldrich, Saint Louis, MO) and for microglia with rabbit antiionized calcium-binding adapter-1 (Iba1) antibody (019–19741; Wako, Osaka, Japan). The experiments were performed on a Ventana Benchmark automated immunostainer (Ventana Medical Systems, Inc., Tucson, AZ) utilizing a Ventana iVIEW DAB Detection kit; the secondary antibody provided in the kit was replaced with Vector goat anti-rabbit biotinylated secondary antibody (1:200 Vector Laboratories, Burlingame, CA). The rabbit polyclonal glial fibrillary acidic protein antibody, specific for astrocyte and glial cell, was diluted 1:400 and the rabbit polyclonal ionized calcium-binding adapter-1 antibody, specific for microglia and macrophage, was diluted 1:500. Sections of the spinal cord were visualized using bright field illumination with a 20X dry objective and a 40X oil immersion objective on a Leica DMR microscope (Leica Microsystems). Images were captured using a Retiga Exi Cooled CCD camera and QCapture Pro imaging software from QImaging.
Statistical analysis
Values are expressed as means ± SEM. Time course data for both the von Frey and Hargreaves tests were analyzed by two-way analysis of variance (ANOVA) with repeated measures to detect interactions between drug treatments and time unless otherwise stated. ANOVAs with statistically significant interactions between drug and time (P < 0.05) were followed by post hoc comparisons using Bonferroni’s t-test when appropriate. The data sets for the peripheral injections are complete. For the intrathecal administration of drugs, a general linear extrapolation was used to compensate for missing observations from an individual animal on a single day. Data for the bar graphs of the peak normalized latency in figures 1 and 2 were compared to the pre-treatment baseline for each drug tested using paired Student’s t-tests (two-tailed). Comparisons among the different drugs are performed using the data for the time courses. The data from the behavioral observations was analyzed by Kruskal-Wallis one-way ANOVA on ranks and followed by post hoc comparisons using Dunn’s Method. Statistical analysis was performed using SigmaStat software (Systat Software Inc., San Jose, CA). For all statistical tests, P < 0.05 was considered to be statistically significant.
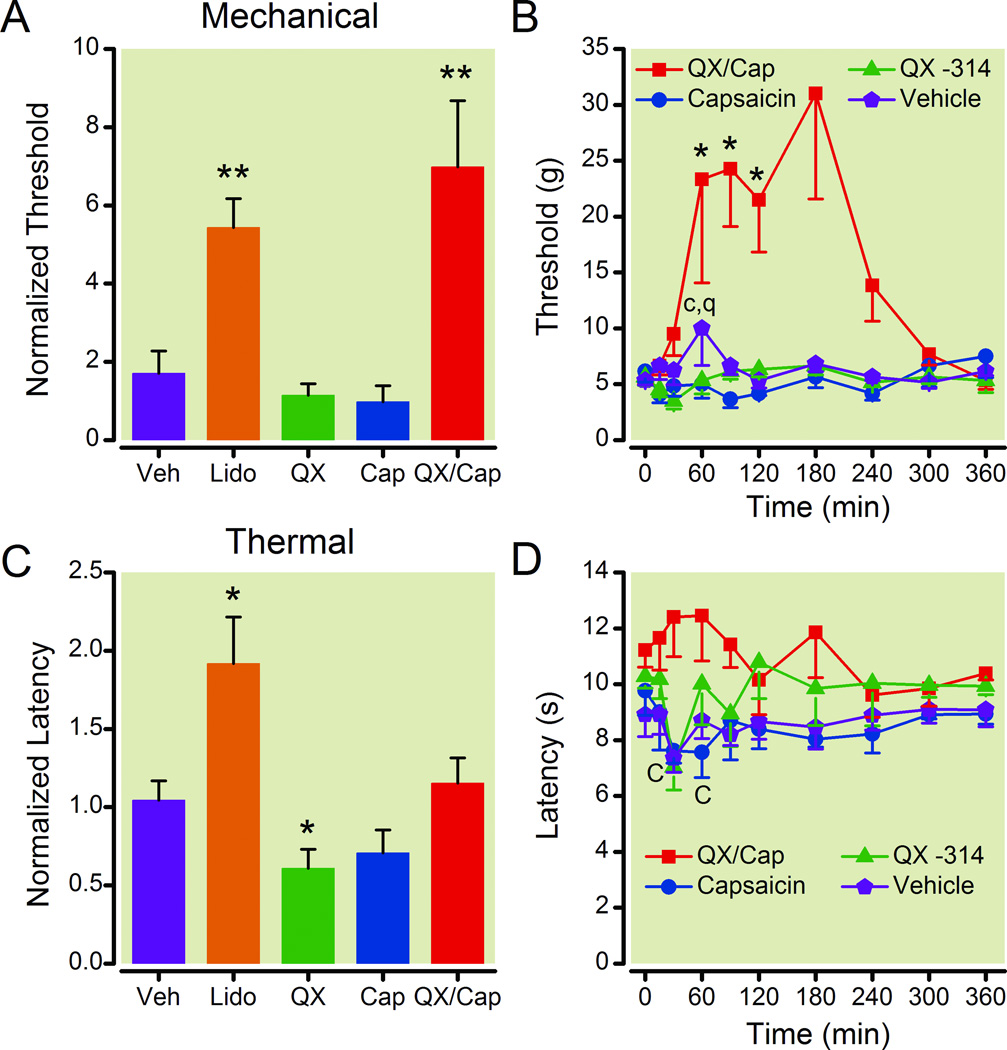
Figure 1.
The QX-314/capsaicin combination reduced the sensitivity of normal rats to mechanical stimuli. The normalized peak threshold (A) and the time course of the threshold to mechanical stimuli (B) show that perisciatic injection of QX-314 (0.2 %) with capsaicin (0.5 mg/ml) increased the withdrawal threshold for ~2 h. The mechanical threshold was also increased by lidocaine (2 %), however lidocaine also caused motor deficits. QX-314 alone or capsaicin alone did not change the normalized peak mechanical threshold. The normalized peak latency (C) and the time course of the latency to thermal stimuli (D) show that perisciatic injection of the QX-314/capsaicin did not change the withdrawal threshold to thermal stimulation. The thermal threshold was increased by lidocaine, whereas it was decreased by QX-314 and capsaicin alone. The peak response to each drug during the time course was normalized to facilitate comparisons between the different groups and graphed as the normalized threshold or latency (A and C). Data graphed in this figure and all subsequent figures are averages ± SEM. For the normalized threshold and latency (A and C), single asterisks indicate that p < 0.05 and double asterisks indicate that p < 0.01 compared to the baseline before the injection. For the time courses (B and D), an asterisk indicates that p < 0.05 for the QX-314/capsaicin combination, q indicates that p < 0.05 for the QX-314 alone, and c indicates that p < 0.05 for the capsaicin alone. For each group, n = 6. Cap = capsaicin; Lido = lidocaine; QX = QX-314; Veh = vehicle.
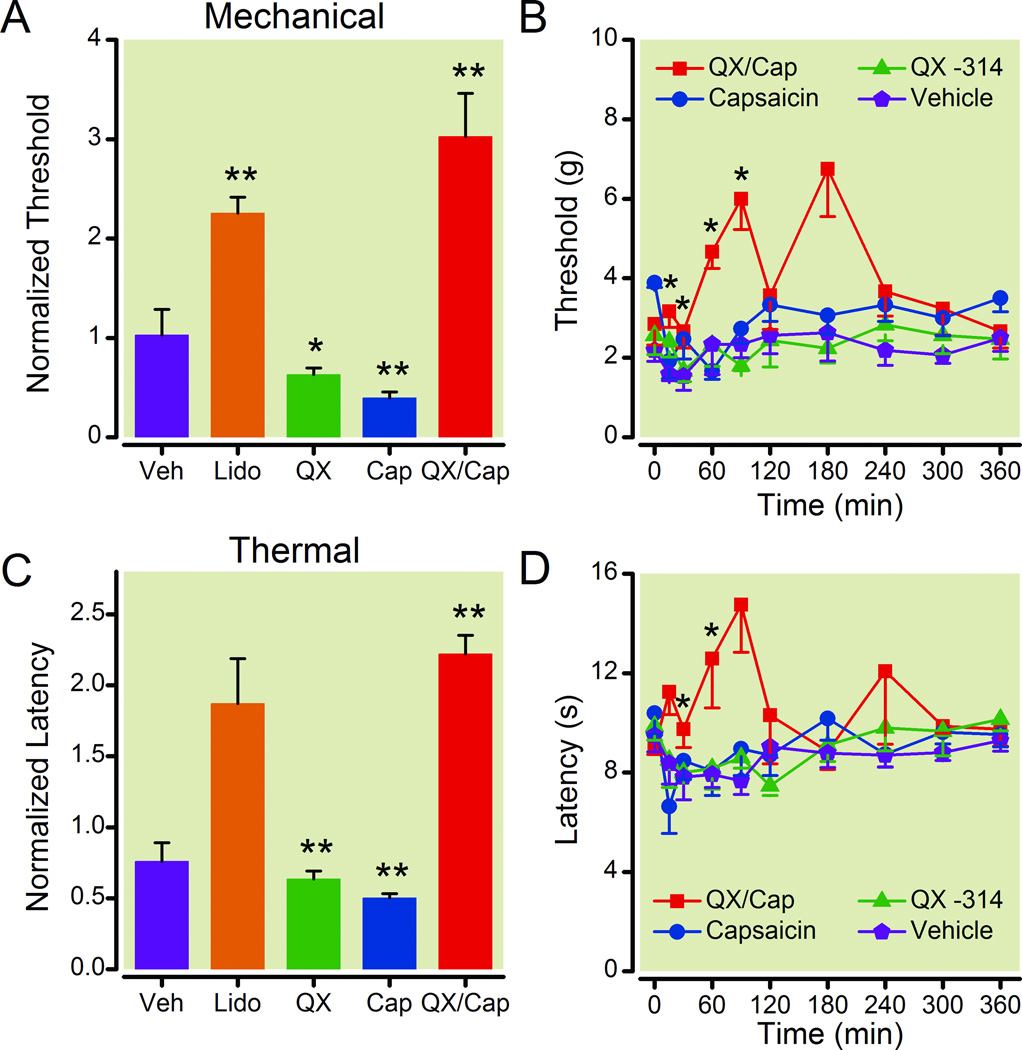
Figure 2.
The QX-314/capsaicin combination reduced the sensitivity of rats with injured sciatic nerves to mechanical and thermal stimuli. The normalized peak threshold (A) and the time course of the threshold to mechanical stimuli (B) show that perisciatic injection of QX-314 (0.2 %) with capsaicin (0.5 mg/ml) increased the withdrawal threshold to mechanical stimulation for ~1 h. The mechanical threshold was also increased by lidocaine (2 %), however lidocaine also caused motor deficits (data not shown). QX-314 alone or capsaicin alone dramatically lowered the mechanical threshold. The normalized peak latency (C) and the time course of the latency to thermal stimuli (D) show that perisciatic injection of the QX-314/capsaicin increased the withdrawal threshold to thermal stimulation. The thermal threshold was decreased by QX-314 and capsaicin alone. The peak response to each drug during the time course was normalized to facilitate comparisons between the different groups and graphed as the normalized threshold or latency (A and C). For the normalized threshold and latency (A and C), single asterisks indicate that p < 0.05 and double asterisks indicate that p < 0.01 compared to the baseline before the injection. For the time courses (B and D), an asterisk indicates that p < 0.05 for the QX-314/capsaicin combination. For each group, n = 6. Cap = capsaicin; Lido = lidocaine; QX = QX-314;Veh = vehicle.
Results
The goal of this study was to determine whether selectively blocking TRPV1-expressing nociceptors would alleviate chronic neuropathic pain in a rat model. We tested the effects of the peripheral and central application of the QX-314/capsacin combination on the withdrawal thresholds for mechanical and thermal stimuli in normal rats and rats with unilateral sciatic nerve ligations.
Peripheral administration of the QX-314/capsaicin combination increased the withdrawal threshold for mechanical stimuli in normal rats
The peripheral injection of the QX-314/capsaicin combination reliably increased the nociceptive threshold to mechanical stimulation. The withdrawal threshold was determined by applying calibrated Semmes-Weinstein von Frey filaments to the plantar surface of the hind footpads. Testing began before the drug injection (0 min) and continued at regular intervals for the next 6 h. The drugs tested were 0.2% QX-314, 2% lidocaine, 0.2% lidocaine, 0.5 mg/ml capsaicin, the QX-314/capsaicin combination and a DMSO containing vehicle. For the coadministration of QX-314 with capsaicin, QX-314 was injected 10 min before capsaicin. The experiment was performed on two different groups of six animals. In the first experiment the drugs were injected without anesthetic and in the second, the rats were briefly anesthetized with isoflurane in order to reduce the stress associated with the restraint and injections. Qualitatively similar results were obtained for both procedures. The isoflurane data was presented in the figures and text.
We found that the withdrawal threshold to mechanical stimulation increased for all of the normal rats in the anesthetized and unanesthetized groups injected with the QX-314/capsaicin combination. The data were normalized to the preinjection baseline and the peak changes for the drugs were averaged and plotted in figure 1A. Only the QX-314/capsaicin combination and the membrane permeable sodium channel inhibitor lidocaine (2 %) produced a statistically significant increase in the threshold to mechanical stimulation in normal rats (paired student’s t-test, p ≤ 0.01, n = 6 each group). The increase was ~ 6-fold compared to preinjection levels for the drugs. We also observed that the increase in the threshold produced by lidocaine was associated with an incomplete motor blockade. Rats injected with 2 % lidocaine were observed to drag their paws in the test chambers for ~ 30 min after the injection. The motor deficit was not quantified because it would interfere with the measurements of the nociceptive thresholds. In contrast, motor deficits were not observed in rats injected with the QX-314/capsaicin combination. The DMSO vehicle, QX-314 alone, and 0.2% capsaicin alone had little effect on the normalized threshold for mechanical stimulation (fig. 1A).
The time course for the effects of perisciatic injections of the vehicle, 0.2% QX-314, 0.5 mg/ml capsaicin, or the QX-314/capsaicin combination on the mechanical threshold in normal rats is shown in figure 1B. We found that the QX-314/capsaicin combination was more effective at increasing the mechanical threshold than either drug alone (two-way ANOVA with repeated measures, p ≤ 0.001 for the interaction between drug and time, n = 6 each group). Post hoc analysis indicated that the increase in the withdrawal threshold to mechanical stimuli reached statistically significant levels 60 min after injecting the drug combination compared to the vehicle and lasted for ~1 h (fig.1B). By comparison, the peripheral application of QX-314 or capsaicin alone produced a statistically significant decrease in the mechanical threshold compared to the vehicle. It is also important to note that the time to peak and the duration of the drug effects was quite variable for individual animals. Although the withdrawal threshold to mechanical stimuli increased for all of the rats injected with the QX-314/capsaicin combination, the effects reached a maximum at different times. While the peak occurred within 60 min after the injection for some animals, it took more than 2 h for others. Therefore, of the drugs tested, only the QX-314/capsaicin combination produced an effective analgesia to noxious stimuli without causing motor deficits in normal rats.
The effects of the QX-314/capsaicin combination on the nociceptive threshold to thermal stimulation were less pronounced. A Basile Plantar test apparatus was used to heat the plantar surface of the hind paw and measure the latency between the activation of the heat source and the foot withdrawal for normal rats. Only lidocaine (2 %) produced a statistically significant increase in the peak normalized latency to thermal stimulation compared to the preinjection baseline (fig. 1C, paired student’s t-test, p ≤ 0.05, n = 6). The increase in the thermal threshold produced by lidocaine was also associated with incomplete motor blockade as described above. In contrast, the peak normalized thermal latency was reduced by the peripheral application of QX-314 alone. The vehicle or capsaicin did not change the thermal latency (fig. 1C). The time course for the effects of perisciatic drug injections on the thermal latency of normal rats is shown in figure 1D. We found that capsaicin reduced the withdrawal latency to thermal stimuli during the first hour after its injection (fig. 1D; two-way ANOVA with repeated measures for the interaction between drug and time, p ≤ 0.012, n = 6). In addition, post hoc analysis also indicated that 2 % lidocaine produced statistically significant increases in the thermal threshold (data not shown). So, similar to previously published reports13, the QX-314/capsaicin combination increased the nociceptive threshold to mechanical stimulation without producing a motor deficit in normal rats. Unlike Binshtok et al.13, we did not observe a statistically significant change in the thermal threshold after perisciatic injection of the QX-314/capsaicin combination.
Peripheral administration of the QX-314/capsaicin combination increased the withdrawal threshold for both mechanical and thermal stimuli in rats with sciatic nerve ligations
Next we tested whether the peripheral application of the QX-314/capsaicin combination increased the mechanical and thermal withdrawal thresholds in a rat model of neuropathic pain. The sciatic nerve was constricted with chromic gut ligatures to induce chronic neuropathic pain (CCI model).24 After recovering from the surgery, the mechanical and thermal thresholds were tested every other day. When hyperalgesia and allodynia developed, we tested the effects of perisciatic nerve drug application on the pain-like behaviors of the neuropathic rats (fig. 2). The peripheral injection of the QX-314/capsaicin combination produced a transient increase in the withdrawal thresholds to mechanical and thermal stimulation in neuropathic rats. We found that the mechanical and thermal withdrawal thresholds increased for all of the neuropathic rats (six anesthetized and six unanesthetized) administered the QX-314/capsaicin combination. Figure 2A shows that, at its peak, the perisciatic injection of the QX-314/capsaicin combination produced an ~3 fold increase in the normalized mechanical threshold of rats with sciatic nerve ligations compared to the preinjection baseline and lidocaine (2 %) produced an ~ 2 fold increase (paired student’s t-test, p ≤ 0.01, n = 6). However, as mentioned above, the rats injected with lidocaine (2 %) were observed to drag the injected limb. No motor deficits were observed in rats injected with the QX-314/capsaicin combination. Unlike normal rats, we found that the QX-314/capsaicin combination also produced an ~2 fold increase in the normalized thermal latency of rats with sciatic nerve ligations compared to the pre-injection baseline (fig. 2C, paired student’s t-test, p ≤ 0.01, n = 6). The effects of the QX-314/capsaicin combination on the mechanical and thermal withdrawal thresholds in neuropathic rats appears to be robust because application of the drugs to different regions of the sciatic nerve (proximal, distal, or at the ligation site) produced nearly identical increases in the withdrawal thresholds (data not shown). We also found that the normalized mechanical threshold and thermal latency were reduced by the administration of QX-314 or capsaicin alone for rats with peripheral nerve injuries. Administration of the vehicle had little effect on the normalized thermal latency or the normalized mechanical threshold (fig. 2A and C). The time course for the effects of the QX-314/capsaicin combination on the mechanical threshold and thermal latency of neuropathic rats indicated that the analgesia produced by the drug combination was transient (fig. 2B and D, two-way ANOVA with repeated measures for the interaction between drug and time, p ≤ 0.001 for mechanical, n = 6; p ≤ 0.012 for thermal, n = 6). Post hoc analysis indicates that the effects of the QX-314/capsaicin combination dissipated in < 3 h when compared to the vehicle. The fluctuation in the time course for the QX-314/capsaicin combination reflects the variation in the time to peak for different animals. Together these results show that the peripheral injections of the QX-314/capsaicin combination and lidocaine alleviated pain-like behaviors in neuropathic rats, whereas application of QX-314 or capsaicin alone intensified these behaviors. Since the QX-314/capsaicin combination and lidocaine are capable of inhibiting signals from peripheral nociceptors, these results suggest that peripheral nociceptors contribute to the mechanical allodynia and thermal hyperalgesia in rats with constriction injuries of the sciatic nerve.
The composition of the vehicle can alter the effectiveness of the QX-314/capsaicin combination
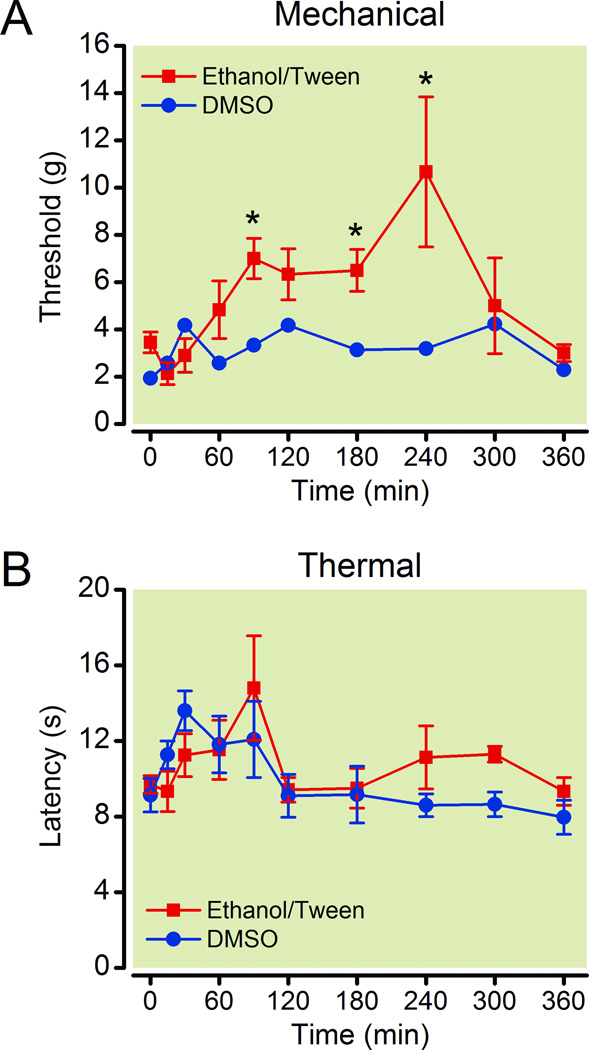
As mentioned above, different results were reported by other labs using the same drug combination dissolved in a different vehicle. Since the composition of the vehicle can alter the availability of drugs in vivo or even have direct effects on the nervous system, we investigated whether different vehicles could alter the effectiveness of the QX-314/capsaicin combination. Capsaicin is insoluble in water and is typically dissolved in a solvent for use in pain studies such as DMSO, Intralipid®, or an ethanol/detergent mixture.13,16,34–36 Here we focused on the two most common vehicles DMSO and an ethanol/detergent mixture. Stock solutions of capsaicin were prepared in either DMSO or ethanol. The capsaicin stock in DMSO (capsaicin/DMSO) was diluted with PBS until the injected solution contained 12.5 % DMSO. The capsaicin stock in ethanol was diluted with a saline containing the detergent Tween 80 until the final concentrations of the vehicles in the injected solution contained 10 % ethanol and 10 % Tween 80 (ethanol/Tween). The analgesic effects of the QX-314/capsaicin combination prepared with DMSO containing vehicle or ethanol/Tween containing vehicle was compared on rats with chronic constriction injuries (fig. 3). We found that, regardless of the vehicle used, the QX-314/capsaicin combination produced a statistically significant increase in the mechanical threshold and thermal latency after the perisciatic injection compared to the vehicle (two-way ANOVA with repeated measures for the interaction between drug and time, p ≤ 0.001 for mechanical and thermal, n = 6). The effectiveness of the analgesia did depend on the vehicle used to deliver the drugs. Post hoc analysis indicated that the QX-314/capsaicin combination was more potent at increasing the withdrawal threshold for mechanical stimuli when it was dissolved in the ethanol/Tween containing vehicle than in the DMSO containing vehicle (fig. 3). The effectiveness of the QX-314/capsaicin combination on thermal stimuli was not affected by the vehicle in rats with nerve ligations.
Figure 3.
Ethanol/Tween containing vehicle enhances the antinociceptive effects of the QX-314/capsaicin combination in rats with a nerve ligation. Time course shows that withdrawal threshold to mechanical stimuli (A) was increased more by the QX-314/capsaicin combination dissolved in ethanol/Tween containing vehicle than dimethyl sulfoxide (DMSO) containing vehicle. The composition of the vehicle had little effect on the withdrawal threshold to thermal stimuli (B). Asterisks indicate that p < 0.05 for ethanol/Tween containing vehicle compared to the DMSO containing vehicle at the same time point. For each group, n = 6.
Based on the known effects of ethanol, it is possible that the ethanol contributed to the observed differences between the two vehicles. Ethanol reduces the excitability of central neurons and can be used as an anesthetic.37,38 The direct application of ethanol to peripheral nerves reduces the amplitude and can even inhibit the conduction of action potentials.34,39 Ethanol may also facilitate the entry of QX-314 into TRPV1-expressing nociceptors because ethanol activates TRPV1 receptors and potentiates their response to capsaicin.40,41 It is also possible the ethanol/Tween saline may facilitate diffusion of the drugs to the appropriate sites on the axons in the sciatic nerve. Therefore, there is a strong possibility that the ethanol/Tween vehicle has a direct effect on the sciatic nerve or that it potentiates the effectiveness of QX-314/capsaicin combination. Consistent with this theory, injection of the ethanol/Tween vehicle intensified the nerve conduction blockade produced by lidocaine or bupivacaine in normal rats.14
Intrathecal application of QX-314 sensitizes the control paw of CCI rats to mechanical stimuli
Clinically it would be extremely useful if neuraxial administration of the QX-314/capsaicin combination produced safe, effective, and selective analgesia without motor blockade or any other adverse side effects. The opioids that are currently extensively employed in clinical practice for subarachnoid and epidural analgesia are also associated with clinically significant, sometimes fatal, adverse outcomes. Therefore, we examined the effects of central administration of the QX-314/capsaicin combination. Capsaicin-sensitive nociceptive neurons transmit sensory information to interneurons located in the dorsal horn of the spinal cord.19,42 The central terminals of these capsaicin-sensitive nociceptors also express functional TRPV1 ion channels and the expression levels of the TRPV1 protein increases after nerve injury in the dorsal horn.17,20–23 We hypothesized that inhibiting the central terminals of the capsaicin-sensitive nociceptors with the QX-314/capsaicin combination would be as effective at reducing neuropathic pain as inhibiting nerve conduction. The drugs were delivered using intrathecal catheters and osmotic pumps to lengthen the duration of drug application, mimicking the clinical scenario of intrathecal pain pump therapy. The pumps were capable of delivering drugs for 2 weeks after the surgery which was important because chronic neuropathic pain lasts for months or years6,43 and the peripheral injections produced effects that only lasted for hours. The drugs were dissolved in the DMSO containing vehicle because the peripheral injection of the QX-314/capsaicin combination dissolved in the DMSO vehicle produced reliable analgesia in normal and neuropathic rats. In addition, unlike ethanol, DMSO does not have anesthetic effects at the concentrations used in this study.32,37,38
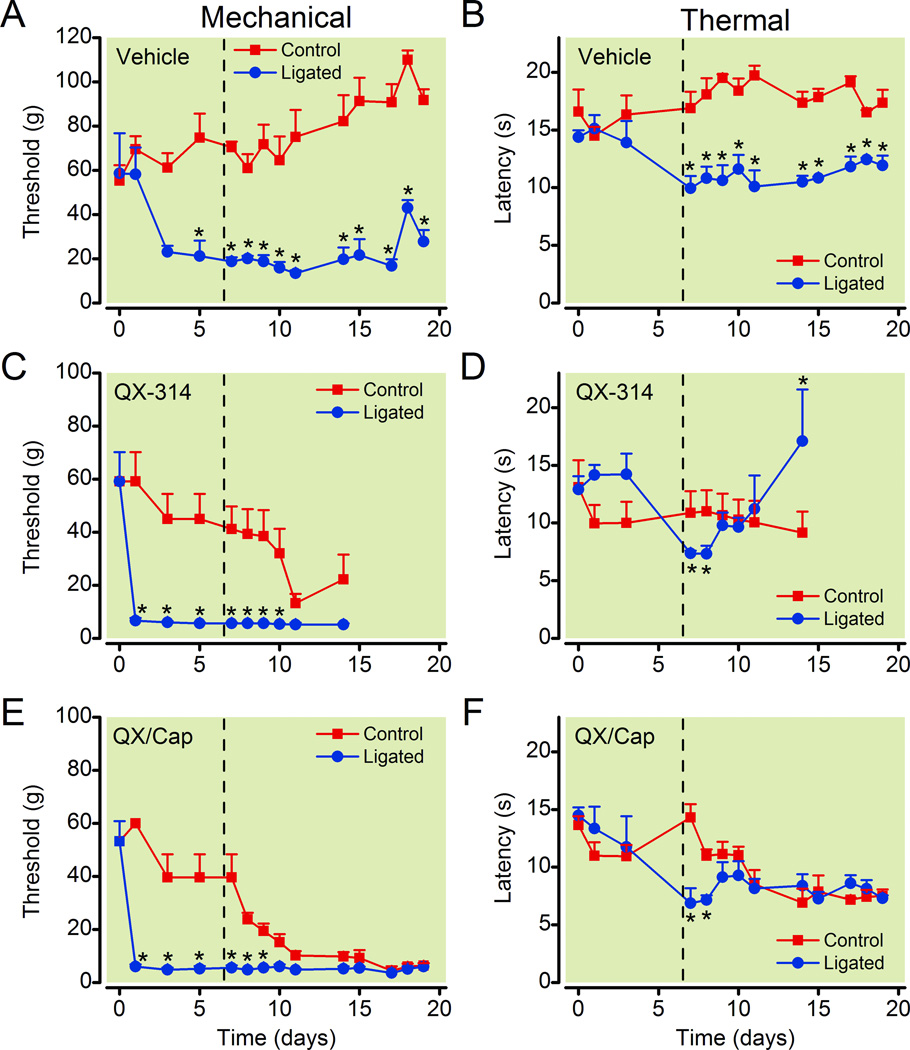
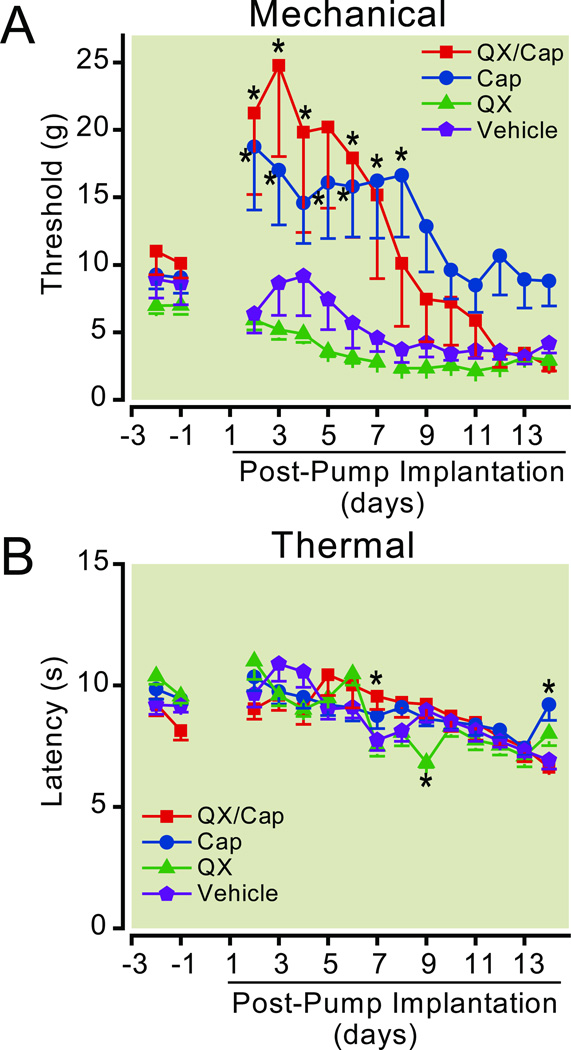
The central administration of the QX-314/capsaicin combination was less effective than peripheral injection at reducing pain-like behaviors for the ligated leg of neuropathic rats (fig. 4). Surprisingly, both the QX-314/capsaicin combination and QX-314 alone were capable of producing statistically significant decreases in the mechanical and/or thermal thresholds of the control leg contralateral to the ligation suggesting that they caused pain-like behaviors (fig. 4, two-way ANOVA with repeated measures for the interaction between drug and time, p ≤ 0.001 for mechanical and thermal, n = 5 or 6). Post hoc analysis indicated that, the mechanical threshold and thermal latency decreased for the paws ipsilateral to the ligation after the nerve ligation on day 0, whereas they remained the same or increased slightly for the contralateral paws (fig. 4). Compared to the control (unligated) paw, the decrease in the withdrawal thresholds of the ligated paw reached statistically significant levels 1 to 5 days after the surgery for mechanical stimuli and 7 days after the surgery for thermal stimuli. The mechanical allodynia and thermal hyperalgesia typically persisted for at least 1 month (data not shown). It is interesting to note that the baseline for the mechanical withdrawal was much higher for this group of animals than we typically observe before any surgeries or drug treatments. However, the mechanical allodynia and thermal hyperalgesia developed normally, so we proceeded with the drug treatment. The QX-314/capsaicin combination or QX-314 were administered by replacing the old pumps with new ones containing the drugs 7 days after the nerve ligation. The pumps were replaced after testing the withdrawal thresholds and confirming that the rats exhibited mechanical allodynia and thermal hyperalgesia.
Figure 4.
Neuropathic pain is not altered by the intrathecal application of the QX-314/capsaicin combination. After ligating the right sciatic nerve, the withdrawal thresholds to mechanical (A, C, and E) and thermal (B, D, and F) stimuli decreased for the side ipsilateral to the nerve ligation (Ligated). The thresholds were not reduced for the paw contralateral to the ligation (Control, n = 5). Typically, this hyperalgesia developed within 4 to 10 days after the surgery. For the paw ipsilateral to the nerve ligation, the QX-314/capsaicin combination (0.2 % QX-314 + 0.5 mg/ml capsaicin, n = 5) did not change the withdrawal threshold to mechanical or thermal stimuli. Surprisingly, the QX-314/capsaicin combination decreased the mechanical threshold of the control (nonligated) leg. QX-314 (0.2 %, n = 6) alone also reduced the mechanical threshold of the control leg without relieving the mechanical allodynia. However, QX-314 did partially reverse the thermal hyperalgesia for the ipsilateral foot. The vehicle had no effect on the mechanical or thermal thresholds for the ligated or control paws. Insertion of osmotic pumps containing drugs is indicated by the dashed line. Asterisks indicate that p < 0.05 compared to the control (unligated) paw. Cap = capsaicin; QX = QX-314.
Unlike the peripheral injections, we found that the central administration of the QX-314/capsaicin combination did not change the mechanical threshold for the ligated leg (fig. 4). Instead, both the QX-314/capsaicin combination and QX-314 alone decreased the mechanical threshold of the control leg contralateral to the ligation (fig. 4). Notice that before the drug application, the withdrawal threshold for mechanical stimuli was lower for the paw ipsilateral to the nerve ligation compared to the contralateral paw. After the administration of the QX-314/capsaicin combination and QX-314 alone, the withdrawal threshold for the control leg decreased until it became indistinguishable from the ligated leg. The QX-314/capsaicin combination had similar effects on the thermal latency. The administration of the QX-314/capsaicin combination had no effect on the thermal latency of the ligated leg, whereas it decreased the latency of the control leg (fig. 4). In contrast to the QX-314/capsaicin combination, QX-314 alone appeared to have an analgesic effect restoring the thermal latency for the paw ipsilateral to the ligation to preligation levels (fig. 4). However, the increase in the thermal latency was also accompanied by severe behavioral side effects as described below. In some cases the behavior was so severe that the animals could not be safely handled and they were euthanized. Data collection for QX-314 treated rats was terminated early because the withdrawal thresholds could not be measured for 3 of the animals after day 14. The vehicle did not have any obvious effects on the withdrawal thresholds for mechanical and thermal stimuli (fig. 4).
All of the animals treated with 0.2 % QX-314 alone (6/6) exhibited abnormal behaviors including agitation, vigorous vocalization, running, circling, rolling and jumping. Rats treated with the QX-314/capsaicin combination also exhibited some agitated behavior, but it was less frequent and less severe. Intrathecal administration of higher concentrations of QX-314 (0.4 %) elicited more severe pain-like behaviors that were not ameliorated by capsaicin. These results suggested that either the intrathecal administration of QX-314 directly induced pain-like behaviors or that it amplified the pain produced by the ligation.
Central administration of QX-314 causes a pain-like behavior in normal rats
In order to determine if QX-314 directly caused pain we implanted intrathecal catheters and osmotic pumps in rats without nerve ligations. Pumps were filled with either the vehicle, QX-314, capsaicin, or the QX-314/capsaicin combination and the behavior of the animals was monitored for the next 2 weeks. In these experiments, no statistically significant differences were detected in the thermal latency between the right and left legs (two-way ANOVA with repeated measures for differences in the means for different sides; p = 0.692 for vehicle, n = 8; p = 0.440 for capsaicin, n = 8; p = 0.613 for QX-314, n = 8; p = 0.789 for the QX-314/capsaicin combination, n = 7). For the mechanical threshold, a difference in the withdrawal responses of the right and left legs was detected only for capsaicin (two-way ANOVA with repeated measures for differences in the means for different sides; p = 0.342 for vehicle, n = 7; p = 0.043 for capsaicin, n = 8; p = 0.739 for QX-314, n = 8; p = 0.375 for the QX-314/capsaicin combination, n = 7). Post hoc analysis indicates that the withdrawal threshold for mechanical stimulation was different on two days when one rat had unusually high thresholds for the right paw. The reason for these high measurements is unknown. Except for this one rat, the similarity in the withdrawal responses for the right and left legs in these experiments suggested that both sides of the spinal cord were similarly affected by the drugs (data not shown).
Interestingly, we found capsaicin, either alone or in the QX-314/capsaicin combination, produced a prolonged increase in the withdrawal threshold to mechanical stimuli compared to the vehicle (two-way ANOVA with repeated measures for the interaction between drug and time, p ≤ 0.001 for mechanical and thermal, p ≤ 0.001, n = 7 or 8). Post hoc analysis revealed that the increase in the mechanical threshold reached a maximum within a few days after the pump was implanted and lasted for ~ 1 week (fig. 5). Intrathecal administration of QX-314 alone had little effect on the mechanical threshold. Data collection for two of the QX-314 treated rats ceased after postsurgery day 7 because the animals could not be safely handled. For this reason, statistical analyses were performed once for all of the groups from the beginning of the experiment to postsurgical day 7 and a second time, without the QX-314 treated group, for full duration of the experiment. None of the drugs produced lasting changes in the withdrawal threshold to thermal stimulation compared to the vehicle (fig. 5).
Figure 5.
Differential changes in the withdrawal thresholds of normal rats were produced by intrathecal QX-314, capsaicin, and the QX-314/capsaicin combination. A. Capsaicin alone and the QX-314/capsaicin combination produced statistically significant elevation of the withdrawal threshold for mechanical stimuli compared to the vehicle controls. QX-314 did not produce statistically significant changes in the mechanical withdrawal threshold. B. QX-314, capsaicin alone, and the QX-314/capsaicin combination had little effect on the withdrawal latency to thermal stimuli. Single asterisks indicate that p < 0.05 compared to the vehicle at the same time point. For mechanical stimulation: n = 7 for vehicle and the QX-314/capsaicin combination, n = 8 for QX-314 alone and capsaicin alone. For thermal stimulation: n = 7 for the QX-314/capsaicin combination, n = 8 for vehicle, QX-314 alone, and capsaicin alone. The break in the line connecting the QX-314 data points indicated that data could not be collected for some of the animals at later test sessions.
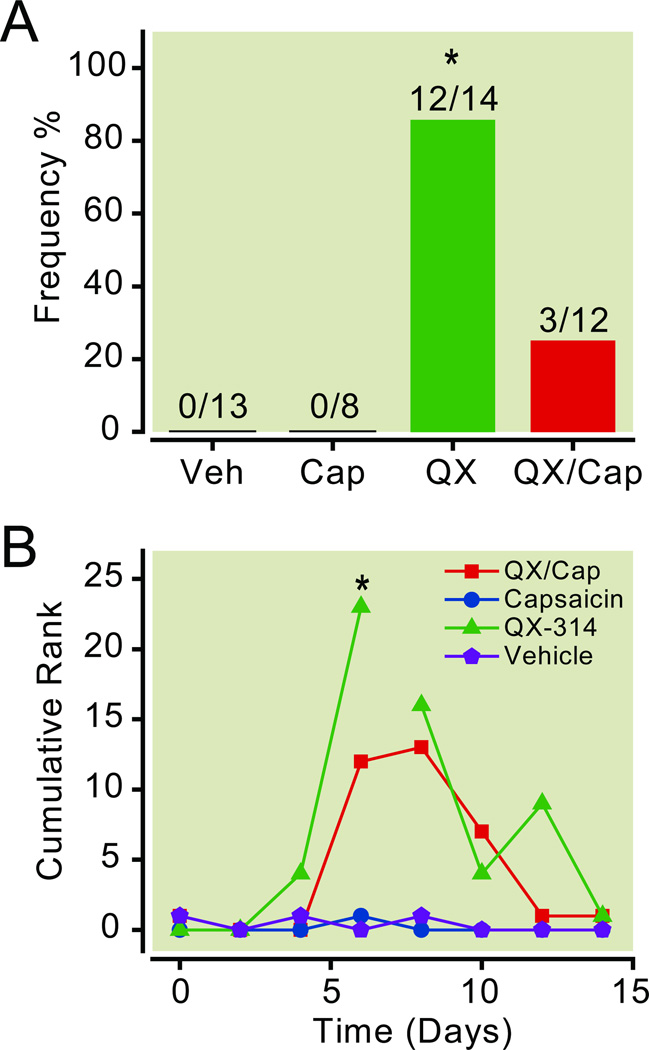
The behavior of the normal rats implanted with intrathecal catheters and osmotic pumps was also monitored and scored. Rats were acclimated to a behavior chamber and then watched for a 10-min period. We found that most of the rats administered QX-314 alone (12/14) exhibited abnormal behaviors such as restless, vocalization, running, circling, rolling, and jumping (fig. 6A). Only 25% of the rats (3/12) administered the QX-314/capsaicin combination had abnormal behaviors. Rats that received vehicle or capsaicin alone typically explored the chamber for the first few minutes and then spent the rest of the time alternating between bouts of grooming and bouts of inactivity (rest/sleep). None of the rats administered vehicle or capsaicin alone vocalized or exhibited circling behaviors (fig. 6A).
Figure 6.
Intrathecal QX-314 induced abnormal motor behaviors. A. Frequency of abnormal agitated behaviors after the intrathecal administration of QX-314, capsaicin, and the QX-314/capsaicin combination. Rats administered QX-314 alone or the QX-314/capsaicin combination were more likely to display agitated behaviors like vocalization, running, circling, and jumping. None of the rats treated with vehicle or capsaicin alone displayed abnormal behaviors. Single asterisks indicate that p < 0.05 (Kruskal-Wallis one-way ANOVA on ranks followed by post hoc comparisons using Dunn’s Method). B. Time course of the agitated behaviors after intrathecal administration of the drugs. The rats were observed and the behavioral responses were ranked for a 10-min period. The scores were recorded for every minute of observation and summed at the end of the experiment. Notice that the cumulative behavioral scores were higher for QX-314 (n = 9) and the QX-314/capsaicin combination (n = 7) groups, whereas they were unchanged for the capsaicin (n = 8) and vehicle (n = 8) groups. Single asterisks indicate that p < 0.05 compared to the baseline before the injection and compared to the vehicle control. The break in the line connecting the QX-314 data points indicated that data could not be collected for some of the animals at later test sessions. Cap = capsaicin; Lido = lidocaine; QX = QX-314; Veh = vehicle.
The behavior of rats receiving vehicle, QX-314, capsaicin, or the QX-314/capsaicin combination was quantified by ranking the behavior for each minute of the 10 min observation period. The behavioral score was based on a graded scale from 0 – 4 with 0 indicating no sign of excitation and 4 representing the most severe behavior (see Methods). The scores for each minute of the observation period were summed at the end of the observation period to generate the cumulative ranks time course shown in figure 6B. We found that the intrathecal administration of QX-314 alone was associated with a statistically significant increase in agitated behaviors (two-way ANOVA with repeated measures for the interaction between drug and time, p = 0.03, n = 9). The abnormal behavior began ~4–5 days after the implantation of the pumps (fig. 6B). Interestingly, the number of rats that had the agitated behavior and the severity of the behavior was lower for the QX-314/capsaicin combination group suggesting that capsaicin can protect rats from this side-effect of QX-314 (fig. 6). For the behavioral time course, severe behavior prevented us from observing the behavior for one rat after the peak on day 6 and a second rat after day 8. Notice that the behavioral scores were still elevated compared to controls after removing the two most extreme rats. The QX-314-induced behaviors appeared to be reversible because the agitated behavior ceased by the end of the experiment when the pumps were depleted of drugs (data not shown).
Effects of QX-314 on spinal cord morphology and immunohistochemistry
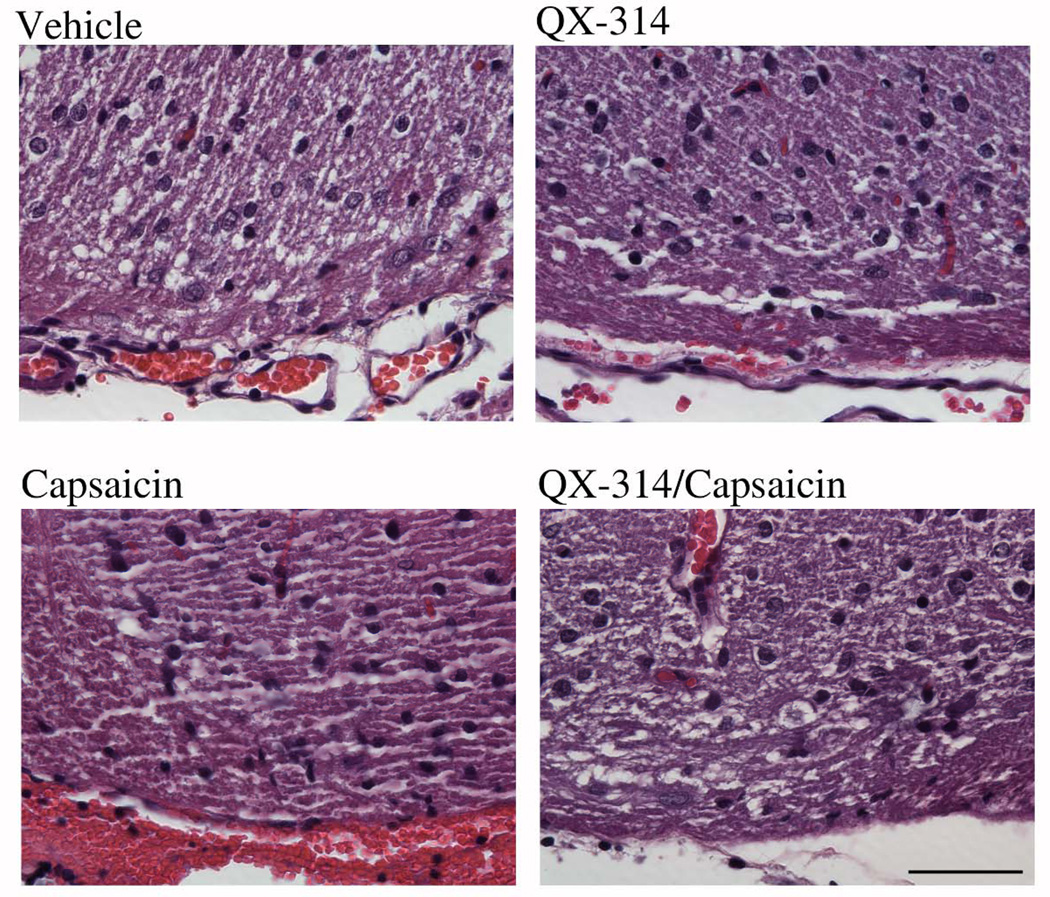
Local anesthetics can be toxic to central neurons and produce lesions of the spinal cord.44–48 Although our study was designed to use low, and presumably safe, concentrations of QX-314 (0.2 %), it is possible that the long-term exposure to QX-314 produced spinal cord lesions that caused the pain-like behaviors. In order to investigate this possibility, we examined spinal cord morphology after finishing the behavioral experiments. First a laminectomy of the lumbar region was performed to determine the location of the tip of the catheter. In all of the animals, the catheter tip was found to be located near the dorsal surface of the spinal cord near the lumbar enlargement. A small segment of the spinal cord near the opening of the catheter was removed, fixed and processed for histology. No obvious lesions were detected in the spinal cord sections from vehicle, QX-314, capsaicin, or the QX-314/capsaicin combination treated animals (fig. 7). This suggests that the behavioral changes were not caused by overt lesions of the spinal cord.
Figure 7.
Intrathecal QX-314 did not produce overt lesions of the spinal cord. A small segment of the spinal cord near the opening of the catheter was removed, fixed and processed for histology. No obvious lesions were detected in the transverse sections of the spinal cord from animals treated with vehicle, QX-314, capsaicin, or the QX-314/capsaicin combination. Scale bar = 50 µm.
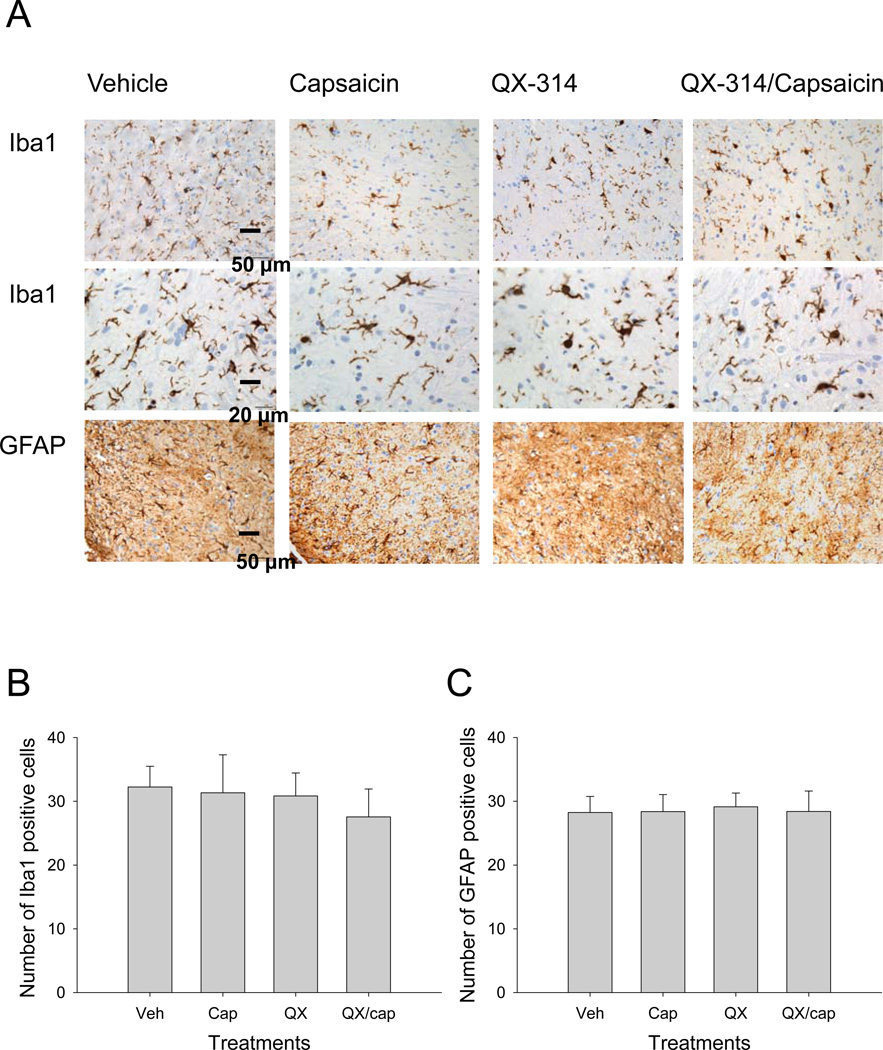
The potential toxic effects of QX-314 were examined further by immunohistochemical staining for two proteins that are considered sensitive markers of neurotoxicity: ionized calcium-binding adapter-1 (Iba1), a cytosolic calcium biding protein expressed in microglia, and glial fibrillary acidic protein, an astrocyte specific marker. The sections used to label microglia and astrocytes were prepared from the same spinal cord samples used to examine the morphology of the dorsal horn. We did not find any obvious differences in ionized calcium-binding adapter-1 or glial fibrillary acidic protein expression pattern in the spinal cord dorsal horn between vehicle, QX-314, capsaicin, or the QX-314 combination treated animals (fig. 8). In addition, there was no evidence of neuroinflammation near the infusion site for the drugs. We counted the number of microglia and astrocytes in the dorsal horn and examined the morphology of the cell bodies and processes. Based on the cell counts and the morphology, we did not detect a statistically significant increase in the activation of microglia or astrocytes in the spinal cord (Kruskal-Wallis one-way ANOVA. p = 0.117 for microglia, n = 4; p = 0.913 for astrocytes, n = 4). However, it should be noted that the effects of the drugs on myelination were not examined and a definitive comment about the toxicity of the drugs cannot be made at this time.
Figure 8.
Intrathecal QX-314 did not produce increased activation of microglia and astrocytes. Antibodies directed against ionized calcium-binding adapter-1 (Iba1) and glial fibrillary acidic protein (GFAP) were used to label microglia and astrocytes in samples from the same segments of the spinal cord used for histology staining. No noticeable differences were detected in ionized calcium-binding adapter-1 or glial fibrillary acidic protein expression patterns in the spinal cord dorsal horn between vehicle, QX-314, capsaicin, or the QX-314/capsaicin combination treated animals. No evidence of increased activation of microglia or astrocytes was detected based on the morphology of the cell bodies and processes (A) and the numbers of microglia (B) and astrocytes (C) counted per microscopic field (at 40X) within the dorsal horn of the spinal cords (Kruskal-Wallis one-way ANOVA. p = 0.117 for microglia, n = 4; p = 0.913 for astrocytes, n = 4). Cap = capsaicin; QX = QX-314; Veh = vehicle.
Discussion
We aimed to investigate whether selectively silencing TRPV1-expressing nociceptors with the QX-314/capsaicin combination would reduce pain-like behaviors in a rat model for neuropathic pain. Surprisingly, we found differential effects for peripheral versus neuraxial co-administration of QX-314 with capsaicin. Perisciatic nerve application produced an effective analgesia to noxious mechanical stimuli, whereas intrathecal administration of the drug combination caused extreme behaviors that resembled spontaneous pain-like behaviors. In addition, our results suggested that extracellular QX-314 was capable of modulating behavior in contradiction to the current belief that QX-314 is impermeable to cell membranes and that it has little or no effect when applied extracellularly at low concentrations.13,15,31,49
Nociceptive inputs and neuropathic pain
Typically nociceptors are polymodal in that they are activated by a wide range of noxious stimuli.50,51 Two reasons for the polymodal activation of nociceptors are: individual transducer channels are activated by several different noxious stimuli and individual nociceptors can express multiple transducer channels. For example, the transducer channel TRPV1, expressed by ~50% of the nociceptors, is activated by capsaicin, heat, acidity, and lipids.19,25,52 TRPV1-expressing nociceptors can also express TRPV2, TRPV3, TRPV4, and TRPA1.51,53–55 This diversity in transducer channel expression and activation has contributed to the often contradictory roles proposed for TRPV1 channels in acute and neuropathic pain. Although some studies have shown that capsaicin-sensitive nociceptors are activated by both noxious mechanical and thermal stimuli in normal rats,13,39,56 there are several conflicting studies indicating that noxious heat and touch are transduced by different pathways and that the TRPV1-expressing nociceptors respond only to noxious thermal stimuli.57–61
In contrast to previous studies, we found that blocking the TRPV1-expressing neurons in normal animals with perisciatic injection of the QX-314/capsaicin combination increased the withdrawal threshold for mechanical stimulation without changing the threshold for thermal stimulation. This data supports the theory that noxious mechanical and thermal stimuli are transduced by different pathways in normal animals. However, unlike a majority of the previous studies, our data suggest that TRPV1-expressing nociceptors are activated by mechanical stimuli. It is important to note that the method used in this study was different from most other studies. Typically, TRPV1-receptor agonists such as capsaicin or resiniferatoxin were used to desensitize or ablate TRPV1-expressing nociceptors. A disadvantage of this technique is that capsaicin analogs initially activate TRPV1-receptors stimulating action potentials and the release of neurotransmitters from nociceptors with dramatic short- and long-term effects on sensory signaling pathways. Instead, our approach was designed to minimize nociceptor activation and neurotransmitter release by using a low dose of capsaicin that did not evoke pain-like behaviors. In addition, when administering the drug combination, QX-314 was applied before capsaicin to minimize the activation of TRPV1 receptors and maximize the antinociceptive effects of the drug combination.13,14
This study suggests that the role of TRPV1 receptors in nociception was modulated by CCI to the sciatic nerve. In normal animals, only the sensitivity to mechanical stimuli was changed by perisciatic injection of the QX-314/capsaicin combination. However, in neuropathic animals the drug combination caused statistically significant increases in the withdrawal thresholds for both mechanical and thermal stimulation. These results indicate that both noxious heat and touch are transduced by TRPV1-expressing neurons in neuropathic rats. The desensitization and/or ablation of TRPV1-expressing neurons with the capsaicin analogue resiniferatoxin produced similar results in animals with CCIs. Systemic administration or plantar injection of multiple doses of resiniferatoxin reduced withdrawal thresholds for both mechanical and thermal stimulation in neuropathic rats.62,63 It has been shown that constriction or transection of the sciatic nerve leads to the ectopic expression of TRPV1 ion channels in non-nociceptive A-fibers22,23,64 and there is also sprouting of the central terminals of A-fibers from deep spinal cord laminae involved in the processing of nonnoxious proprioception to superficial laminae involved in processing noxious nociceptive stimuli.65–68 Both the increased expression of the TRPV1 ion channels and the sprouting of nonnociceptive A-fibers could contribute to the increased effectiveness of the QX-314/capsaicin combination in neuropathic rats.
Permeation of QX-314
Our results suggest that QX-314 is capable of modulating behavior through mechanisms that are independent of TRPV1 channel activation. Lidocaine and its derivatives are believed to block the voltage-gated sodium channels by binding to their intracellular domains.29,30 Therefore, charged derivatives of lidocaine like QX-314 that are impermeable to cell membrane should theoretically have little or no effect on the neuronal excitability when applied extracellularly. Consistent with this theory, the extracellular application of QX-314 does not inhibit action potentials in cultured dorsal root ganglion neurons13 or the squid giant axon.31 However, we have clearly shown that in an intact animal, the extracellular administration of QX-314 caused abnormal behaviors. This result is consistent with the observations that QX-314 alone produced long lasting local anesthesia at similar concentrations to lidocaine,16,49,69–71 and reduced the hyperexcitability of dorsal root ganglion neurons in two different pain models.72,73 Therefore, QX-314 is either capable of acting at extracellular sites or entering cells by mechanisms that are independent of TRPV1 channel activation.
Selectivity of the QX-314/capsaicin combination
The selectivity of the QX-314/capsaicin combination depends on how it is administered. For peripheral injections, the predominant effect of the QX-314/capsaicin combination was to inhibit the conduction of action potentials in TRPV1-expressing neurons and selectively block the transmission of nociceptive information to the central nervous system.13–16 We did not detect any adverse behaviors after the perisciatic administration of QX-314 alone or the QX-314/capsaicin combination. In contrast, the intrathecal administration of QX-314 severely altered the behavior of the rats. Long-term exposure to QX-314 is not necessary for producing the agitated behaviors because a single bolus injection of QX-314 into the lumbar intrathecal space produces similar behaviors in mice.48 Interestingly, injection of QX-314 into the rostral ventromedial medulla reversed the tactile and thermal hyperexcitability caused by nerve injury without producing any agitated behaviors.70 Therefore, the spinal cord neural networks appear to be important for generating the pain-like behaviors caused by QX-314.
The mechanisms underlying the agitated behaviors induced by QX-314 are currently unknown. One possible explanation is that QX-314 is toxic to the spinal cord because bolus intrathecal injections of QX-314 rapidly induce severe signs of irritation and even cause death in mice.48 The dose of QX-314 used in our study was 200 to 10,000 fold lower than the bolus injections that produced acute irritation in mice (adjusting for body weight). However, it is possible that the continuous infusion of low doses of QX-314 caused toxic effects with a delayed onset. The time course of the agitated behavior after implanting pumps containing QX-314 was consistent with delayed toxicity. In order to directly access the toxic effects of long-term infusion of 0.2 % QX-314 we examined the morphology of the dorsal horn near the infusion site using hematoxylin and eosin stained sections. We further tested for markers indicative of neuroinflammation such as astrocyte or microglial activation that have been implicated in the development of acute and chronic pain in animal models.74,75 No overt lesions or signs of increased neuroinflammation were detected indicating that the 0.2 % QX-314 was not highly toxic inducing general cell death. Nevertheless it is important to remember that we did not examine myelination and glial activation was examined only at the end of the experiment. Therefore, a definitive conclusion about the toxicity of QX-314 cannot be made at this time.
It is also possible that the pain-like behaviors induced by QX-314 were caused by an imbalance between the excitatory and inhibitory inputs to spinal interneurons in the pain pathway.76,77 In addition to blocking voltage-gated sodium channels, QX-314 can inhibit potassium channels activated by γ-aminobutyric acid receptors,78–80 N-methyl-D-aspartate receptors,81,82 and nicotinic acetylcholine receptors.83,84 The neurons in the pain pathway use the gabaergic, glutamatergic, and cholinergic transmitter systems to process sensory information and the disruption of these systems has been linked to chronic neuropathic pain.18,85,86 Furthermore, QX-314 disrupts G protein-coupled receptor signaling.87–90 Considering the prevalence of G protein-coupled receptors in nociceptive pathways,91–93 it is also possible that the agitated behaviors were caused by the direct effects of QX-314 on G proteins. Alternatively, some lidocaine derivatives activate TRPV1 currents and potentiate the response of dorsal root ganglion neurons to capsaicin and heat.94,95 However, extracellular QX-314 does not activate TRPV1 ion channels and partially blocks capsaicin-evoked inward currents instead. It is unlikely that the activation of TRPV1 ion channels contributes to the QX-314-induced behavioral changes.
Here we have shown that the peripheral, but not central, application of the QX-314/capsaicin combination selectively inhibits nociceptors in naÔve and neuropathic rats. When applied intrathecally, QX-314 alone or the QX-314/capsaicin combination has “nonspecific” effects that cause abnormal pain-like behaviors in normal rats. The severity of these behaviors indicates that QX-314 is unsuitable as a spinal analgesic unless the causes of its side effects can be identified and controlled.
Summary statement.
Peripheral blockade of transient receptor potential vanilloid receptor 1-expressing nociceptive afferents by perisciatic injection of the QX-314/capsaicin combination reduced neuropathic allodynia and hyperalgesia. Central blockade by intrathecal injection invoked spontaneous pain-like behaviors in rats.
Final Boxed Summary Statement.
What we already know about this topic
Capsaicin, by opening channels in pain-related peripheral nerves, allows permanently charged local anesthetics, like the compound QX-314, to enter nerves and produce selective blockade of just those expressing this pain related channel
Whether coapplication of capsaicin and QX-314 could alter neuropathic pain is not known
What this article tells us that is new
In rats with sciatic nerve inflammation and hypersensitivity, perineural coapplication of capsaicin and QX-314 reduced both thermal and mechanical hypersensitivity. In contrast, spinal application resulted in spontaneous pain behaviors
This combination deserves further study for selective blockade in neuropathic pain
Acknowledgements
This work was supported by grants from the National Institute of Neurological Disorders and Stroke (Bethesda, Maryland; R01 NS052372) and internal grants to Jianguo Cheng from the Cleveland Clinic Institute of Anesthesiology (Cleveland, Ohio). Drs. Fox and Shen contributed equally to this investigation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Toth C, Lander J, Wiebe S. The prevalence and impact of chronic pain with neuropathic pain symptoms in the general population. Pain Med. 2009;10:918–929. doi: 10.1111/j.1526-4637.2009.00655.x. [DOI] [PubMed] [Google Scholar]
- 2.Jensen MP, Chodroff MJ, Dworkin RH. The impact of neuropathic pain on health-related quality of life: Review and implications. Neurology. 2007;68:1178–1182. doi: 10.1212/01.wnl.0000259085.61898.9e. [DOI] [PubMed] [Google Scholar]
- 3.O'Connor AB. Neuropathic pain: Quality-of-life impact, costs and cost effectiveness of therapy. Pharmacoeconomics. 2009;27:95–112. doi: 10.2165/00019053-200927020-00002. [DOI] [PubMed] [Google Scholar]
- 4.Baron R, Binder A, Wasner G. Neuropathic pain: Diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010;9:807–819. doi: 10.1016/S1474-4422(10)70143-5. [DOI] [PubMed] [Google Scholar]
- 5.Attal N, Cruccu G, Haanpää M, Hansson P, Jensen TS, Nurmikko T, Sampaio C, Sindrup S, Wiffen P. EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol. 2006;13:1153–1169. doi: 10.1111/j.1468-1331.2006.01511.x. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy JD. Neuropathic pain: Molecular complexity underlies continuing unmet medical need. J Med Chem. 2007;50:2547–2556. doi: 10.1021/jm061023c. [DOI] [PubMed] [Google Scholar]
- 7.O'Connor AB, Dworkin RH. Treatment of neuropathic pain: An overview of recent guidelines. Am J Med. 2009;122(10 Suppl):S22–S32. doi: 10.1016/j.amjmed.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 8.Santiago-Figueroa J, Kuffler DP. Reducing and eliminating neuropathic pain. P R Health Sci J. 2009;28:289–300. [PubMed] [Google Scholar]
- 9.Van Boxem K, Cheng J, Patijn J, van Kleef M, Lataster A, Mekhail N, Van Zundert J. Lumbosacral radicular pain. Pain Pract. 2010;10:339–358. doi: 10.1111/j.1533-2500.2010.00370.x. [DOI] [PubMed] [Google Scholar]
- 10.Pluijms W, Huygen F, Cheng J, Mekhail N, van Kleef M, Van Zundert J, van Dongen R. Evidence-based interventional pain medicine according to clinical diagnoses 18: Diabetic polyneuropathy. Pain Pract. 2011;11:191–198. doi: 10.1111/j.1533-2500.2010.00435.x. [DOI] [PubMed] [Google Scholar]
- 11.Mekhail NA, Cheng J, Narouze S, Kapural L, Mekhail MN, Deer T. Clinical applications of neurostimulation: Forty years later. Pain Pract. 2010;10:103–112. doi: 10.1111/j.1533-2500.2009.00341.x. [DOI] [PubMed] [Google Scholar]
- 12.Xiao L, Cheng J, Xiao D, Zhang D. Botulinum toxin decreases hyperalgesia and inhibits P2X3 receptor over-expression in sensory neurons induced by ventral root transection in rats. Pain Med. 2011;12:1385–1394. doi: 10.1111/j.1526-4637.2011.01182.x. [DOI] [PubMed] [Google Scholar]
- 13.Binshtok AM, Bean BP, Woolf CJ. Inhibition of nociceptors by TRPV1-mediated entry of impermeant sodium channel blockers. Nature. 2007;449:607–610. doi: 10.1038/nature06191. [DOI] [PubMed] [Google Scholar]
- 14.Gerner P, Binshtok AM, Wang CF, Hevelone ND, Bean BP, Woolf CJ, Wang GK. Capsaicin combined with local anesthetics preferentially prolongs sensory/nociceptive block in rat sciatic nerve. Anesthesiology. 2008;109:872–878. doi: 10.1097/ALN.0b013e31818958f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Binshtok AM, Gerner P, Oh SB, Puopolo M, Suzuki S, Roberson DP, Herbert T, Wang CF, Kim D, Chung G, Mitani AA, Wang GK, Bean BP, Woolf CJ. Coapplication of lidocaine and the permanently charged sodium channel blocker QX-314 produces a long-lasting nociceptive blockade in rodents. Anesthesiology. 2009;111:127–137. doi: 10.1097/ALN.0b013e3181a915e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ries CR, Pillai R, Chung CC, Wang JT, MacLeod BA, Schwarz SK. QX-314 produces long-lasting local anesthesia modulated by transient receptor potential vanilloid receptors in mice. Anesthesiology. 2009;111:122–126. doi: 10.1097/ALN.0b013e3181a9160e. [DOI] [PubMed] [Google Scholar]
- 17.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: A maladaptive response of the nervous system to damage. Ann Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Latremoliere A, Woolf CJ. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 20.Valtschanoff JG, Rustioni A, Guo A, Hwang SJ. Vanilloid receptor VR1 is both presynaptic and postsynaptic in the superficial laminae of the rat dorsal horn. J Comp Neurol. 2001;436:225–235. [PubMed] [Google Scholar]
- 21.Doly S, Fischer J, Conrath M. The vanilloid receptor-1 (TRPV1) is expressed in some rat dorsal horn NK1 cells. Brain Res. 2004;1004:203–227. doi: 10.1016/j.brainres.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 22.Wilson-Gerwing TD, Dmyterko MV, Zochodne DW, Johnston JM, Verge VM. Neurotrophin-3 suppresses thermal hyperalgesia associated with neuropathic pain and attenuates transient receptor potential vanilloid receptor-1 expression in adult sensory neurons. J Neurosci. 2005;25:758–767. doi: 10.1523/JNEUROSCI.3909-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanai Y, Nakazato E, Fujiuchi A, Hara T, Imai A. Involvement of an increased spinal TRPV1 sensitization through its up-regulation in mechanical allodynia of CCI rats. Neuropharmacology. 2005;49:977–984. doi: 10.1016/j.neuropharm.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 25.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sørgård M, Di Marzo V, Julius D, Högestätt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 26.Tominaga M, Tominaga T. Structure and function of TRPV1. Pflugers Arch. 2005;451:143–150. doi: 10.1007/s00424-005-1457-8. [DOI] [PubMed] [Google Scholar]
- 27.Meyers JR, MacDonald RB, Duggan A, Lenzi D, Standaert DG, Corwin JT, Corey DP. Lighting up the senses: FM1-43 loading of sensory cells through nonselective ion channels. J Neurosci. 2003;23:4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Myrdal SE, Steyger PS. TRPV1 regulators mediate gentamicin penetration of cultured kidney cells. Hear Res. 2005;204:170–182. doi: 10.1016/j.heares.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hille B. Local anesthetics: Hydrophilic and hydrophobic pathways for the drug-receptor reaction. J Gen Physiol. 1977;69:497–515. doi: 10.1085/jgp.69.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Butterworth JF, 4th, Strichartz GR. Molecular mechanisms of local anesthesia: A review. Anesthesiology. 1990;72:711–734. doi: 10.1097/00000542-199004000-00022. [DOI] [PubMed] [Google Scholar]
- 31.Frazier DT, Narahashi T, Yamada M. The site of action and active form of local anesthetics. II. Experiments with quaternary compounds. J Pharmacol Exp Ther. 1970;171:45–51. [PubMed] [Google Scholar]
- 32.Lavrov I, Cheng J. Methodological optimization of applying neuroactive agents for the study of locomotor-like activity in the mudpuppies (Necturus Maculatus) J Neurosc Methods. 2008;174:97–102. doi: 10.1016/j.jneumeth.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 34.Wall PD, Fitzgerald M. Effects of capsaicin applied locally to adult peripheral nerve. I. Physiology of peripheral nerve and spinal cord. Pain. 1981;11:363–377. doi: 10.1016/0304-3959(81)90636-9. [DOI] [PubMed] [Google Scholar]
- 35.Dickenson A, Hughes C, Rueff A, Dray A. A spinal mechanism of action is involved in the antinociception produced by the capsaicin analogue NE 19550 (olvanil) Pain. 1990;43:353–362. doi: 10.1016/0304-3959(90)90032-9. [DOI] [PubMed] [Google Scholar]
- 36.Shin HC, Park HJ, Raymond SA, Strichartz GR. Potentiation by capsaicin of lidocaine's tonic impulse block in isolated rat sciatic nerve. Neurosci Lett. 1994;174:14–16. doi: 10.1016/0304-3940(94)90107-4. [DOI] [PubMed] [Google Scholar]
- 37.Dundee JW, Isaac M, Clarke RS. Use of alcohol in anesthesia. Anesth Analg. 1969;48:665–669. [PubMed] [Google Scholar]
- 38.Fang Z, Gong D, Ionescu P, Laster MJ, Eger EI, 2nd, Kendig J. Maturation decreases ethanol minimum alveolar anesthetic concentration (MAC) more than desflurane MAC in rats. Anesth Analg. 1997;84:852–858. doi: 10.1097/00000539-199704000-00028. [DOI] [PubMed] [Google Scholar]
- 39.Petsche U, Fleischer E, Lembeck F, Handwerker HO. The effect of capsaicin application to a peripheral nerve on impulse conduction in functionally identified afferent nerve fibres. Brain Res. 1983;265:233–240. doi: 10.1016/0006-8993(83)90337-2. [DOI] [PubMed] [Google Scholar]
- 40.Trevisani M, Smart D, Gunthorpe MJ, Tognetto M, Barbieri M, Campi B, Amadesi S, Gray J, Jerman JC, Brough SJ, Owen D, Smith GD, Randall AD, Harrison S, Bianchi A, Davis JB, Geppetti P. Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nat Neurosci. 2002;5:546–551. doi: 10.1038/nn0602-852. [DOI] [PubMed] [Google Scholar]
- 41.Vetter I, Wyse BD, Roberts-Thomson SJ, Monteith GR, Cabot PJ. Mechanisms involved in potentiation of transient receptor potential vanilloid 1 responses by ethanol. Eur J Pain. 2008;12:441–454. doi: 10.1016/j.ejpain.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 42.Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): Relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- 43.Bridges D, Thompson SW, Rice AS. Mechanisms of neuropathic pain. Br J Anaesth. 2001;87:12–26. doi: 10.1093/bja/87.1.12. [DOI] [PubMed] [Google Scholar]
- 44.Li DF, Bahar M, Cole G, Rosen M. Neurological toxicity of the subarachnoid infusion of bupivacaine, lignocaine or 2-chloroprocaine in the rat. Br J Anaesth. 1985;57:424–429. doi: 10.1093/bja/57.4.424. [DOI] [PubMed] [Google Scholar]
- 45.Pollock JE. Neurotoxicity of intrathecal local anaesthetics and transient neurological symptoms. Best Pract Res Clin Anaesthesiol. 2003;17:471–484. doi: 10.1016/s1521-6896(02)00113-1. [DOI] [PubMed] [Google Scholar]
- 46.Takenami T, Yagishita S, Nara Y, Hoka S. Intrathecal mepivacaine and prilocaine are less neurotoxic than lidocaine in a rat intrathecal model. Reg Anesth Pain Med. 2004;29:446–453. doi: 10.1016/j.rapm.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 47.Kissin I. Vanilloid-induced conduction analgesia: Selective, dose-dependent, long-lasting, with a low level of potential neurotoxicity. Anesth Analg. 2008;107:271–281. doi: 10.1213/ane.0b013e318162cfa3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwarz SK, Cheung HM, Ries CR, Lee SM, Wang JT, MacLeod BA. Lumbar intrathecal administration of the quaternary lidocaine derivative, QX-314, produces irritation and death in mice. Anesthesiology. 2010;113:438–444. doi: 10.1097/ALN.0b013e3181dfd31b. [DOI] [PubMed] [Google Scholar]
- 49.Lim TK, Macleod BA, Ries CR, Schwarz SK. The quaternary lidocaine derivative, QX-314, produces long-lasting local anesthesia in animal models in vivo. Anesthesiology. 2007;107:305–311. doi: 10.1097/01.anes.0000270758.77314.b4. [DOI] [PubMed] [Google Scholar]
- 50.Patapoutian A, Tate S, Woolf CJ. Transient receptor potential channels: Targeting pain at the source. Nat Rev Drug Discov. 2009;8:55–68. doi: 10.1038/nrd2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: Endogenous capsaicin-like substances. Proc Natl Acad Sci U S A. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 54.Rutter AR, Ma QP, Leveridge M, Bonnert TP. Heteromerization and colocalization of TrpV1 and TrpV2 in mammalian cell lines and rat dorsal root ganglia. Neuroreport. 2005;16:1735–1739. doi: 10.1097/01.wnr.0000185958.03841.0f. [DOI] [PubMed] [Google Scholar]
- 55.Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with A-delta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- 56.Pini A, Baranowski R, Lynn B. Long-term reduction in the number of C-fibre nociceptors following capsaicin treatment of a cutaneous nerve in adult rats. Eur J Neurosci. 1990;2:89–97. doi: 10.1111/j.1460-9568.1990.tb00384.x. [DOI] [PubMed] [Google Scholar]
- 57.Yaksh TL, Farb DH, Leeman SE, Jessell TM. Intrathecal capsaicin depletes substance P in the rat spinal cord and produces prolonged thermal analgesia. Science. 1979;206:481–483. doi: 10.1126/science.228392. [DOI] [PubMed] [Google Scholar]
- 58.Fitzgerald M, Woolf CJ. The time course and specificity of the changes in the behavioural and dorsal horn cell responses to noxious stimuli following peripheral nerve capsaicin treatment in the rat. Neuroscience. 1982;7:2051–2056. doi: 10.1016/0306-4522(82)90119-1. [DOI] [PubMed] [Google Scholar]
- 59.Dray A, Bettaney J, Forster P. Capsaicin desensitization of peripheral nociceptive fibres does not impair sensitivity to other noxious stimuli. Neurosci Lett. 1989;99:50–54. doi: 10.1016/0304-3940(89)90263-2. [DOI] [PubMed] [Google Scholar]
- 60.Lawson JJ, McIlwrath SL, Woodbury CJ, Davis BM, Koerber HR. TRPV1 unlike TRPV2 is restricted to a subset of mechanically insensitive cutaneous nociceptors responding to heat. J Pain. 2008;9:298–308. doi: 10.1016/j.jpain.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci U S A. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Field MJ, Bramwell S, Hughes J, Singh L. Detection of static and dynamic components of mechanical allodynia in rat models of neuropathic pain: Are they signalled by distinct primary sensory neurones? Pain. 1999;83:303–311. doi: 10.1016/s0304-3959(99)00111-6. [DOI] [PubMed] [Google Scholar]
- 63.Yamamoto W, Sugiura A, Nakazato-Imasato E, Kita Y. Characterization of primary sensory neurons mediating static and dynamic allodynia in rat chronic constriction injury model. J Pharm Pharmacol. 2008;60:717–722. doi: 10.1211/jpp.60.6.0006. [DOI] [PubMed] [Google Scholar]
- 64.Hudson LJ, Bevan S, Wotherspoon G, Gentry C, Fox A, Winter J. VR1 protein expression increases in undamaged DRG neurons after partial nerve injury. Eur J Neurosci. 2001;13:2105–2114. doi: 10.1046/j.0953-816x.2001.01591.x. [DOI] [PubMed] [Google Scholar]
- 65.Woolf CJ, Shortland P, Coggeshall RE. Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature. 1992;355:75–78. doi: 10.1038/355075a0. [DOI] [PubMed] [Google Scholar]
- 66.Shortland P, Kinman E, Molander C. Sprouting of A-fibre primary afferents into lamina II in two rat models of neuropathic pain. Eur J Pain. 1997;1:215–227. doi: 10.1016/s1090-3801(97)90107-5. [DOI] [PubMed] [Google Scholar]
- 67.Nakamura S, Myers RR. Myelinated afferents sprout into lamina II of L3-5 dorsal horn following chronic constriction nerve injury in rats. Brain Res. 1999;818:285–290. doi: 10.1016/s0006-8993(98)01291-8. [DOI] [PubMed] [Google Scholar]
- 68.Hayashi M, Kamiya Y, Itoh H, Higashi T, Miyazaki T, Funakoshi K, Yamashita N, Goshima Y, Andoh T, Yamada Y, Goto T. Intrathecally administered Sema3A protein attenuates neuropathic pain behavior in rats with chronic constriction injury of the sciatic nerve. Neurosci Res. 2011;69:17–24. doi: 10.1016/j.neures.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 69.Jasmin L, Kohan L, Franssen M, Janni G, Goff JR. The cold plate as a test of nociceptive behaviors: Description and application to the study of chronic neuropathic and inflammatory pain models. Pain. 1998;75:367–382. doi: 10.1016/s0304-3959(98)00017-7. [DOI] [PubMed] [Google Scholar]
- 70.Chen Q, King T, Vanderah TW, Ossipov MH, Malan TP, Jr, Lai J, Porreca F. Differential blockade of nerve injury-induced thermal and tactile hypersensitivity by systemically administered brain-penetrating and peripherally restricted local anesthetics. J Pain. 2004;5:281–289. doi: 10.1016/j.jpain.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 71.Sagie I, Kohane DS. Prolonged sensory-selective nerve blockade. Proc Natl Acad Sci U S A. 2010;107:3740–3745. doi: 10.1073/pnas.0911542107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Omana-Zapata I, Khabbaz MA, Hunter JC, Bley KR. QX-314 inhibits ectopic nerve activity associated with neuropathic pain. Brain Res. 1997;771:228–237. doi: 10.1016/s0006-8993(97)00770-1. [DOI] [PubMed] [Google Scholar]
- 73.Kawamata M, Sugino S, Narimatsu E, Yamauchi M, Kiya T, Furuse S, Namiki A. Effects of systemic administration of lidocaine and QX-314 on hyperexcitability of spinal dorsal horn neurons after incision in the rat. Pain. 2006;122:68–80. doi: 10.1016/j.pain.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 74.Narita M, Yoshida T, Nakajima M, Narita M, Miyatake M, Takagi T, Yajima Y, Suzuki T. Direct evidence for spinal cord microglia in the development of a neuropathic pain-like state in mice. J Neurochemistry. 2006;97:1337–1348. doi: 10.1111/j.1471-4159.2006.03808.x. [DOI] [PubMed] [Google Scholar]
- 75.Romero-Sandoval A, Chai N, Nutile-McMenemy N, Deleo JA. A comparison of spinal Iba1 and GFAP expression in rodent models of acute and chronic pain. Brain Res. 2008;1219:116–126. doi: 10.1016/j.brainres.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wen ZH, Yang LC, Wang JJ, Chang YC, Hsing CH, Chen JY, Wong CS. Intrathecal pertussis toxin induces thermal hyperalgesia: Involvement of excitatory and inhibitory amino acids. Neuroscience. 2003;116:871–878. doi: 10.1016/s0306-4522(02)00758-3. [DOI] [PubMed] [Google Scholar]
- 77.Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR. Opioid-induced glial activation: Mechanisms of activation and implications for opioid analgesia, dependence, and reward. ScientificWorldJournal. 2007;7:98–111. doi: 10.1100/tsw.2007.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nathan T, Jensen MS, Lambert JD. The slow inhibitory postsynaptic potential in rat hippocampal CA1 neurones is blocked by intracellular injection of QX-314. Neurosci Lett. 1990;110:309–313. doi: 10.1016/0304-3940(90)90865-7. [DOI] [PubMed] [Google Scholar]
- 79.Andrade R. Blockade of neurotransmitter-activated K+ conductance by QX-314 in the rat hippocampus. Eur J Pharmacol. 1991;199:259–262. doi: 10.1016/0014-2999(91)90467-5. [DOI] [PubMed] [Google Scholar]
- 80.Olivéras JL, Montagne-Clavel J. The GABAA receptor antagonist picrotoxin induces a 'pain-like' behavior when administered into the thalamic reticular nucleus of the behaving rat: A possible model for 'central' pain? Neurosci Lett. 1994;179:21–24. doi: 10.1016/0304-3940(94)90925-3. [DOI] [PubMed] [Google Scholar]
- 81.Nishizawa N, Shirasaki T, Nakao S, Matsuda H, Shingu K. The inhibition of the N-methyl-D-aspartate receptor channel by local anesthetics in mouse CA1 pyramidal neurons. Anesth Analg. 2002;94:325–330. doi: 10.1097/00000539-200202000-00017. [DOI] [PubMed] [Google Scholar]
- 82.Hahnenkamp K, Durieux ME, Hahnenkamp A, Schauerte SK, Hoenemann CW, Vegh V, Theilmeier G, Hollmann MW. Local anaesthetics inhibit signalling of human NMDA receptors recombinantly expressed in Xenopus laevis oocytes: Role of protein kinase C. Br J Anaesth. 2006;96:77–87. doi: 10.1093/bja/aei271. [DOI] [PubMed] [Google Scholar]
- 83.Neher E, Steinbach JH. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Horn R, Brodwick MS, Dickey WD. Asymmetry of the acetylcholine channel revealed by quaternary anesthetics. Science. 1980;210:205–207. doi: 10.1126/science.6251552. [DOI] [PubMed] [Google Scholar]
- 85.Millan MJ. The induction of pain: An integrative review. Prog Neurobiol. 1999;57:1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- 86.Fürst S. Transmitters involved in antinociception in the spinal cord. Brain Res Bull Jan. 1999;48:129–141. doi: 10.1016/s0361-9230(98)00159-2. [DOI] [PubMed] [Google Scholar]
- 87.Hollmann MW, Wieczorek KS, Berger A, Durieux ME. Local anesthetic inhibition of G protein-coupled receptor signaling by interference with Galpha(q) protein function. Mol Pharmacol. 2001;59:294–301. doi: 10.1124/mol.59.2.294. [DOI] [PubMed] [Google Scholar]
- 88.Hollmann MW, McIntire WE, Garrison JC, Durieux ME. Inhibition of mammalian Gq protein function by local anesthetics. Anesthesiology. 2002;97:1451–1457. doi: 10.1097/00000542-200212000-00017. [DOI] [PubMed] [Google Scholar]
- 89.Hollmann MW, Herroeder S, Kurz KS, Hoenemann CW, Struemper D, Hahnenkamp K, Durieux ME. Time-dependent inhibition of G protein-coupled receptor signaling by local anesthetics. Anesthesiology. 2004;100:852–860. doi: 10.1097/00000542-200404000-00015. [DOI] [PubMed] [Google Scholar]
- 90.Benkwitz C, Garrison JC, Linden J, Durieux ME, Hollmann MW. Lidocaine enhances Galphai protein function. Anesthesiology. 2003;99:1093–1101. doi: 10.1097/00000542-200311000-00015. [DOI] [PubMed] [Google Scholar]
- 91.Okuse K. Pain signalling pathways: From cytokines to ion channels. Int J Biochem Cell Biol. 2007;39:490–496. doi: 10.1016/j.biocel.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 92.Goudet C, Magnaghi V, Landry M, Nagy F, Gereau RW, 4th, Pin JP. Metabotropic receptors for glutamate and GABA in pain. Brain Res Rev. 2009;60:43–56. doi: 10.1016/j.brainresrev.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 93.Seybold VS. The role of peptides in central sensitization. Handb Exp Pharmacol. 2009;194:451–491. doi: 10.1007/978-3-540-79090-7_13. [DOI] [PubMed] [Google Scholar]
- 94.Komai H, McDowell TS. Differential effects of bupivacaine and tetracaine on capsaicin-induced currents in dorsal root ganglion neurons. Neurosci Lett. 2005;380:21–25. doi: 10.1016/j.neulet.2005.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leffler A, Fischer MJ, Rehner D, Kienel S, Kistner K, Sauer SK, Gavva NR, Reeh PW, Nau C. The vanilloid receptor TRPV1 is activated and sensitized by local anesthetics in rodent sensory neurons. J Clin Invest. 2008;118:763–776. doi: 10.1172/JCI32751. [DOI] [PMC free article] [PubMed] [Google Scholar]