Abstract
The metallo-β-lactamase GIM-1 (German imipenemase) has been found so far only in clinical isolates of Pseudomonas aeruginosa from Germany. Here we report the detection of blaGIM-1 in a clinical strain of Serratia marcescens that was isolated from urine, blood, and wound samples over a period of 20 months. The strain was repeatedly isolated from one patient in two German hospitals and an outpatient department located in the region in which all previously described GIM-1-producing P. aeruginosa strains were identified.
TEXT
Within Serratia spp. belonging to the Enterobacteriaceae family, Serratia marcescens is the most commonly detected species associated with nosocomial infections of the respiratory tract, urinary tract, and bloodstream. Outbreaks of Serratia spp. were caused by contamination of medical products (intravenous fluids, catheters) and equipment (apparatuses), transmitted mainly by the clinical personnel (11).
Resistance to many β-lactams due to β-lactamase production in combination with resistance to various other antimicrobial agents has become a serious threat. In the last decade, various extended-spectrum β-lactamases (ESBLs) (TEM, SHV, CTX-M, GES, and BES types) and different carbapenemases (SME, KPC, OXA-48, VIM, and IMP types) were identified in S. marcescens (2, 6, 7, 10, 14, 15, 17, 19, 21). Here we report the isolation of multidrug-resistant GIM-1-producing S. marcescens from Germany.
A 53-year-old patient suffering from chronic renal insufficiency was hospitalized with urosepsis in January 2009. From blood culture and urine, an S. marcescens strain (MG2504) was isolated. An empirical antimicrobial therapy with imipenem was successful and was not modified upon receiving the microbiology results. Altogether, seven isolates (blood, n = 1; urine, n = 4; hypogastric wound, n = 2) that were clonally identical by XbaI macrorestriction followed by pulsed-field gel electrophoresis (PFGE) (data not shown) were collected from this patient within a period of 20 months from two hospitals and one outpatient department in which the patient was hospitalized. No S. marcescens isolate except the one from blood caused an infection, so further antibiotic treatment was not necessary. The three health care facilities are located within a distance of 25 km in the federal state of North Rhine-Westphalia, Germany.
The isolated strain MG2504 was identified as S. marcescens with the Vitek2 system (Vitek2 GN card; bioMérieux, Brussels, Belgium) and confirmed by using mass spectrometry (matrix-assisted laser desorption ionization–time of flight [MALDI-TOF]; Bruker, Bellerica, MA). Antimicrobial susceptibilities were determined according to the guidelines of the Clinical and Laboratory Standards Institute (5) using the Vitek2 AST-N118 and AST-N110 cards and by Etest (bioMérieux, Nuertingen, Germany). The strain S. marcescens MG2504 was resistant to various β-lactams. However, the strain was susceptible to aztreonam and cefepime (Table 1). The confirmatory tests for carbapenemases (modified Hodge test [8]) and metallo-β-lactamases (Etest-MBL; bioMérieux, Nuertingen, Germany) showed ambiguous results and could not clearly confirm the presence of a carbapenem-hydrolyzing enzyme. During an occurrence of S. marcescens strain MG2504 in 2009, we recovered 1,024 further nonduplicate S. marcescens isolates from different hospitals and outpatient departments in North Rhine-Westphalia. Altogether, 20 isolates (2.0%) were resistant to expanded-spectrum cephalosporins (ceftazidime, cefotaxime). In 2010, we recovered 998 isolates, with 59 (6%) isolates being resistant to ceftazidime and/or cefotaxime, and in 2011, the resistance rate increased to 10% (102 of 1,047 isolates). However, S. marcescens MG2504, isolated in 2009 and 2010, was the only strain exhibiting reduced susceptibility to carbapenems.
Table 1.
S. marcescens MG2504 MICs
| Antimicrobial agent | MIC (μg/ml) |
|---|---|
| Ampicillin | >32 |
| Ampicillin-sulbactam | >32 |
| Piperacillin-tazobactam | >128 |
| Cefepime | ≤1 |
| Cefotaxime | >64 |
| Ceftazidime | 32 |
| Aztreonam | ≤1 |
| Gentamicin | 4 |
| Amikacin | ≤2 |
| Ciprofloxacin | >32 |
| Trimethoprim-sulfamethoxazole | >320 |
| Fosfomycin | 64 |
| Tigecycline | 4 |
| Meropenem | 1 |
| Imipenem | 2 |
| Ertapenema | 1.5 |
Tested by Etest (bioMérieux, Nuertingen, Germany).
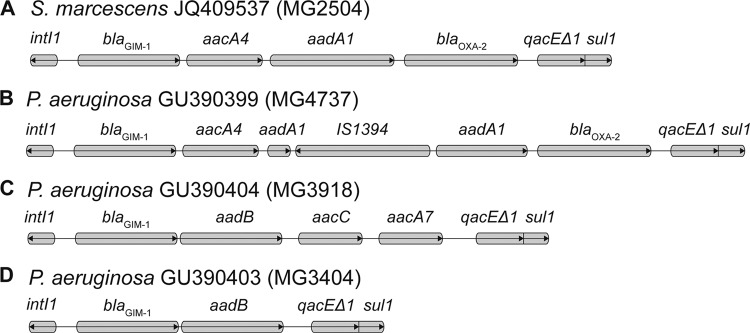
Molecular screening by PCR and sequencing was performed for different extended-spectrum β-lactamase (ESBL) genes (blaTEM, blaSHV, blaCTX-M, and blaBES) and carbapenemase genes (blaOXA-48, blaSME,blaGES, and blaKPC) as well as for the most-commonly detected metallo-β-lactamase (MBL) genes (blaIMP, blaVIM, and blaNDM) and for locally occurring types (blaGIM, blaSPM, and blaSIM) as described previously (2, 6, 9, 13, 14, 18). Using PCR and sequence analysis, we identified the MBL gene blaGIM-1 in S. marcescens MG2504. Detailed investigation of the blaGIM-1 genetic environment by PCR mapping (primer walking [4]) showed an integron structure comparable to structures that have been identified in blaGIM-harboring Pseudomonas aeruginosa isolates collected in 2002 and 2009 to 2010 in North Rhine-Westphalia, Germany (4, 20). In contrast to these P. aeruginosa integron structures, in S. marcescens, the aadA1 gene was not interrupted by a copy of the insertion sequence IS1394 (Fig. 1A and B).
Fig 1.
Comparison of blaGIM-1-containing integron structures in S. marcescens and P. aeruginosa. Genes are indicated by boxes. Arrows in the boxes show the direction of transcription.
S. marcescens isolates producing MBLs of the VIM and IMP types have been reported from South Korea, Japan, Taiwan, and Australia (10, 22, 12, 16). The GIM-1 enzyme that we found in S. marcescens MG2504 was described so far in only six clinical P. aeruginosa strains from a localized region in Germany (4, 20). Interestingly, in two hospitals in which the patient was treated, we identified two different multidrug-resistant P. aeruginosa strains harboring blaGIM-1. One P. aeruginosa strain was isolated 4 months before the first detection of the S. marcescens strain in another ward from a patient with colonization. The second P. aeruginosa isolate was recovered from lower-respiratory-tract specimens 9 months after detection of the S. marcescens strain in the same ward, isolated from a patient with pneumonia. However, in both P. aeruginosa isolates, blaGIM-1 was located within integron structures widely different from those of S. marcescens (Fig. 1C and D).
Transferability of the blaGIM-1 gene was tested by broth mating assays using sodium azide-resistant Escherichia coli K12J53 as the recipient. Selection of transconjugants was performed on Mueller-Hinton agar plates that contained sodium azide (200 mg/liter) and ampicillin (100 mg/liter) or meropenem (0.5 mg/liter). For determination of plasmid size, whole genomic DNA was digested with S1 nuclease and subjected to pulsed-field gel electrophoresis (PFGE) as described previously (1). Southern hybridization using digoxigenin-dUTP-labeled probes and signal detection using CDP-Star were performed following the manufacturer's guidelines (Roche Diagnostics Ltd., West Sussex, United Kingdom). The broth mate conjugation experiment in the present study was not successful, but we detected blaGIM-1 on a plasmid with a size of ca. 22 kb in the clinical S. marcescens MG2504 strain. Replicon typing for identification of the plasmid was performed as described previously (3). However, a replicon type could not be determined.
In the first GIM-1-positive P. aeruginosa isolate from 2002, blaGIM-1 was found to be located on a 22-kb plasmid (4). We speculate that a blaGIM-1-carrying plasmid was transferred between species, from P. aeruginosa to S. marcescens. Since the source of the blaGIM-1 gene and the blaGIM-1-carrying plasmid is still unknown, we assume that the spread in other Gram-negative species is possible and probably ongoing. A problem is the slightly increased MIC values for meropenem and imipenem, leading to ambiguous results of phenotypic carbapenemase tests, which substantially complicates the diagnostics of these strains in common microbiological laboratories without having the possibility of using PCR. Therefore, the prevalence of GIM-1-producing S. marcescens and P. aeruginosa and the presence of other GIM-1-possessing Gram-negative species in Germany are most probably underestimated.
The present multidrug-resistant isolate of S. marcescens (MG2504) contained the MBL gene blaGIM-1, previously described only in P. aeruginosa. This indicates the potential of transmission of blaGIM-1-carrying mobile genetic elements or plasmids between different Gram-negative species. Extensive resistance surveillance, including molecular epidemiological investigations, is needed to learn more about the emergence and dissemination of GIM-1-producing bacteria in Germany.
Nucleotide sequence accession number.
The nucleotide sequence of the S. marcescens MG2504 integron structure has been registered in the GenBank database under accession number JQ409537.
ACKNOWLEDGMENTS
This work was funded by the Ministry of Health, Germany.
We extend special thanks to George A. Jacoby for providing the E. coli J53 azide-resistant strain. We thank Sybille Mueller-Bertling for excellent technical support.
Footnotes
Published ahead of print 18 June 2012
REFERENCES
- 1. Barton BM, Harding GP, Zuccarelli AJ. 1995. A general method for detecting and sizing large plasmids. Anal. Biochem. 226:235–240 [DOI] [PubMed] [Google Scholar]
- 2. Bonnin RA, Poirel L, Sampaio JL, Nordmann P. 2012. Complete sequence of broad-host-range plasmid pRIO-5 harboring the extended-spectrum β-lactamase gene blaBES-1. Antimicrob. Agents Chemother. 56:1116–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carattoli A, et al. 2005. Identification of plasmids by PCR-based replicon typing. J. Microbiol. Methods 63:219–228 [DOI] [PubMed] [Google Scholar]
- 4. Castanheira M, Toleman MA, Jones RN, Schmidt FJ, Walsh TR. 2004. Molecular characterization of a β-lactamase gene, blaGIM-1, encoding a new subclass of metallo-β-lactamase. Antimicrob. Agents Chemother. 48:4654–4661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clinical and Laboratory Standards Institute 2012. Performance standards for antimicrobial susceptibility testing. CLSI M100-S22. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 6. de Vries JJ, et al. 2006. Outbreak of Serratia marcescens colonization and infection traced to a healthcare worker with long-term carriage on the hands. Infect. Control Hosp. Epidemiol. 27:1153–1158 [DOI] [PubMed] [Google Scholar]
- 7. Dhawan B, et al. 2003. Infection with an extended-spectrum β-lactamase-producing strain of Serratia marcescens following tongue reconstruction. J. Clin. Microbiol. 41:2233–2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Girlich D, Poirel L, Nordmann P. 2012. Value of the modified Hodge test for detection of emerging carbapenemases in Enterobacteriaceae. J. Clin. Microbiol. 50:477–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gröbner S, et al. 2009. Emergence of carbapenem-non-susceptible extended-spectrum β-lactamase-producing Klebsiella pneumoniae isolates at the university hospital of Tübingen, Germany. J. Med. Microbiol. 58:912–922 [DOI] [PubMed] [Google Scholar]
- 10. Ito H, et al. 1995. Plasmid-mediated dissemination of the metallo-β-lactamase gene blaIMP among clinically isolated strains of Serratia marcescens. Antimicrob. Agents Chemother. 39:824–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Janda JM, Abbott SL. 2005. The enterobacteria, 2nd ed ASM Press, Washington, DC [Google Scholar]
- 12. Lee MF, Peng CF, Hsu HJ, Chen YH. 2008. Molecular characterisation of the metallo-beta-lactamase genes in imipenem-resistant gram-negative bacteria from a university hospital in southern Taiwan. Int. J. Antimicrob. Agents 32:475–480 [DOI] [PubMed] [Google Scholar]
- 13. Mendes RE, et al. 2007. Rapid detection and identification of metallo-β-lactamase-encoding genes by multiplex real-time PCR assay and melt curve analysis. J. Clin. Microbiol. 45:544–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Naas T, Vandel L, Sougakoff W, Livermore DM, Nordmann P. 1994. Cloning and sequence analysis of the gene for a carbapenem-hydrolyzing class A β-lactamase, Sme-1, from Serratia marcescens S6. Antimicrob. Agents Chemother. 38:1262–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park YJ, et al. 2009. Spread of Serratia marcescens coharboring aac(6′)-Ib-cr, blaCTX-M, armA, and blaOXA-1 carried by conjugative IncL/M type plasmid in Korean hospitals. Microb. Drug Resist. 15:97–102 [DOI] [PubMed] [Google Scholar]
- 16. Peleg AY, Franklin C, Bell JM, Spelman DW. 2005. Dissemination of the metallo-β-lactamase gene blaIMP-4 among gram-negative pathogens in a clinical setting in Australia. Clin. Infect. Dis. 41:1549–1556 [DOI] [PubMed] [Google Scholar]
- 17. Perilli M, et al. 1997. Characterization of a new TEM-derived β-lactamase produced in a Serratia marcescens strain. Antimicrob. Agents Chemother. 41:2374–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pfeifer Y, et al. 2011. Molecular characterisation of blaNDM-1 in an Acinetobacter baumannii strain isolated in Germany in 2007. J. Antimicrob. Chemother. 66:1998–2001 [DOI] [PubMed] [Google Scholar]
- 19. Potron A, Poirel L, Bussy F, Nordmann P. 2011. Occurrence of the carbapenem-hydrolyzing β-lactamase gene blaOXA-48 in the environment in Morocco. Antimicrob. Agents Chemother. 55:5413–5414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rieber H, Frontzek A, von Baum H, Pfeifer Y. 2012. Emergence of metallo-β-lactamases GIM-1 and VIM in multidrug-resistant Pseudomonas aeruginosa in North Rhine-Westphalia, Germany. J. Antimicrob. Chemother. 67:1043–1045 [DOI] [PubMed] [Google Scholar]
- 21. Tsakris A, et al. 2010. In vivo acquisition of a plasmid-mediated blaKPC-2 gene among clonal isolates of Serratia marcescens. J. Clin. Microbiol. 48:2546–2549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yum JH, Yong D, Lee K, Kim H-S, Chong Y. 2002. A new integron carrying VIM-2 metallo-β-lactamase gene cassette in a Serratia marcescens isolate. Diagn. Microbiol. Infect. Dis. 42:217–219 [DOI] [PubMed] [Google Scholar]