Abstract
Bovicin HC5 is a lantibiotic produced by Streptococcus bovis HC5 that targets the cell wall precursor lipid II. An understanding of the modes of action against target bacteria can help broadening the clinical applicability of lantibiotics in human and veterinary medicine. In this study, the interaction of bovicin HC5 with lipid II was examined using tryptophan fluorescence and circular dichroism spectroscopy with model membrane systems that do or do not allow pore formation by bovicin HC5. In the presence of lipid II, a blue-shift of 12 nm could be observed for the fluorescence emission maximum of the tryptophan residue for all of the membrane systems tested. This change in fluorescence emission was paralleled by a decrease in accessibility toward acrylamide and phospholipids carrying a spin-label at the acyl chain; the tryptophan residue of bovicin HC5 was located near the twelfth position of the membrane phospholipid acyl chains. Moreover, the binding of lipid II by bovicin HC5 induced remarkable conformational changes in the bovicin HC5 structure. The interaction of bovicin HC5 with lipid II was highly stable even at pH 2.0. These results indicate that bovicin HC5 interacts directly with lipid II and that the topology of this interaction changes under different conditions, which is relevant for the biological activity of the peptide. To our knowledge, bovicin HC5 is the only bacteriocin described thus far that is able to interact with its target in extreme pH values, and this fact might be related to its unique structure and stability.
INTRODUCTION
Antimicrobial peptides are widespread in nature as a defensive mechanism of higher organisms (animals and plants) and single-cell prokaryotes. The ribosomally synthesized antimicrobial peptides produced by Bacteria and Archaea are named bacteriocins. The bacteriocin production confers ecological advantages in complex bacterial communities, allows better access to limited nutrients, and ensures the survival and perpetuation of the bacteriocin-producer cells (26). Bacteriocins can act in small concentrations and show bactericidal or bacteriostatic activity (9). They differ in biochemical properties, mechanisms of action, and spectrum of activity, being effective against food-borne pathogens, spoilage bacteria, as well as human and veterinary pathogens, which makes the bacteriocins potentially useful for food and clinical applications (6, 11).
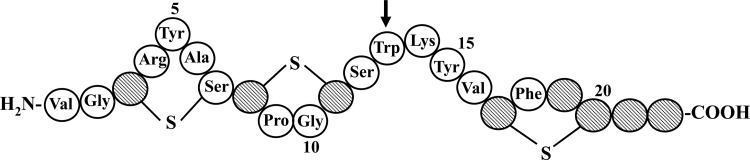
In previous work, we demonstrated the usefulness of bovicin HC5, a bacteriocin of Streptococcus bovis HC5, to manipulate ruminal fermentation and to control spoilage bacteria in fruit juices (7, 8, 23). Bovicin HC5 is a posttranslationally modified bacteriocin (Fig. 1) that shares some similarities with nisin, the most well-known lantibiotic. The mode of action of bovicin HC5 is based on the interaction with lipid II, the bacterial cell wall precursor. However, unlike nisin, the ability of bovicin HC5 to permeabilize thick lipid bilayers is limited, since its pore-forming activity is detected only in thinner membranes (29). Independent of the membrane thickness influence, bovicin HC5 is able to cluster lipid II into domains, with consequent inhibition of the bacterial cell wall biosynthesis (29).
Fig 1.
Primary structure of bovicin HC5. An arrow indicates the single tryptophan residue, used in the tryptophan spectroscopy experiments. The residues indicated by striped circles do not correspond to any of the 20 amino acids commonly found in proteins, and they represent putative posttranslational modified amino acid residues. The suggested positions of lanthionine linkages are indicated by the lines.
The biosynthesis of bacterial cell wall provides many targets for currently used antibiotics, such as natural and semisynthetic penicillins, bacitracin, and glycopeptide antibiotics, which interfere with different steps of this pathway (12, 33). Lipid II plays an essential role in peptidoglycan synthesis, since it is the carrier molecule responsible for transferring sugar-peptide subunits from the cytosolic side of the membrane to the peptidoglycan synthesis machinery outside the cell (14). In this sense, lipid II can be considered a potent target for therapeutic agents, since it is part of a specific pathway present only in bacterial cells and absent in eukaryotic organisms; moreover, modification of lipid II structure has been rarely reported (3).
It has been demonstrated that the conjugation of the lipid II binding moiety of antimicrobial agents with molecules that target the bacterial cell envelope could enhance the potency and specificity of such agents, which could be important for clinical applications. When temporin L, an antimicrobial peptide derived from the European red frog, was conjugated by click chemistry with vancomycin, a glycopeptide antibiotic known to act by binding to the C-terminal peptide of the lipid II, the pore-forming ability of the vancomycin-linked derivatives of temporin L increased >20-fold, as indicated by carboxyfluorescein leakage from mixed DOPC/DOPG vesicles containing lipid II (4). Therefore, understanding the mechanisms of interaction between antimicrobial peptides and membrane-bound lipid II could potentially lead to the development of new drugs that target this important bacterial cell molecule.
In order to broaden the range of suitable molecules used as models for lipid II binding and foster the development of more effective drugs, the interaction of antimicrobial peptides that target lipid II has been examined in detail (35, 37). In the present study, tryptophan fluorescence spectroscopy was used to characterize the interaction between bovicin HC5 and lipid II in liposomes that allow (DLPC/DMoPC vesicles) or do not allow pore formation (DOPC vesicles) by bovicin HC5. In addition, circular dichroism (CD) spectroscopy experiments provided insight into the conformational changes of bovicin HC5 in solution and upon binding to lipid II. These techniques allowed us to determine the factors affecting the interaction between bovicin HC5 and lipid II and evaluate changes in topology of bovicin HC5 upon binding to lipid II under different environmental conditions that are relevant for its activity in vivo.
We show that the peptide-ligand interaction and the consequent permeabilization of the membrane occurred independently of the membrane composition. Moreover, the lipid II binding resulted in substantial conformational changes on bovicin HC5 structure, and these changes were pH dependent. These results reveal that bovicin HC5 has unique stability and a distinct mechanism of lipid II binding. Because bovicin HC5 is highly active against bacterial pathogens (e.g., Staphylococcus aureus) and resistance does not seem to be easily developed by sensitive bacteria, bovicin HC5 could be an attractive candidate for clinical applications.
MATERIALS AND METHODS
Chemicals and materials.
The phospholipids 1,2-dioleoyl-sn-glycero-3-phosphocoline (C18:1; DOPC), 1,2-dioleoyl-sn-glycero-3-phosphoglycerol (DOPG), and 1,2-dimyristoleoyl-sn-glycero-3-phosphocoline (C14:1; DMoPC) and the spin-labeled phospholipids 1,2-dioleoyl-sn-glycero-3-TEMPO-phosphocoline (TEMPO-PC), 1-palmitoyl-2-stearoyl (5-DOXYL)-sn-glycero-3-phosphocoline (5DOX-PC), and 1-palmitoyl-2-stearoyl (12-DOXYL)-sn-glycero-3-phosphocoline (12DOX-PC) were purchased from Avanti-Polar Lipids, Inc. Lipid II and water-soluble lipid II (3-LII) were synthesized and purified as described previously (2). Phospholipids were dissolved in chloroform-methanol (1:1) to a stock concentration of 10 mM and stored at −20°C. After the destruction of phospholipids by perchloric acid, the sample concentrations were determined by inorganic phosphate analysis (32). All other chemicals used were of analytical or reagent grade.
Bovicin HC5 production and purification.
Streptococcus bovis HC5 was cultivated overnight at 39°C, under anaerobic conditions, in basal medium containing 0.292 g of K2HPO4, 0.292 g of KH2PO4, 0.48 g of (NH4)2SO4, 0.48 g of NaCl, 0.1 g of MgSO4·7H2O, 0.064 g of CaCl2·2H2O, 0.5 g of cysteine hydrochloride, 4 g of Na2CO3, 1 g of Trypticase, and 0.5 g of yeast extract per liter. Glucose was added as a carbon source (4 g/liter). Extracts of bovicin HC5 were prepared as previously described (27). Purification of bovicin HC5 was performed by reversed-phase high-pressure liquid chromatography (HPLC) using a semipreparative column (Shimadzu C18; 5 μm, 150 by 4.6 mm [inner diameter]). The column was equilibrated with buffer A (0.1% trifluoroacetic acid [TFA] in ultrapure water), and the peptide was eluted using a linear gradient of 35 to 50% buffer B (80% acetonitrile and 0.1% TFA in ultrapure water) at 22°C and a flow rate of 1 ml min−1. The absorbance was monitored at 214 and 280 nm, and the eluted fraction corresponding to pure bovicin HC5 was lyophilized. The purity of bovicin HC5 was confirmed by using analytical HPLC and electrospray mass spectrometry. Bovicin HC5 stock solution (1 mM in 0.05% acetic acid) was stored at −20°C until use. The protein concentration was determined using a bicinchoninic acid protein assay reagent (Pierce Chemical Corp., Bonn, Germany), with bovine serum albumin as a standard.
LUVs.
Large unilamellar vesicles (LUVs) containing DOPC, DOPC/3 mol% DOPG or DLPC/DMoPC (1:1 [mol/mol]), with or without lipid II, were prepared by the extrusion technique (18). Buffer containing 10 mM Tris and 150 mM NaCl (pH 7.5) or buffer containing 10 mM potassium phosphate and 40 mM potassium sulfate (pH 6.0) was used to hydrate the dried lipid films, depending on the experiment to be performed (tryptophan and CD spectroscopy, respectively) and followed by vigorous stirring. The LUVs were prepared by repeated extrusion through polycarbonate filters with a 0.2-μm pore size (Isopore membrane filters; Millipore, Ireland). Lipid and vesicle concentrations were based on inorganic phosphate determination (32). The final vesicle concentration used was 25 μM (final lipid-Pi), and the final concentration of lipid II varied according to the experiment performed (1 or 4 mol% of the total lipid amount for tryptophan or CD spectroscopy, respectively). Control experiments were performed using vesicles without lipid II but containing the same phospholipid composition.
Emission spectra and intensity measurements.
The fluorescence emission spectra of the bovicin HC5 tryptophan residue were determined on a SLM Aminco spectrofluorimeter (SPF-500C), with spectral recordings between 300 and 450 nm (bandwidth, 5 nm), as well as single-wavelength recordings at 340 nm, with excitation at 280 nm (bandwidth, 5 nm), in the absence or presence of vesicles (100 μM lipid-Pi). The measurements were performed after an incubation time of 5 min, and the spectral changes were followed for 6 min. The samples were continuously stirred in a 10 × 4 mm quartz cuvette and kept at 20°C, using a continuous circulation water bath. Bovicin HC5 was used at a concentration of 1 μM, and fluorescence spectra and single-wavelength recordings were corrected by blank subtraction. The bovicin HC5/lipid II ratio was 1:1.
Titration experiments using individual samples were performed to investigate the concentration dependency of the spectral changes observed in the fluorescence spectra of bovicin HC5. Fluorescence intensities were measured as a function of the amount of added vesicles and averaged over recording times of 20 s. The intensities and complete spectra were measured at 340 nm before and after the addition of various amounts of lipid II-containing membranes (0 to 200 μM vesicles, corresponding to 0 to 2 μM lipid II). The lipid II/bovicin HC5 ratios used for the individual samples varied from 0:1 to 4:1, and the experiments were performed in quadruplicate.
Acrylamide quenching.
Insertion of bovicin HC5 into lipid II-containing membranes was investigated with the water-soluble quencher acrylamide (10). Acrylamide quenching experiments were carried out at an excitation wavelength of 280 nm, recorded at 340 nm and averaged over measuring times of 20 s. Small aliquots from an aqueous 3 M acrylamide stock solution were added stepwise, and an equilibration time of 60 s was taken to allow homogeneous distribution of the acrylamide. Acrylamide titration of bovicin samples, in the absence or in the presence of lipid II-containing membranes, was started 5 min after the addition of the samples.
Titrations were performed in triplicate, and the data were analyzed according to the Stern-Volmer equation (10): F0/F = 1 + KSV × [Q], where F0 is the fluorescence in the absence, and F is the fluorescence in the presence of increasing quencher concentrations ([Q]). The Stern-Volmer quenching constant KSV is the product of the bimolecular quenching rate kq and the fluorescence lifetime τ0: KSV = kq × τ0. Both the Stern-Volmer constant and the bimolecular quenching rate can be used as a measure for the accessibility of the tryptophan residue to acrylamide. The KSV was calculated as the slope of the Stern-Volmer plot, which is linear for acrylamide concentrations up to 60 mM.
Spin-labeled lipid quenching.
The position of the tryptophan residue of bovicin HC5 in the lipid II-containing membranes was assessed by considering the quenching effect of three different spin-labeled phospholipids on the fluorescence of bovicin HC5 tryptophan residue. Single-wavelength recordings (340 nm) and spectral recordings (300 to 450 nm) were performed in the absence or presence of DOPC or DLPC/DMoPC vesicles containing 1 mol% of lipid II and an additional amount of 25 mol% of one of the spin-labeled phospholipids (TEMPO-PC, 5DOX-PC, or 12DOX-PC) (25). The change in fluorescence intensity after the addition of 100 μM lipid-Pi of the spin-labeled vesicles to a solution of 1 μM bovicin HC5 was compared to the fluorescence intensity change after the addition of the same amount of vesicles containing unlabeled phospholipids (F0). The data were analyzed as quenching efficiency (Qef) of each spin-labeled lipid, via the equation: Qef = (1 − Fh/F0) × 100, where Fh is the fluorescence in the presence of quencher at depth h and F0 is the fluorescence in the absence of quencher (21).
CD measurements.
The CD spectra of bovicin HC5 were recorded on a Jasco-810 CD spectrometer in a quartz cuvette of 1-mm light path. The temperature was kept at 20°C by a Jasco Peltier CDF 426S, and the samples were scanned in the wavelength range of 195 to 280 nm. The data were digitally collected every 0.2 nm at a scan speed of 20 nm/min, with a 1-s response time and a 2-mm bandwidth. The spectra were recorded at a bovicin HC5 concentration of 20 μM in 10 mM potassium phosphate–40 mM potassium sulfate buffer (different pH values) or a 0 to 30 μM lipid II final concentration or 0 to 700 μM vesicles without lipid II (concentrations corresponding to the amount of lipid II-containing vesicles used in the same experiments). Five recordings were averaged for each wavelength range, and the final CD spectrum was obtained by subtracting the scans of the buffer blank measured in the same cuvette used for the samples. The effect of lipid II on the structure of bovicin HC5 was determined by comparison of the bovicin HC5 individual spectrum obtained in buffer and the spectrum obtained in the presence of lipid II. The results were given in degrees cm−2 dmol−1.
RESULTS
Tryptophan fluorescence spectroscopy can be considered a valuable tool for studying the association of peptides with biological membranes (1). In the present study, in order to analyze the interaction of bovicin HC5 with model membranes containing lipid II, we measured its intrinsic tryptophan fluorescence before and after exposure to lipid II-containing vesicles with different phospholipid compositions. This approach would allow us to examine conditions where bovicin HC5 could form pores (DLPC/DMoPC vesicles, 1:1 [mol/mol]) and also study situations where pore formation has not been demonstrated (DOPC vesicles) (29).
Tryptophan fluorescence and emission spectra.
When bovicin HC5 was dissolved in buffer, the emission spectrum of the tryptophan showed a fluorescence emission maximum at 353 nm (Fig. 2, dotted line). Upon the addition of lipid II-containing vesicles, a blue-shift of the emission maximum of 12 nm (maximal emission at 341 nm) could be observed, which was paralleled by an increase in fluorescence intensity (2.45- and 2.12-fold in the presence of DOPC and DLPC/DMoPC vesicles, respectively) (Fig. 2, lines A and B). The two model membranes also showed a consistent difference in fluorescence intensity, in that the emission spectrum for the DLPC/DMoPC was less intense than that observed for DOPC vesicles. No effect was observed when the emission spectra were recorded in the presence of vesicles without lipid II or in the presence of negatively charged liposomes composed of DOPC and 3 mol% of DOPG (data not shown).
Fig 2.
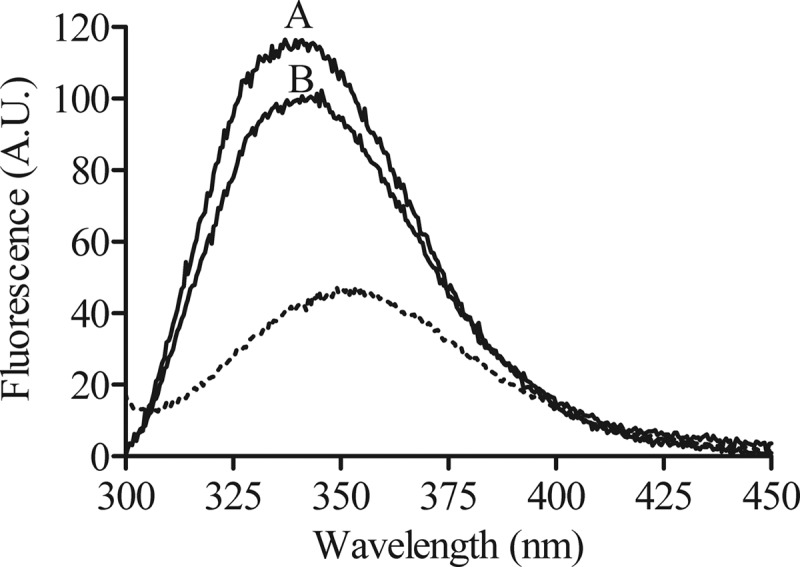
Fluorescence emission spectra of the tryptophan residue of bovicin HC5 in the absence or presence of model membranes. Spectra were recorded for 1 μM bovicin HC5 in the absence (dotted line) or presence of model membranes composed of DOPC (line A) or DLPC/DMoPC (line B) containing 1 mol% of lipid II. An excitation wavelength of 280 nm was applied, and emission was recorded at between 300 and 450 nm. Spectra were corrected by blank subtraction. The vesicle final concentration used was 100 μM vesicles (lipid-Pi). A.U., arbitrary units.
In order to investigate how the bovicin HC5 tryptophan fluorescence quantitatively depended on the presence of lipid II molecule, a solution of 1 μM bovicin HC5 was titrated into lipid II-containing vesicles. Increasing concentrations of lipid II-containing vesicles resulted in increased intensity of the tryptophan fluorescence, until the lipid II/bovicin HC5 ratio reached 0.5. After this point, the addition of lipid II-containing vesicles did not influence the tryptophan fluorescence (Fig. 3). Also in this experiment, a consistent difference in fluorescence intensity between the two vesicle systems was observed. No differences were detected when the titration was performed with vesicles that did not contain lipid II (data not shown).
Fig 3.
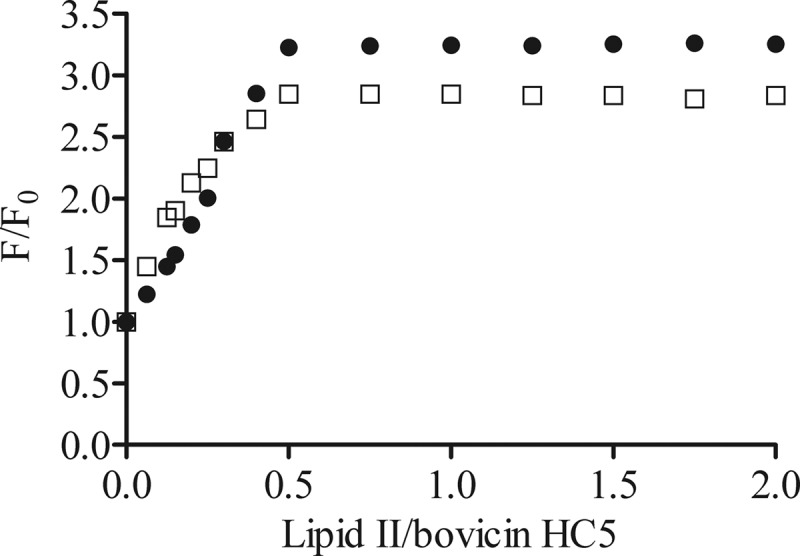
Lipid II/bovicin HC5 ratio-dependent change in the tryptophan fluorescence of bovicin HC5. Different amounts of DOPC (●) or DLPC/DMoPC (□) vesicles containing 1 mol% of lipid II were added to 1 μM bovicin HC5. Single-wavelength recordings were performed at 340 nm using an excitation wavelength of 280 nm. The fluorescence intensities before (F0) and after addition of lipid II-containing membranes (F) were used to calculate the F/F0 values, which were plotted against the lipid II/bovicin HC5 ratio.
Acrylamide quenching.
The blue-shifts of the tryptophan residue indicated that it was inserted into the lipid phase of DOPC and DLPC/DMoPC lipid II-containing vesicles. To assess this in a more direct manner, the accessibility of bovicin HC5 tryptophan to acrylamide was determined in the presence or absence of DOPC and DLPC/DMoPC vesicles, with or without lipid II. Acrylamide is a neutral water soluble quencher of the tryptophan fluorescence, and no charge interactions occur with the headgroup of negatively charged lipids.
In the absence of vesicles, the addition of acrylamide caused an efficient quenching of the bovicin HC5 tryptophan fluorescence, as demonstrated by an increase in the F0/F ratio (Fig. 4, filled triangles), indicating that the tryptophan residue is readily accessible when bovicin HC5 is in solution. In the presence of lipid II-containing membranes, protection of quenching by acrylamide was observed, and this reduction was the same, independent on the membrane composition (DOPC [Fig. 4, filled circles] or DLPC/DMoPC [Fig. 4, open squares]). Control experiments using vesicles composed of DOPC and 3% DOPG (without lipid II) revealed the same quenching effect as that observed in buffer (data not shown).
Fig 4.
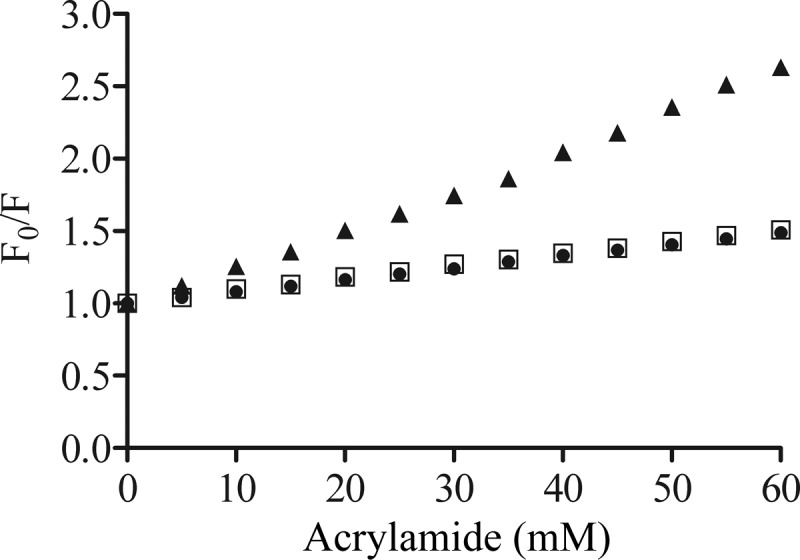
Stern-Volmer plot, showing the acrylamide quenching of the bovicin HC5 tryptophan residue measured for samples containing 1 μM bovicin HC5 in the absence (▲) or presence of DOPC (●) or DLPC/DMoPC (□) vesicles containing 1 mol% of lipid II (100 μM lipid-Pi). Single-wavelength recordings were performed at 340 nm using an excitation wavelength of 280 nm. F0, fluorescence measured in the absence of the quencher; F, fluorescence measured in the presence of the quencher.
The F0/F plots were used to calculate the Stern-Volmer quenching constant (Ksv), as described in Materials and Methods. When bovicin HC5 was in solution (the absence of vesicles), the Stern-Volmer quenching constant was 27.9 M−1, and in the presence of lipid II-containing membranes the Ksv values were significantly lower (8.2 and 8.4 M−1 for DOPC and DLPC/DMoPC vesicles, respectively). This result demonstrates that the tryptophan residue was significantly less accessible for acrylamide when lipid II was present and indicated that bovicin HC5 was inserted into the membrane in a lipid II-dependent way.
Spin-labeled lipid quenching.
Depth-dependent quenching of fluorescence by spin-labeled phospholipids was used in order to obtain more information about the depth of insertion of the tryptophan residue of bovicin HC5 in lipid II-containing membranes. In this method, quenching depends on the distance between the tryptophan residue and the spin-label and occurs over a very short distance. Spin-labeled phospholipids consisted of a covalently linked nitroxide group with an unpaired electron (spin-label) positioned either at the head group (TEMPO-PC) or attached to the hydrocarbon chain at C-atom 5 (5DOX-PC) or 12 (12DOX-PC).
In the presence of vesicles containing spin-labeled phospholipids, the fluorescence intensity of tryptophan residue became reduced compared to the fluorescence in the presence of vesicles of the same composition, but without spin-labeled lipids. In both model membrane systems evaluated, the tryptophan fluorescence intensity was more effectively quenched by 12DOX-PC, the deepest quencher tested here. However, in DOPC system (Fig. 5, black bars), the quenching efficiency was lower compared to the DLPC/DMoPC system (Fig. 5, white bars), although the relative differences were larger within the DOPC system, when the different spin-labeled phospholipids were compared. The presence of spin-labeled phospholipids in DOPC/DOPG vesicles did not affect the tryptophan fluorescence (data not shown).
Fig 5.
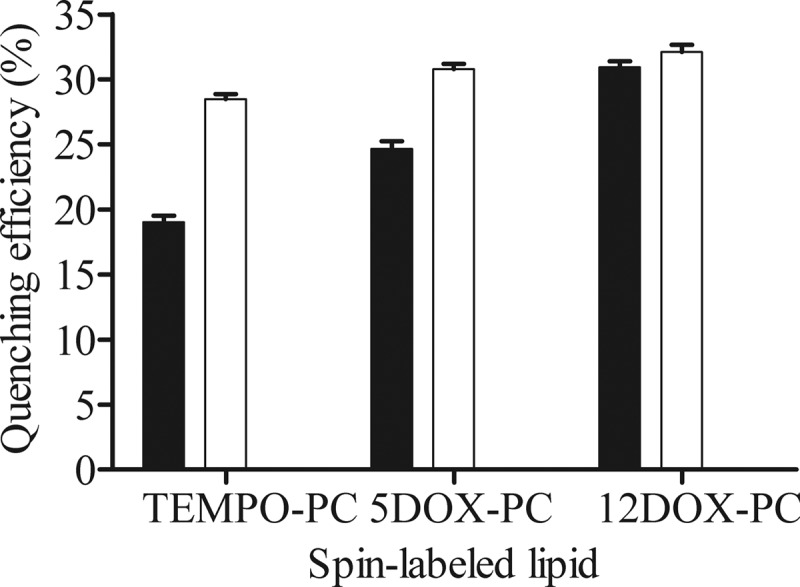
Quenching efficiency of the fluorescence emitted by the tryptophan residue of bovicin HC5 by spin-labeled lipids (TEMPO-PC, 5DOX-PC, and 12DOX-PC) incorporated at 25 mol% in DOPC (■) or DLPC/DMoPC (□) vesicles containing 1 mol% of lipid II. Single-wavelength recordings were performed at 340 nm using an excitation wavelength of 280 nm. The quenching efficiencies were calculated from the tryptophan fluorescence in the presence of membranes with or without spin-labeled lipids (n = 5).
CD spectroscopy.
CD is a good method for gathering information about the secondary structure and folding of proteins, since the proteins' spectra are dependent on their conformation. This is possible because all amino acids residues (except glycine) are chiral and right-handedly and left-handedly polarized light differently. This difference is presented as the ellipticity, which is a measure for CD (13, 30).
The CD spectra of bovicin HC5 were recorded in different environments, i.e., in solution, in DOPC or DLPC/DMoPC vesicles (with or without lipid II), or in the presence of 3-LII. When the spectra of bovicin HC5 were assessed in solution (buffer), no structural change was verified, independently of the pH tested (from 2 to 10), and the minimum was obtained at 198 nm (Fig. 6).
Fig 6.
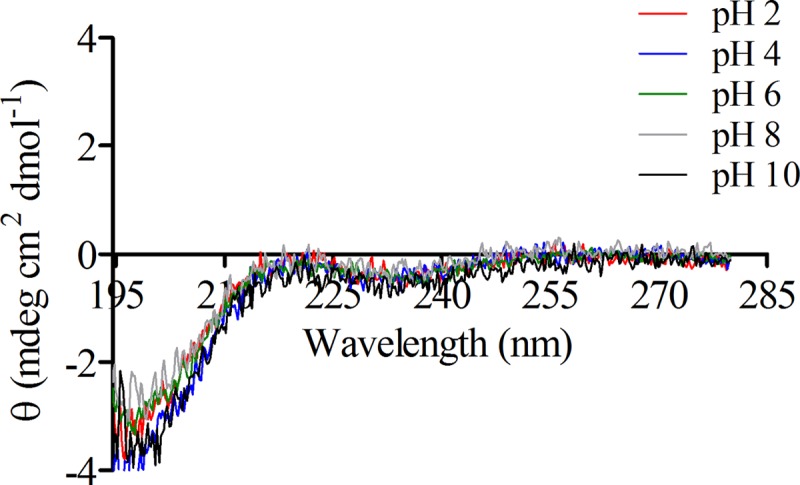
CD spectra of 20 μM bovicin HC5 in solution (10 mM potassium phosphate, 40 mM potassium sulfate) and at different pHs (from 2 to 10). The samples were scanned from 195 to 280 nm at 20°C. Each spectrum represents an average of five recordings after subtraction of the blank spectrum from each bovicin HC5 spectrum.
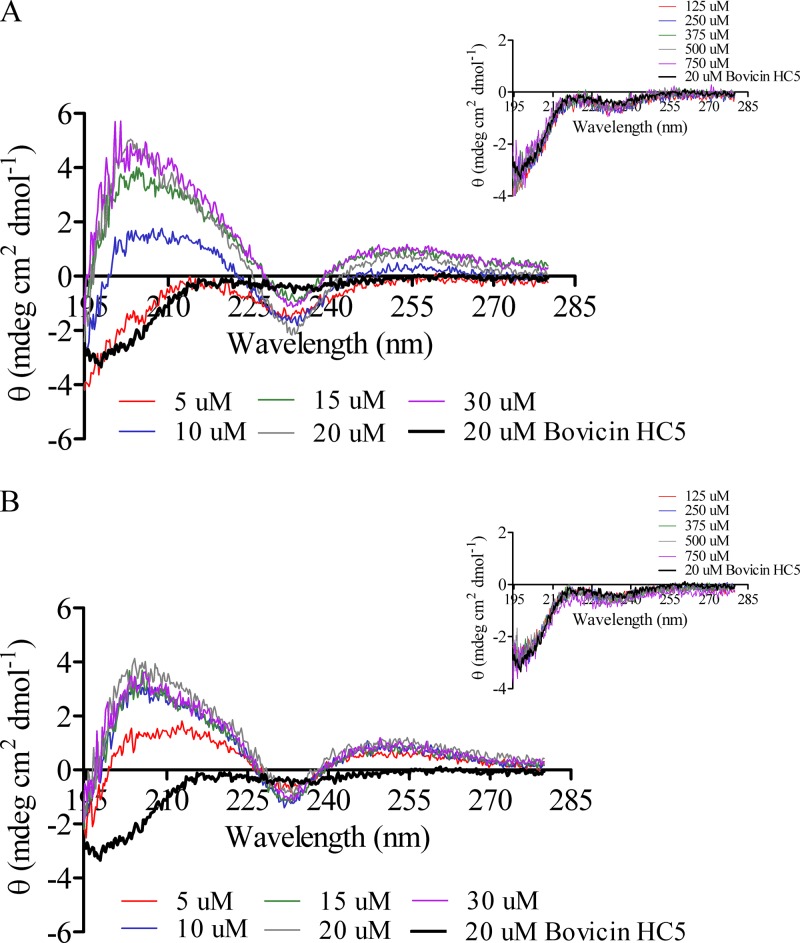
The spectrum of bovicin HC5 was significantly changed upon the addition of lipid II-containing vesicles or 3-LII. In the presence of model membranes containing lipid II (DOPC [Fig. 7A] or DLPC/DMoPC [Fig. 7B]), the minimum appeared to be centered at ∼234 nm. This minimum was flanked by two maxima, one at ∼250 nm and another at ∼204 nm. The changes in CD signal were specifically caused by the interaction of bovicin HC5 with lipid II present in both model membranes, because the addition of vesicles lacking lipid II did not induce any spectral changes (Fig. 7, insets). Parallel control experiments were performed with negatively charged membranes (composed of DOPC and 3 mol% of DOPG), and no changes in the spectrum of bovicin HC5 were observed (data not shown).
Fig 7.
CD spectra of bovicin HC5 in the presence of model membranes composed of DOPC (A) or DLPC/DMoPC (B) containing lipid II. Each spectrum was recorded for bovicin HC5 (20 μM) in buffer at pH 6 and in the presence of 5 to 30 μM lipid II. The samples were scanned from 195 to 280 nm at 20°C. Each spectrum represents an average of five recordings after subtraction of the blank spectrum from each bovicin HC5 spectrum. The insets show the spectra of bovicin HC5 in the presence of increasing concentrations of vesicles (125 to 750 μM expressed as lipid-Pi equivalents) without lipid II.
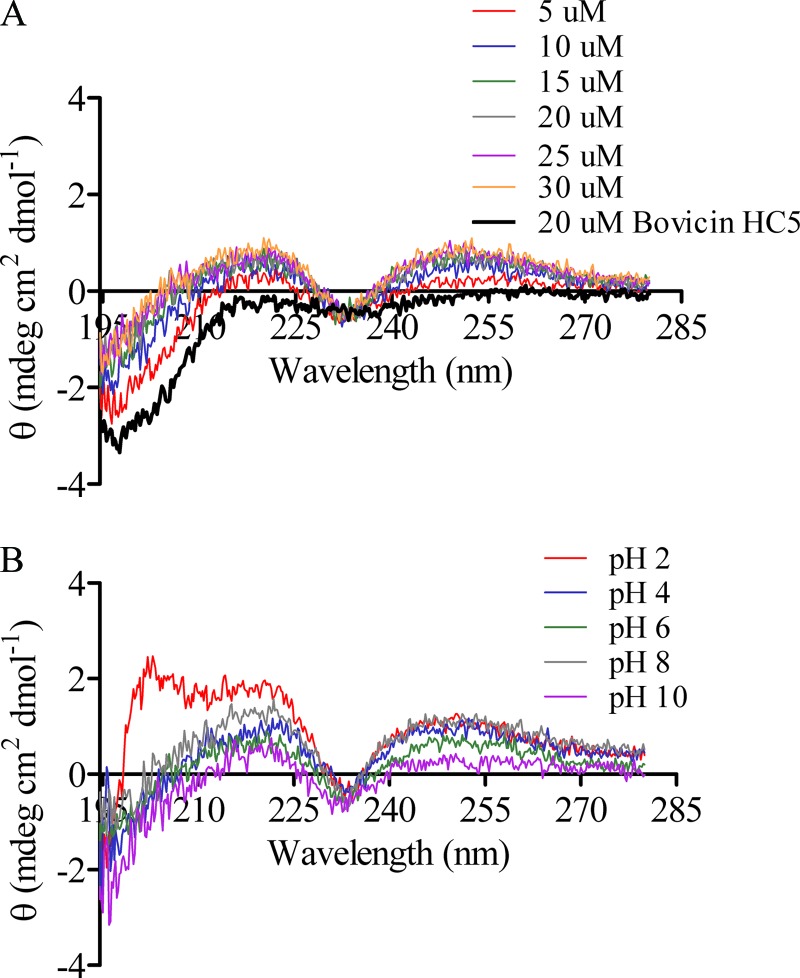
When the CD spectrum of bovicin HC5 was recorded in the presence of 3-LII, at pH 6.0, the inflection demonstrated in the presence of lipid II-containing vesicles, between 225 and 240 nm (minimum centered at ∼234 nm), was also observed. However, in the presence of 3-LII, the spectrum of bovicin HC5 was flanked by two maxima at 220 and 250 nm (Fig. 8A), which contrasted the effects on peptide's spectrum observed in the presence of vesicles containing lipid II, in which a maximum at ∼204 nm was also observed (Fig. 7). Using increasing concentrations of 3-LII, we could determine the stoichiometry of the bovicin HC5-lipid II interaction as 1:1, since the intensity and the pattern of the bovicin HC5‘s spectrum did not alter when recorded with concentrations of 3-LII above 20 μM (Fig. 8A).
Fig 8.
CD spectra of bovicin HC5 in the presence of water-soluble lipid II (3-LII). (A) CD spectrum recorded for bovicin HC5 (20 μM) in buffer at pH 6 and in the presence of 5 to 30 μM 3-LII. (B) CD spectrum recorded in the presence of 20 μM bovicin HC5 and 20 μM 3-LII at different pH values. The samples were scanned from 195 to 280 nm at 20°C. Each spectrum represents an average of five recordings after subtraction of the blank spectrum from each bovicin HC5 spectrum.
Based on these results and in order to compare the influence of pH on the stability of the complexes formed by bovicin HC5 and lipid II, the spectrum of bovicin HC5 was recorded in equimolar concentrations of 3-LII but at different pH values (from 2.0 to 10.0). At all of the additional pH values tested, the same inflection between 225 and 240 nm was observed. Nevertheless, at pH 2.0 the spectrum of bovicin HC5 was not flanked by two maxima, as observed at pH 4 to 10, but a maxima wavelength was observed at 203 nm (Fig. 8B).
DISCUSSION
With the increase in antibiotic resistance among bacterial species frequently isolated from human and animal infections, the study of alternatives to “classical” antibiotics used for therapeutic applications is warranted. To be considered for practical applications in human and veterinary medicine, an antimicrobial agent must meet at least two criteria: effectiveness and safety. Bacteriocins, especially the lantibiotics produced by lactic acid bacteria, have been suggested as suitable candidates to fulfill both requirements. Some lantibiotics (e.g., nisin and bovicin HC5) have a dual mechanism of action, which is not shared by other compounds already used for therapeutic applications (16, 29).
It has been demonstrated that nisin has a singular lipid II-binding mechanism and the pyrophosphate moiety in the lipid II molecule is considered the primary binding site for nisin. Upon binding to lipid II, the N-terminal part of nisin folds back onto the first two lanthionine rings, forming a cage-like structure (20). This lipid II-recognition mechanism may be generalized to other lantibiotics that have the conserved N-terminal lanthionine rings, such as bovicin HC5.
Bovicin HC5 can induce potassium efflux (27) and uses lipid II as a target in sensitive cell membranes (29). Previous studies demonstrated that after interacting with lipid II, bovicin HC5 is able to form pores in a membrane-thickness-dependent way (29). However, it was not clear how the peptide interacted with its target molecule and how environmental conditions affected the stability of this interaction. Because lipid II is a key component in cell wall biosynthesis, the evaluation of antimicrobial agents that interact with lipid II could lead to development of new strategies to control clinically relevant bacteria in both human and veterinary settings. We studied here the biochemical aspects of the interaction between bovicin HC5 and lipid II in model membranes that either allow (thin lipid membranes composed of DLPC/DMoPC phospholipids) or do not allow (thick lipid membranes composed of DOPC phospholipids) pore formation in model membranes. In addition, we addressed how this interaction was affected by pH values that are known to influence the biological activity of lantibiotics.
In order to determine whether the orientation and depth of insertion of bovicin HC5 in model membranes was affected upon binding to lipid II, we used tryptophan fluorescence spectroscopy, a technique that had been successfully applied to study the orientation of bacteriocins in model membranes, such as nisin (1, 28, 35) and pediocin PA-1 (5). In solution (absence of vesicles), the fluorescence maximum of the tryptophan spectra occurred at 353 nm, which indicates that the tryptophan residue of bovicin HC5 was localized in a hydrophilic environment (22). Independent on the membrane phospholipid composition, upon addition of lipid II-containing vesicles, the fluorescence maximum of the tryptophan residue displayed a blue-shift (maximally 12 nm) and a concomitant intensity increase, indicating that the tryptophan residue became located in a more hydrophobic environment, i.e., in the lipid bilayer.
The two model membranes tested showed a consistent difference in fluorescence intensity, i.e., the emission spectrum for the DLPC/DMoPC was less intense than the observed for DOPC vesicles. This result may be due to the pore assembly of bovicin HC5 in thinner membranes, where in the pore configuration the tryptophan residue is probably quenched via self-quenching or by another residue from the neighboring bovicin HC5.
The lipid II/bovicin HC5 ratio was determined to be 0.5, which means that the stoichiometry of this interaction might be 2:1, with two bovicin HC5 molecules interacting with one lipid II molecule. The KSV values showed that the tryptophan residue became much less accessible to acrylamide when lipid II-containing vesicles were present (reductions of 70.6 and 69.9% in KSV values obtained in the presence of DOPC and DLPC/DMoPC vesicles, respectively, compared to that obtained in solution), reinforcing that the bovicin HC5 tryptophan residue became inserted into the membrane when bovicin HC5 interacts with lipid II.
Quenching of fluorescence by spin-labeled phospholipids has been used as a valuable tool to determine the topology and depth of penetration of tryptophan- or tyrosine-containing peptides in model membranes, since quenching depends on the distance between the tryptophan or tyrosine residue and the spin-label, occurring only over a short distance (24, 31). In the present study, quenching by spin-labeled phospholipids indicated that the tryptophan residue of bovicin HC5 was located near the twelfth position of the acyl chains of the membrane phospholipids, the deepest position checked in the present study, placing the bovicin HC5 in the middle of the bilayer.
Because spin-labeled lipids are longer (C18) than DLPC or DMoPC, the efficiency of quenching obtained in the presence of DLPC/DMoPC membranes was quite similar, regardless of the position of the spin-label (head group or hydrocarbon chain). This effect contrasts with the results obtained with DOPC vesicles, since in these model membranes the quenching efficiency increased as phospholipids containing spin-label in deeper positions were used.
The changes in the tryptophan emission spectrum, in addition to the results from quenching experiments, suggest a plausible model for the orientation adopted by bovicin HC5 in membranes containing lipid II. Similarly to the orientation of nisin reported previously (35), the interaction with lipid II appears to change the orientation of bovicin HC5 on the membrane surface from parallel to a perpendicular orientation.
CD spectroscopy enables the determination of secondary structures of proteins (17) and is also a good tool for monitoring conformational changes due to temperature, mutations, heat, denaturants, or binding interactions (13). CD spectroscopy was used here as a second approach to investigate potential changes in bovicin HC5 conformation induced by lipid II binding and to substantiate our tryptophan spectroscopy analysis. In addition, stoichiometry and pH dependency on the bovicin HC5-lipid II interaction were examined using CD.
The presence of posttranslational modified amino acids residues and lanthionine rings within the primary structure of a peptide, as observed in bovicin HC5, prevent the quantitative structural analysis from the spectra obtained by CD measurements (34). Moreover, as a consequence of its small size (22 amino acids residues), bovicin HC5 did not adopt a defined structure in aqueous solution, since in this condition the individual spectrum of bovicin HC5 was similar to the archetypal random coil spectrum (very low ellipticity above 210 nm and negative bands near 195 nm [36]).
Because we could not calculate the average secondary structure of bovicin HC5 from the CD data, comparisons of individual spectra and spectra in the presence of lipid II provided information about the structural changes of bovicin HC5 upon interaction with its receptor. Earlier studies with nisin also used CD measurements for qualitative purposes to gain insight into changes in the nisin molecule after its interaction with lipid II (15).
The CD spectrum of bovicin HC5 in aqueous solution was not altered in a wide range of pH values tested. Regarding the measurements in the presence of lipid II-containing membranes, the CD spectrum of bovicin HC5 was significantly changed compared to that in solution, but it was basically the same in both model membranes tested, confirming that there are indeed conformational changes in bovicin HC5 in the presence of lipid II and that the peptide-ligand interaction is not influenced by the membrane composition.
The maximum centered at 204 nm observed on the spectrum of bovicin HC5 in the presence of lipid II-containing vesicles was probably due to the effect of the interaction between the lateral chains of bovicin HC5 amino acid residues and the phospholipids that compose the model membranes tested. In addition, the inflection observed in the same spectra was caused strictly by the interaction between bovicin HC5 and lipid II, since the same spectral change was demonstrated in the presence of soluble lipid II.
Water soluble lipid II (3-LII) is a lipid II variant that consists of a shortened prenyl chain, with three isoprene repeats, instead of eleven isoprene repeats detected on the natural occurring full-length lipid II. According to the spectrum of bovicin HC5 obtained in the presence of increasing concentrations of 3-LII, the stoichiometry of the interaction between bovicin HC5 and lipid II was determined to be 1:1, which contrasts with the 2:1 stoichiometry determined in tryptophan spectroscopy experiments. The two different stoichiometries determined for the bovicin HC5-lipid II interaction can be attributed to the location of lipid II molecules available for binding to bovicin HC5: either in solution or embedded in model membranes.
Since the experiments with 3-LII were performed in the absence of phospholipid membranes, the stoichiometry of 1:1 may reflect the initial interaction between a single bovicin HC5 and lipid II. On the other hand, the final pore structure would be composed of the double of bovicin HC5 molecules compared to the lipid II molecules (2:1). Regarding the CD spectra, the first binding to lipid II induced a conformational change (range of 225 to 240 nm) and then the subsequent formation of the pore structure induced a further change (range of 195 to 210 nm).
Most lantibiotics are not stable over a wide pH range, since under basic conditions many peptides undergo the oxidation of the lan/melan thioethers, resulting in a loss of antimicrobial activity (38). This lack of activity does not seem to occur to bovicin HC5, since we demonstrated here that bovicin HC5 maintains the ability to bind to its target lipid II over a wide range of pHs (from 2 to 10).
Nisin-lipid II interaction is not observed at pH values below 4, and the changes observed at nisin spectrum upon binding to lipid II are more evident at pH 6 (E. Breukink, unpublished results). In contrast, not only does bovicin HC5 maintain its affinity for lipid II in highly acidic environments, but also the conformation adopted by this peptide upon binding to its target is different in acidic conditions.
At pH 2, the CD spectrum of bovicin HC5 recorded in the presence of 3-LII has changed compared to the spectra recorded at pH 4 to 10, and an additional spectral change was detected in the region between 195 and 210 nm. Interestingly, this change is located in the same region where spectral changes were observed when bovicin HC5 was incubated in the presence of vesicles containing lipid II.
These results suggest that, in acidic conditions, bovicin HC5 changes its interaction with lipid II, which may help explain the greater in vivo activity of bovicin HC5 against several bacterial strains in such conditions (19). This is also an important feature to be considered in view of the practical applications for bovicin HC5, especially for the preservation of acidic food products (e.g., tropical fruit juices).
In conclusion, the results obtained in the present study demonstrate that bovicin HC5-lipid II interaction occurs even in the absence of pore formation and that, upon interaction with lipid II, bovicin HC5 changes its conformation, probably adopting a transmembrane orientation. The binding of bovicin HC5 to lipid II occurs over a wide pH range, and this interaction is distinct under acidic conditions, which corroborates with greater a stability and biological activity of bovicin HC5 at low pH values. Because the phosphate groups of lipid II are expected to be protonated at pH 2.0, it appears that bovicin HC5 does not rely only on the binding to the pyrophosphate moiety of lipid II to interact with its target molecule. Growth experiments indicated that the antimicrobial activity of bovicin HC5 has greater persistence against target bacteria compared to nisin and that even nisin-resistant Staphylococcus cells were inhibited by bovicin HC5 (29). These latter observations and the results reported here emphasize the potential of bovicin HC5 as a useful molecule for controlling harmful bacteria of industrial, agricultural, or medical relevance.
ACKNOWLEDGMENTS
This study was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; Brasília, Brazil) and the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG; Belo Horizonte, Brazil). A.D.P. was supported by a fellowship (201179/2009-1) from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; Brasília, Brazil).
Footnotes
Published ahead of print 11 June 2012
REFERENCES
- 1. Breukink E, et al. 1998. The orientation of nisin in membranes. Biochemistry 37:8153–8162 [DOI] [PubMed] [Google Scholar]
- 2. Breukink E, et al. 2003. Lipid II is an intrinsic component of the pore induced by nisin in bacterial membranes. J. Biol. Chem. 278:19898–19903 [DOI] [PubMed] [Google Scholar]
- 3. Breukink E, de Kruijff B. 2006. Lipid II as a target for antibiotics. Nat. Rev. Drug Discov. 5:321–323 [DOI] [PubMed] [Google Scholar]
- 4. Chamorro C, Boerman MA, Arnusch CJ, Breukink E, Pieters RJ. 2012. Enhancing membrane disruption by targeting and multivalent presentation of antimicrobial peptides. Biochim. Biophys. Acta 1818:2171–2174 [DOI] [PubMed] [Google Scholar]
- 5. Chen Y, Ludescher RD, Montville TJ. 1997. Electrostatic interactions, but not the YGNGV consensus motif, govern the binding of pediocin PA-1 and its fragments to phospholipid vesicles. Appl. Environ. Microbiol. 63:4770–4777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cotter PD, Hill C, Ross RP. 2005. Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3:777–788 [DOI] [PubMed] [Google Scholar]
- 7. de Carvalho AAT, Costa ED, Mantovani HC, Vanetti MCD. 2007. Effect of bovicin HC5 on growth and spore germination of Bacillus cereus and Bacillus thuringiensis isolated from spoiled mango pulp. J. Appl. Microbiol. 102:1000–1009 [DOI] [PubMed] [Google Scholar]
- 8. de Carvalho AAT, Mantovani HC, Vanetti MCD. 2007. Bactericidal effect of bovicin HC5 and nisin against Clostridium tyrobutyricum isolated from spoiled mango pulp. Let. Appl. Microbiol. 45:68–74 [DOI] [PubMed] [Google Scholar]
- 9. Drosinos EH, Mataragas M, Metaxopoulos J. 2006. Modeling of growth and bacteriocin production by Leuconostoc mesenteroides E131. Meat Sci. 74:690–696 [DOI] [PubMed] [Google Scholar]
- 10. Eftink MR, Ghiron CA. 1976. Exposure of tryptophanyl residues in proteins: quantitative determination by fluorescence quenching studies. Biochemistry 15:672–680 [DOI] [PubMed] [Google Scholar]
- 11. Gálvez A, López RL, Abriouel H, Valdivia E, Ben Omar N. 2008. Application of bacteriocins in the control of foodborne pathogenic and spoilage bacteria. Crit. Rev. Biotechnol. 28:125–152 [DOI] [PubMed] [Google Scholar]
- 12. Green DW. 2002. The bacterial cell wall as a source of antibacterial targets. Expert Opin. Ther. Targets 6:1–19 [DOI] [PubMed] [Google Scholar]
- 13. Greenfield NJ. 2006. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 1:2876–2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harz H, Burgdorf K, Holtje JV. 1990. 1990. Isolation and separation of the glycan strands from murein of Escherichia coli by reversed-phase high-performance liquid chromatography. Anal. Biochem. 190:120–128 [DOI] [PubMed] [Google Scholar]
- 15. Hasper HE, de Kruijff B, Breukink E. 2004. Assembly and stability of nisin-lipid II pores. Biochemistry 43:11567–11575 [DOI] [PubMed] [Google Scholar]
- 16. Hasper HE, et al. 2006. An alternative bactericidal mechanism of action for lantibiotic peptides that target lipid II. Science 313:1636–1637 [DOI] [PubMed] [Google Scholar]
- 17. Hennessey JP, Jr, Scarborough GA. 1988. Secondary structure of the Neurospora crassa plasma membrane H+-ATPase as estimated by circular dichroism. J. Biol. Chem. 263:3123–3130 [PubMed] [Google Scholar]
- 18. Hope M, Bally MB, Webb G, Cullis PR. 1985. Production of large unilamellar vesicles by a rapid extrusion procedure: characterization of size distribution, trapped volume and ability to maintain a membrane potential. Biochim. Biophys. Acta 812:55–65 [DOI] [PubMed] [Google Scholar]
- 19. Houlihan AJ, Mantovani HC, Russell JB. 2004. Effect of pH on the activity of bovicin HC5, a bacteriocin from Streptococcus bovis HC5. FEMS Microbiol. Lett. 231:27–32 [DOI] [PubMed] [Google Scholar]
- 20. Hsu STD, et al. 2004. The nisin-lipid II complex reveals a pyrophosphate cage that provides a blueprint for novel antibiotics. Nat. Struct. Mol. Biol. 11:963–967 [DOI] [PubMed] [Google Scholar]
- 21. Ladokhin AS. 1999. Analysis of protein and peptide penetration into membranes by depth-dependent fluorescence quenching: theoretical considerations. Biophys. J. 76:946–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lakowicz JR. 1999. Principles of fluorescence spectroscopy. Kluwer Academic/Plenum Publishers, New York, NY [Google Scholar]
- 23. Lima JR, Ribon AOB, Russell JB, Mantovani HC. 2009. Bovicin HC5 inhibits wasteful amino acid degradation by mixed ruminal bacteria in vitro. FEMS Microbiol. Lett. 292:78–84 [DOI] [PubMed] [Google Scholar]
- 24. Liu LP, Deber CM. 1997. Anionic phospholipids modulate peptide insertion into membranes. Biochemistry 36:5476–5482 [DOI] [PubMed] [Google Scholar]
- 25. London E, Feigenson GW. 1981. Fluorescence quenching in model membranes. 1. Characterization of quenching by a spin-labeled phospholipid. Biochemistry 20:1932–1938 [DOI] [PubMed] [Google Scholar]
- 26. Majeed H, Gillor O, Kerr B, Riley MA. 2011. Competitive interactions in Escherichia coli populations: the role of bacteriocins. ISME J. 5:71–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mantovani H, Hu H, Worobo RW, Russell JB. 2002. Bovicin HC5, a bacteriocin from Streptococcus bovis HC5. Microbiology 148:3347–3352 [DOI] [PubMed] [Google Scholar]
- 28. Martin I, Ruysschaert JM, Sanders D, Giffard CJ. 1996. Interaction of the lantibiotic nisin with membranes revealed by fluorescence quenching of an introduced tryptophan. Eur. J. Biochem. 239:156–164 [DOI] [PubMed] [Google Scholar]
- 29. Paiva AD, Breukink E, Mantovani HC. 2011. Role of Lipid II and membrane thickness in the mechanism of action of the lantibiotic bovicin HC5. Antimicrob. Agents Chemother. 55:5284–5293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pelton JT, McLean LR. 2000. Spectroscopic methods for analysis of protein secondary structure. Anal. Biochem. 277:167–176 [DOI] [PubMed] [Google Scholar]
- 31. Ren J, Lew S, Wang Z, London E. 1997. Transmembrane orientation of hydrophobic-helices is regulated by the relationship of helix length to bilayer thickness and by cholesterol concentration. Biochemistry 36:10213–10220 [DOI] [PubMed] [Google Scholar]
- 32. Rouser G, Fkeischer S, Yamamoto A. 1970. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorous analysis of spots. Lipids 5:494–496 [DOI] [PubMed] [Google Scholar]
- 33. Silver LL. 2003. Novel inhibitors of bacterial cell wall synthesis. Curr. Opin. Microbiol. 6:431–438 [DOI] [PubMed] [Google Scholar]
- 34. van den Hooven HW, et al. 1993. NMR and circular dichroism studies of the lantibiotic nisin in non-aqueous environments. FEBS Lett. 319:189–194 [DOI] [PubMed] [Google Scholar]
- 35. van Heusden HE, de Kruijff B, Breukink E. 2002. Lipid II induces a transmembrane orientation of the pore-forming peptide lantibiotic nisin. Biochemistry 41:12171–12178 [DOI] [PubMed] [Google Scholar]
- 36. Venyaminov S, Baikalov IA, Shen ZM, Wu CS, Yang JT. 1993. Circular dichroic analysis of denatured proteins: inclusion of denatured proteins in the reference set. Anal. Biochem. 214:17–24 [DOI] [PubMed] [Google Scholar]
- 37. Wiedemann I, et al. 2006. The mode of action of the lantibiotic lacticin 3147: a complex mechanism involving specific interaction of two peptides and the cell wall precursor lipid II. Mol. Microbiol. 6:285–296 [DOI] [PubMed] [Google Scholar]
- 38. Wilson-Stanford S, et al. 2009. Oxidation of lanthionines renders the lantibiotic nisin inactive. Appl. Environ. Microbiol. 75:1381–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]