Abstract
Catheter-associated urinary tract infections (CAUTIs) constitute the majority of nosocomial urinary tract infections (UTIs) and pose significant clinical challenges. These infections are polymicrobial in nature and are often associated with multidrug-resistant pathogens, including uropathogenic Escherichia coli (UPEC). Urinary catheterization elicits major histological and immunological alterations in the bladder that can favor microbial colonization and dissemination in the urinary tract. We report that these biological perturbations impact UPEC pathogenesis and that bacterial reservoirs established during a previous UPEC infection, in which bacteriuria had resolved, can serve as a nidus for subsequent urinary catheter colonization. Mannosides, small molecule inhibitors of the type 1 pilus adhesin, FimH, provided significant protection against UPEC CAUTI by preventing bacterial invasion and shifting the UPEC niche primarily to the extracellular milieu and on the foreign body. By doing so, mannosides potentiated the action of trimethoprim-sulfamethoxazole in the prevention and treatment of CAUTI. In this study, we provide novel insights into UPEC pathogenesis in the context of urinary catheterization, and demonstrate the efficacy of novel therapies that target critical mechanisms for this infection. Thus, we establish a proof-of-principle for the development of mannosides to prevent and eventually treat these infections in the face of rising antibiotic-resistant uropathogens.
INTRODUCTION
Catheter-associated urinary tract infections (CAUTIs) often involve Gram-positive and/or Gram-negative bacterial colonization and biofilm aggregation on the surface of indwelling urologic devices such as urinary catheters. These biofilms and the increasing occurrence of multidrug-resistant pathogens render treatment very difficult (22, 49, 57). Uropathogenic Escherichia coli (UPEC), the primary cause of community-acquired urinary tract infections (UTIs), account for 50% of nosocomial UTIs, including CAUTIs (27). However, very little is known about its pathogenesis after urinary catheterization, which results in the disruption of the normal mechanical and antimicrobial defenses of the bladder (9, 17, 43, 44). Previous reports using human biopsy specimens and rodent models of infections have shown that the catheterized bladder is edematous and highly inflamed with immune cell infiltration and high levels of proinflammatory cytokines, an environment very different from that which UPEC initially encounters upon infection of a noncatheterized bladder (17, 18, 28, 44). We hypothesized that these profound catheter-related changes may affect UPEC pathogenesis.
The UPEC pathogenic cascade has been extensively characterized in a noncatheterized murine model of cystitis (21, 25, 30, 31, 35, 37, 38, 62). UPEC strains encode numerous adhesive pilus fibers assembled by the chaperone/usher pathway (CUP) (50, 58) that are often tipped with adhesins that bind to receptors with stereochemical specificity (10, 23), thus facilitating colonization and biofilm formation (31, 45, 62). Almost all UPEC strains encode the class of CUP pili known as type 1 pili (24) that are tipped with the mannose-binding FimH adhesin (23). FimH is known to bind highly homologous mannosylated uroplakins that coat the luminal surface of both the human and murine bladder (55, 64, 65) and N-linked oligosaccharides on β1 and α3 integrins, which are expressed throughout the urothelium (13). Further, FimH has been shown to facilitate the bacterial colonization and invasion of human bladder tissue culture cells (5, 25, 41, 62), human ureter tissues, and mouse bladder epithelial cells. Internalized UPEC are exocytosed in a TLR4-dependent process (54); however, bacteria within superficial epithelial cells can escape into the host cell cytoplasm, subverting expulsion and innate defenses. Within the cytoplasm, UPEC strains replicate and aggregate into large biofilm-like intracellular bacterial communities (IBCs) in a FimH-dependent process (2, 25, 62). Subsequently, UPEC strains disperse from mature IBCs, escape into the bladder lumen, and reinitiate the process by binding and invading naive epithelial cells. In addition to IBCs, UPEC strains are also able to establish quiescent intracellular reservoirs (QIRs), consisting of small numbers of intracellular UPEC that can persist in a dormant state, tolerant to antibiotic therapy, and which can subsequently serve as seeds for recurrent infection (36, 37, 52). Filamentation and IBC formation has been documented in samples from human clinical studies (47). In agreement with these findings and in support of a role for FimH in humans, it has been shown that the fimH gene is under positive selection in human clinical UPEC isolates (5, 46, 60).
Detailed understanding of the critical steps of this pathogenic cascade has led to the development of small-molecule inhibitors called mannosides (20) that bind to FimH with affinities 200,000-fold greater than mannose (20), thus inhibiting UPEC binding and invasion of the superficial umbrella cells (in UTIs) (20). Mannosides such as mannoside 6 (20) have been shown to be orally bioavailable in a murine model (8, 19). Oral administration is able to efficaciously treat chronic UTI via a potent fast-acting mechanism (8) that is capable of potentiating the efficacy of trimethoprim-sulfamethoxazole (TMP-SMZ) (8).
In this report, an optimized murine model of foreign body-associated UTI that closely mimics CAUTI (18) was used to investigate the consequences of urinary catheterization on the pathophysiology of UPEC infection. For these studies, the contributions of several UPEC virulence mechanisms, including type 1 pili, IBC formation, and UPEC reservoirs from prior UTI episodes, were assessed. The results obtained indicate that urinary catheterization provides UPEC with a more favorable environment for extracellular colonization of the urinary tract with the opportunity to exploit the ability to form type 1 pilus-mediated biofilms on the surface of the foreign body. Prophylactic administration of mannosides, which blocked biofilm formation on silicone in vitro, potentiated the efficacy of TMP-SMZ in preventing murine CAUTI. This report provides important insights into the mechanisms underlying UPEC-mediated CAUTI and informs efforts to design better therapeutic approaches to prevent and potentially treat these infections.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All strains used in the present study and their characteristics are listed in the Table 1. Unless otherwise specified, a single colony of E. coli grown on a Luria-Bertani (LB) agar (Becton Dickinson) plate supplemented with appropriate antibiotics was inoculated into LB broth and grown statically at 37°C for 18 h before use in the indicated assays.
Table 1.
Strains used in this study
| Strain | Relevant antibiotic resistancea | Characteristics | Source or reference |
|---|---|---|---|
| UTI89 | Parental UPEC UTI89 strain, cystitis isolate | 3 | |
| UTI89ΔfimH | UTI89 with an in-frame deletion of fimH, type 1 pilus defective | 4 | |
| UTI89ΔsfaA-H | UTI89 with an in-frame deletion of sfa operon, S pilus defective | This study | |
| UTI89ΔsfaA-HΔfimB-H | Kanr Cmr | UTI89 with in-frame deletions of the sfa operon and the fim operon from fimB to fimH, S and type 1 pilus defective | This study |
| UTI89ΔcsgA | UTI89 with an in-frame deletion of csgA, curli deficient | 1 | |
| UTI89ΔcsgBΔcsgG | UTI89 with in-frame deletions of csgB and csgG, curli deficient | 1 | |
| UTI89HK::GFP | Kanr | UTI89 with an insertion of a kanamycin cassette and GFP at the HK site | 5 |
| UTI89pCOMGFP | Kanr | UTI89 ectopically expressing GFP from pCOM plasmid | 2, 5 |
Cmr, chloramphenicol resistance; Kanr, kanamycin resistance.
In vitro cultivation and quantification of biofilms.
Biofilms on all-silicone Foley catheters (Bard Medical, GA) or silicone tubing (Thermo Fisher Scientific Inc., PA) were grown as described by Ferrieres et al. (14) and modified as follows. All tubing and connectors in the system were autoclaved and ethanol sterilized prior to use. The system was assembled similar to the previously described flow chamber system (6). Priming of the catheter or the silicone tubing occurred at 37°C for 20 min by flowing prewarmed pooled human urine. Urine was collected from healthy volunteers as approved by the Institutional Review Board of Washington University in St. Louis. Pooled samples were spun at 10,000 × g for 15 min, supernatants were then filter-sterilized through 33-μm-pore-size filters, and, if necessary, stored at 4°C for no more than 3 days. Portions (3 ml) of stationary-phase E. coli from overnight cultures diluted to 1 × 106 to 2 × 106 CFU/ml in human urine were injected into the catheter or silicone tubing using a 30-ml gauge needle. The bacteria were allowed to attach to the substratum for 1 h before urine flow via Watson-Marlow peristaltic pump 205S was resumed at 0.5 ml min−1. When indicated, urine was supplemented with 1% methyl mannose (Sigma, St Louis, MO) prior to the experiment. After 24 h, the remaining medium was exchanged for sterile double-distilled H2O (ddH2O) that was allowed to flow at 0.5 ml min−1 to remove the residual urine and nonadherent bacteria in the system. The liquid from the catheter or silicone tubing was then removed by capillary action onto absorbent paper. The tubing was aseptically cut into pieces for CFU enumeration or crystal violet staining, respectively. For CFU enumeration, at least three pieces (1 cm in length) of incubated tubing were separately further cut into smaller pieces and placed into 1 ml of phosphate-buffered saline (PBS). Adherent cells were detached by sonication (10 min) and vigorous vortexing (3 min). Viable bacterial counts were assessed by serial dilution on LB plates with appropriate antibiotics. Crystal violet staining was used to determine biofilm biomass. At least three pieces of incubated tubing (3 cm in length) were filled with 0.5% crystal violet at room temperature for 10 min. Excess dye was removed by three washes with ddH2O and dried by capillary action on absorbent paper. The bound crystal violet was then dissolved in 200 μl of 33% acetic acid, and the absorbance was measured at 595 nm. Biofilm was quantitated as CFU/ml per cm2 or as the A595/cm2. The experiment was repeated at least three times with different urine samples.
Animal implantation and infections.
Six- to seven-week-old female wild-type C57BL/6Ncr mice purchased from the National Cancer Institute (NCI) were used in the present study. Experiments were performed after 1-week adaptation in the animal facility after arrival from the NCI. Animals were implanted and infected with the indicated bacterial strain as previously described (18). Briefly, 7- to 8-week-old female mice were anesthetized by inhalation of isoflurane and transurethrally implanted with platinum-cured silicone tubing (4 to 5 mm in length). Immediately after implantation, 50 μl of ca. 1 × 107 to 2 × 107 CFU bacteria in 1× PBS were introduced into the bladder lumen by transurethral inoculation. Nonimplanted animals were inoculated in the same manner. Animals were sacrificed at the indicated time points by cervical dislocation under anesthesia inhalation. Bladders and kidneys were aseptically harvested. Subsequently, the silicone implant was retrieved from the bladder when present, placed in PBS, sonicated for 10 min, and then vortexed at maximum speed for 3 min. The bladder and kidneys from each mouse were homogenized in PBS. Samples were serially diluted and plated on LB agar plates supplemented with appropriate antibiotics. CFU were enumerated after 24 h of incubation at 37°C. In all cases, experiments were performed at least twice with n = 5 mice/strain/condition. All studies and procedures were approved by the Animal Studies Committee at Washington University School of Medicine.
Mannoside and antibiotic treatment.
For pretreatment experiments, 50 μl of mannoside (mannoside 6; 5 mg/kg [mouse body weight]) or PBS was administered intraperitoneally (i.p.) 30 min prior to implantation as previously described (8). As indicated for preinfection treatment, trimethoprim-sulfamethoxazole (TMP-SMZ) was added to the drinking water for 3 days prior to infection at 54 and 270 μg/ml, respectively. The drinking water was changed every 24 h. To assess the effects of mannoside 6 and/or TMP-SMZ on established infections, animals were implanted and infected for 24 h. At 24 h postinfection (hpi), TMP-SMZ was added to the drinking water at the concentrations indicated above, and mannoside 6 or PBS was administered i.p. 6 h prior to sacrifice. Animals were sacrificed 48 hpi.
UPEC reservoir-derived colonization.
Nonimplanted animals were infected with UTI89HK::GFP as described above. At 14 days postinfection (dpi), urine was collected, serially diluted, and plated for CFU. Animals with bacterial loads greater than the limits of detection in each experiment (200 or 400 CFU/ml) were determined to be bacteriuric and eliminated from further analyses (see Fig. S3A in the supplemental material). A subset of the remaining nonbacteriuric animals was implanted for 3 or 5 days, while the others remained nonimplanted and served as a control group for reinfection events. Bacterial reservoir-mediated colonization after implantation was assessed by CFU enumeration of bacteria on implants and in the bladders 3 or 5 days postimplantation (17 or 19 dpi). Only abacteriuric animals with UTI89HK::GFP titers >104 CFU on implants or bladders at 17 or 19 dpi were considered reemergence events.
IBC enumeration and visualization.
Implanted and nonimplanted animals were infected with UTI89 for 6 h. When indicated, mannoside 6 (5 mg/kg) or PBS was administered i.p. at 30 min prior to implantation. At 6 hpi, the bladders were harvested, bisected, splayed on silicone plates, and fixed in 2% paraformaldehyde. LacZ staining of whole bladders was performed as previously described (26). Punctate violet spots characteristic of IBCs were enumerated by light microscopy.
For IBC visualization, animals were infected with UTI89 constitutively expressing green fluorescent protein (GFP), i.e., UTI89pCOM-GFP. At the indicated time point, bladders were removed, bisected, splayed, and fixed as described above. The splayed bladders were then incubated for 20 min at room temperature with Alexa Fluor 633-conjugated wheat germ agglutinin (WGA [Molecular Probes]; 1:1,000 in PBS) for staining of the bladder surface and, when indicated, SYTO83 (1:1,000 in PBS) to stain the bacteria. Bladders were rinsed with PBS, mounted using Prolong Gold antifade reagent (Invitrogen), and examined with a Zeiss LSM510 confocal laser scanning microscope under a 63× objective lens. SYTO83 and WGA were excited at 543 and 633 nm, respectively.
Gentamicin protection assay.
To quantify intracellular and extracellular bacteria, bladders were aseptically harvested at 3 and 6 hpi. Bladders were cut in four parts and washed three times in 500 μl of PBS. The wash fractions were pooled and centrifuged at 500 rpm for 5 min to pellet exfoliated bladder cells. The supernatants were then serially diluted and plated on LB agar supplemented with appropriate antibiotics, which were incubated at 37°C for 24 h to obtain extracellular bacterial CFU counts. Rinsed bladders were then treated with 100 μg of gentamicin/ml for 90 min at 37°C. After gentamicin treatment, the bladder tissue was washed twice with PBS to eliminate residual antibiotics and homogenized in 1 ml of PBS, and bacterial CFU counts of were determined as described above to determine the levels of intracellular bacteria (protected from gentamicin killing).
Statistical methods.
Comparisons between groups were conducted by nonparametric Mann-Whitney U test using GraphPad Prism (GraphPad software, version 5). Values below the limit of detection for in vivo experiments (20 CFU for organs and 10 CFU for implants) were assigned the appropriate limit of detection for statistical analyses. All tests were two tailed, and a P value < 0.05 was considered significant. Colonization and infection were defined as organs or implants with bacterial titers above the limit of detection.
RESULTS
UPEC adherence, invasion, and IBC morphology are unaltered in catheterized bladders.
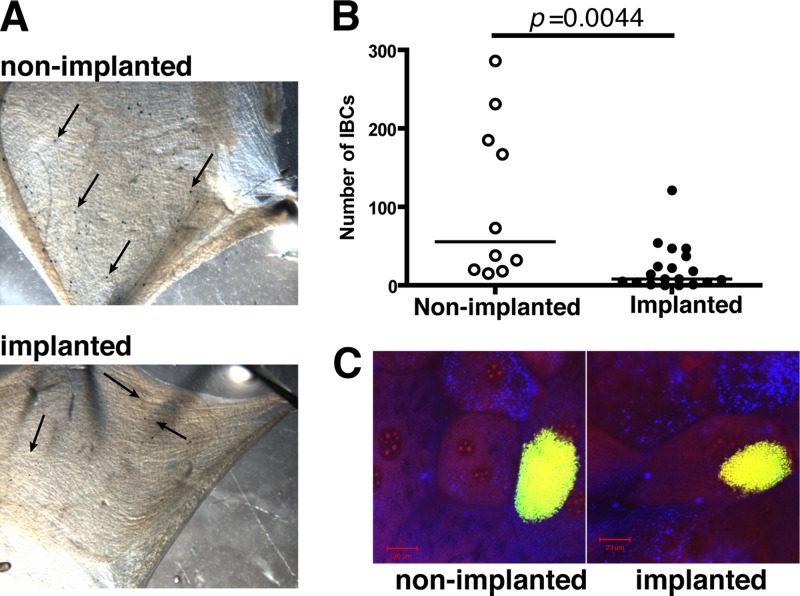
IBC formation occurs in the pathogenesis of UPEC in noncatheterized patients (47) and has been shown in mouse models to be critical for infection (25, 62). To assess the effects of urinary catheterization on IBC formation, 4- to 5-mm platinum-cured silicone tubing sections were implanted in the bladders of C57BL/6Ncr female mice, which were then immediately infected with 1 × 107 to 2 × 107 CFU of the well-characterized virulent UPEC strain UTI89 by transurethral catheterization. Gentamicin protection assays performed at 3 hpi revealed no significant difference (P > 0.05) in either the extracellular or intracellular UPEC populations in the presence or absence of implants (see Fig. S1 in the supplemental material), indicating no gross defect in bacterial invasion in implanted animals in the first 3 h of infection. However, by 6 hpi, IBC formation was significantly reduced in implanted bladders compared to nonimplanted animals, as assessed by LacZ staining and confocal scanning laser microscopy (CSLM) (Fig. 1A). Implanted animals had a median of 8 IBCs/bladder (P = 0.0044; Fig. 1B) compared to nonimplanted animals, in which IBC numbers ranged up to >250 IBCs/bladder with a median of 55 IBCs/bladder. IBC morphology did not account for this difference in implanted animals since the IBCs formed in implanted animals were observed to have the same dimensions as those produced in nonimplanted bladders (Fig. 1C), as seen by CLSM analysis. However, the bladders of implanted animals appeared to have more extensive exfoliation than nonimplanted animals at 6 hpi with UPEC colonization selectively localized in the remaining umbrella cells and not in the exposed underlying epithelium, as determined by CLSM analysis (see Fig. S2 in the supplemental material). The bacterial CFU were similar in the bladders of implanted and nonimplanted animals (data not shown, but see Fig. 3). The discrepancy between IBC number and CFU in implanted bladders suggested that in the implanted mice, an increased proportion of the CFU were comprised of niches outside of IBCs such as in extracellular niches or non-IBC intracellular niches. Together, these findings indicate that urinary catheterization alters the pathogenic cascade of UPEC resulting in decreased IBC formation. This is likely due to increased exfoliation in catheterized bladders.
Fig 1.
Uropathogenic E. coli produces IBCs in the superficial umbrella cells of implanted bladders. (A) Representative images of splayed bladders of female C57BL/6Ncr mice infected with UTI89 at 6 hpi in the absence (nonimplanted) or presence (implanted) of implants after LacZ staining. Each black arrow indicates a purple speck, indicative of an IBC. (B) Quantification of IBC formation following LacZ staining at 6 hpi. Each symbol represents IBC number from a single mouse from two independent experiments with n = 5/group. The P value was obtained using the Mann-Whitney U test. (C) Representative CLSM images of whole bladders from nonimplanted and implanted animals infected with UTI89 ectopically expressing GFP (green), stained with DNA dye SYTO83 (red), and an Alexa Fluor 633 conjugate of WGA (blue) reveal the presence of IBC within umbrella cells. Scale bar, 20 μm.
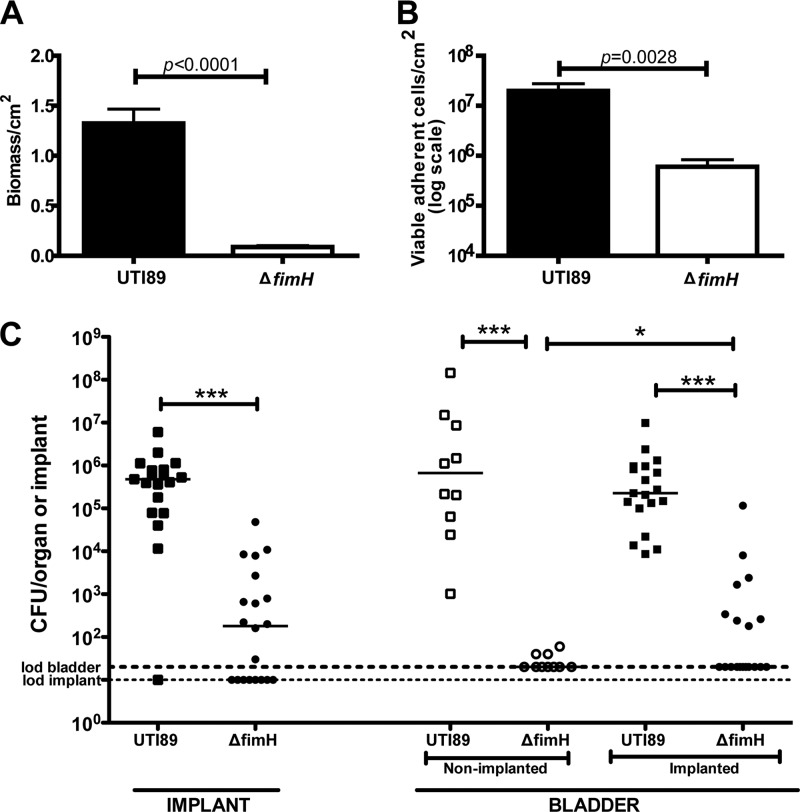
Fig 3.
Deletion of fimH reduces biofilm formation and attenuates UPEC virulence. Graphs represent crystal violet-based quantification (A) and CFU enumeration in a logarithmic scale (log scale) (B) of 24-h-old UTI89 and UTI89ΔfimH (ΔfimH) biofilms under human urine flow on silicone tubing at 37°C, indicating that the ΔfimH strain is defective in biofilm formation under these conditions. The bars represent means of three independent experiments, and error bars indicate the standard errors of the mean. The P values were determined using the Mann-Whitney U test. (C) Graph representing bacterial titers in log scale recovered from implants and homogenized bladders of nonimplanted (open symbols) and implanted (closed symbols) infected with either UTI89 (squares) or UTI89ΔfimH (circles) for 24 h. Horizontal dashed lines represent the limit of detection for viable bacteria. Each symbol represents a mouse from at least two independent experiments with n = 5/group. The horizontal bars represent the median of each data set. *, P < 0.05; ***, P < 0.0005 (as determined by the Mann-Whitney U test).
Bacteria originating from existing UPEC reservoirs can seed urinary implant colonization.
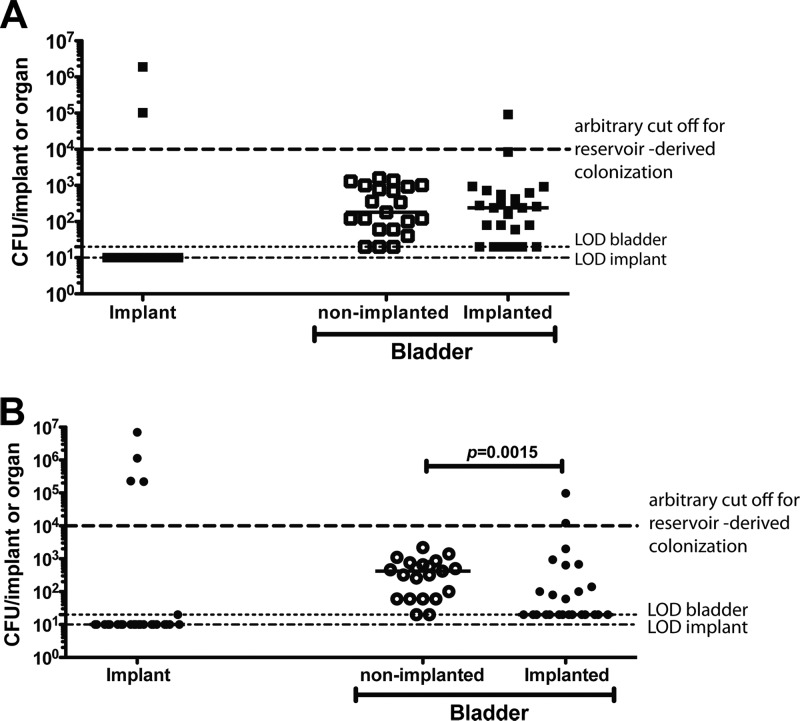
One troubling possible outcome of the UPEC pathogenic cascade is the establishment of quiescent intracellular reservoirs (QIRs) in the underlying epithelial layers. QIRs have been defined as intracellular bacterial reservoirs of 101 and 104 CFU/bladder established during the course of an active UTI that persist in the bladder tissue even after clearance of bacteriuria (36, 37, 52). Bacteria from these QIRs can reemerge upon stimulation of uroepithelial turnover (37) and have been shown to seed recurrent UTIs (rUTIs) (52). We have previously shown that urinary catheterization causes damage to the protective uroepithelial layer (9, 17, 18, 28, 44). Thus, we hypothesized that urinary catheterization might perturb existing UPEC reservoirs and result in bacteriuria, catheter colonization, and further dissemination. To test this hypothesis, mice were infected with 1 × 107 to 2 × 107 CFU UTI89HK::GFP, and infection was allowed to resolve over the course of 2 weeks. On day 14 postinfection, urine was collected from each animal to assess their bacteriuric state (see Fig. S3A in the supplemental material) prior to urinary implantation of a subset of the animals. Animals with urine titers above the limit of detection (200 or 400 CFU/ml) in each experiment were considered bacteriuric with active (unresolved and or recurrent) infection and were removed from further analyses. Only abacteriuric animals (i.e., with urine titers at the limit of detection) presumed to either have completely cleared the infection or to have bacterial reservoirs within the bladder were used for further analyses. At 3 or 5 days postimplantation, bacterial titers from implants and bladder were assessed to determine whether a previously established reservoir could serve as a nidus for CAUTI. On day 3 postimplantation, UPEC UTI89HK::GFP biofilms had formed on implants in 2 of 25 implanted mice (∼8%). One implanted mouse had bladder colonization greater than 104 CFU/ml compared to none of the 21 similarly infected but nonimplanted abacteriuric animals (Fig. 2A). There was no significant difference in bladder colonization between groups at 3 dpi. For mice assessed at 5 days postimplantation, UTI89HK::GFP biofilms were recovered from implants of 4 of 31 implanted animals (∼13%) with 2 of the mice having bladder titers >104 CFU/ml (Fig. 2B) compared to 0 of 21 in nonimplanted animals. Interestingly, there were overall significantly fewer bacteria recovered from the bladders of implanted animals compared to nonimplanted animals at 5 dpi (P = 0.0015), possibly the result of implant induced exfoliation of the uroepithelium, which is thought to be part of an innate defense to clear tissue associated bacteria (35). Treatment of UPEC-infected mice at 14 dpi with protamine sulfate, a chemical that leads to exfoliation of the superficial umbrella cells of the uroepithelium, resulted in clearance of tissue-associated bacteria in 100% of treated animals (37). Figure S3B in the supplemental material matches the urine titers on day 14, which were at the level of detection, with the implant and bladder titers on day 17 or 19 postinfection for each of the reinfected animals. Together, these findings indicate that bacteria from previously established reservoirs can reemerge and colonize the urinary catheter.
Fig 2.
UPEC reservoir reactivation can lead to urinary implant and bladder colonization. Graphs represent bacterial titers in log scale recovered from implants and homogenized bladders of nonimplanted or implanted for 3 days (A) or 5 days (B) animals that were nonbacteriuric and previously infected for 14 dpi with UTI89HK::GFP. Horizontal dashed lines represent the limit of detection for viable bacteria. Each symbol represents a mouse from two independent experiments with n = 10 to 20/group/experiment. The horizontal bars represent the median of each data set. The P value was determined by using the Mann-Whitney U test.
FimH is required for biofilm formation and UPEC colonization of the urinary tract after catheter implantation.
Biofilm formation is a critical component of CAUTI pathophysiology (49, 57). We have previously shown that UPEC is able to produce biofilms on the surface of the foreign body and is recovered from the catheterized murine bladder at high titers with a median of 1.9 × 105 at 24 hpi (18). As discussed above, type 1 pili are UPEC virulence factors (42) that have been shown to be critical in bladder colonization, IBC formation, and other aspects of UPEC uropathogenesis. In addition, type 1 pili have been shown to be important in biofilm formation in vitro (48). Other UPEC fibers associated with biofilm formation include S pili, curli, and flagella (4, 11, 32, 39, 63). Thus, we assessed the contribution of each of these fibers to biofilm formation in vitro on silicone tubing in filtered human urine under flow conditions and UPEC-mediated CAUTI in vivo. Deletion of the fimH gene significantly reduced biofilm formation on silicone tubing in human urine in vitro (P < 0.0001) as measured by lower biomass (Fig. 3A) and an ∼2-fold reduction in adherent viable bacteria (Fig. 3B). In contrast, deletions of the sfa operon (S pili), csgA (curli) or fliC (flagella) had no effect on biofilm formation under these conditions (data not shown).
In vivo, UTI89ΔfimH was severely attenuated in the murine model of foreign body-associated UTI (Fig. 3C) similar to what has been seen previously in the murine model of cystitis (5, 31, 35, 62). UTI89ΔfimH displayed >3 log fewer CFU in the bladder at 24 hpi and did not ascend to the kidneys (see Fig. S4A in the supplemental material). Further, deletion of fimH significantly reduced the ability of UTI89 to colonize the implants (P < 0.0001). Similar to the in vitro experiments, S pili and curli were not required for CAUTI. The strains UTI89ΔsfaA-H and UTI89ΔcsgA behaved identically to wild-type UTI89 (see Fig. S4A and B in the supplemental material). A double deletion of both sfaA-H and fimB-H recapitulated the UTI89ΔfimH phenotype (see Fig. S4A in the supplemental material). Although colonization of the implants was significantly reduced in the ΔfimH strain, residual binding to implants and bladders was detected, which was therefore presumably mediated by other pili or biofilm determinants. Together, these data strongly argue that the type 1 pilus tip adhesin FimH is a critical determinant of UPEC virulence in mediating biofilm formation and virulence during CAUTI.
Mannoside treatment reduces IBC formation.
Having established that FimH is required for UPEC virulence in implanted bladders, we investigated type 1 pili as a potential therapeutic target for CAUTI using small molecule inhibitors called mannosides designed to block the function of the type 1 pilus tip adhesin FimH. We first assessed the ability of methyl-α-d-mannopyranoside (methyl mannose) to block UTI89 biofilm formation on silicone tubing in human urine under flow conditions. 1% methyl mannose significantly blocked the ability of UTI89 to form biofilms, as measured by reduced biomass (P = 0.0022) and reduced biofilm-adherent cells (P = 0.0012), compared to untreated controls (see Fig. S5 in the supplemental material), an effect similar to what was observed for the deletion of fimH (Fig. 3A). Methyl mannose is a FimH antagonist, thus confirming the critical role of type 1 pili in biofilm formation on silicone tubing in urine as was previously described for biofilms formed in LB medium (45).
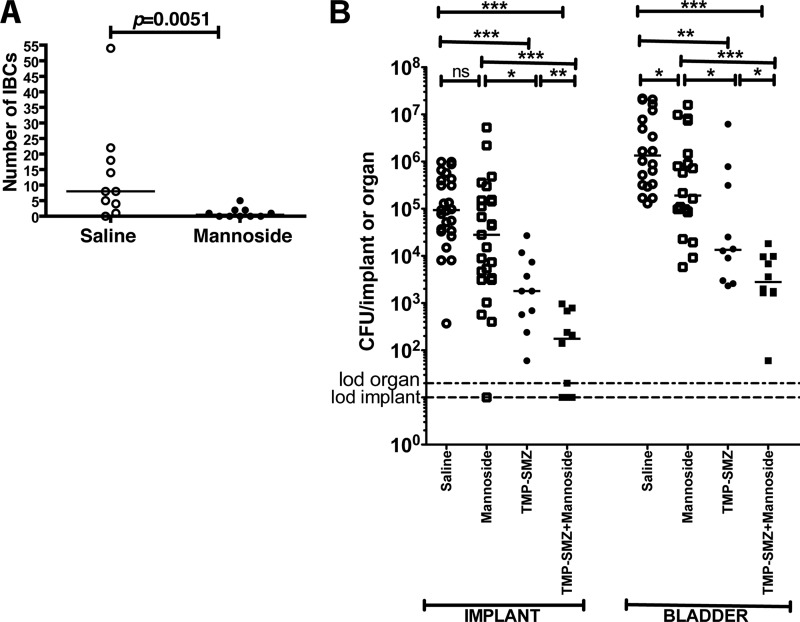
Methyl mannose exhibits poor bioavailability (1), whereas mannoside compounds 6, 8, and 10 have recently been shown to be orally bioavailable (8, 19) and potent therapeutics in the treatment and prevention of acute and chronic UPEC infections (8). Mannoside 6 is ∼1,000-fold more active in inhibiting FimH than are methyl mannose and heptyl mannose (which is a more potent inhibitor of FimH than methyl mannose) both in vitro and in vivo (8, 15, 20, 61). Thus, mannoside 6 was tested for its therapeutic potential to prevent CAUTI. Mice were treated i.p. with saline or 5 mg of mannoside 6/kg at 30 min prior to urinary implantation. Catheter implantation was immediately followed by transurethral inoculation of UTI89. Based on pharmacokinetic studies, this dose of mannoside 6 has been shown to be present in the bladder above the 50% inhibitory concentrations for 8 h (8). Thus, IBC formation was assayed by LacZ staining at 6 hpi. As shown in Fig. 4A, mannoside treatment significantly reduced IBC formation (P = 0.0051) in implanted animals. These findings indicate that mannoside 6 prevents bacterial invasion into the bladder tissue. If translated to a human application, prevention of bacterial invasion into bladder tissue could enhance the efficacy of current management protocols of removing the catheter (56) by preventing bladder colonization and thus preventing CAUTI.
Fig 4.
Mannoside treatment prevents IBC formation and UPEC virulence when used in combination with TMP-SMZ. (A) Graph representing IBC enumeration from LacZ staining of splayed bladders of female C57BL/6Ncr mice treated i.p. with mannoside or saline prior to transurethral implantation and inoculation with UTI89 at 6 hpi. Each symbol represents the IBC number from a single mouse from two independent experiments with n = 5/group. The P value was determined using the Mann-Whitney U test. (B) Graph representing bacterial titers in log scale recovered at 6 hpi from implants and homogenized bladders of animals treated with saline (○), mannoside (□),TMP-SMZ (●), and TMP-SMZ plus mannoside (■) prior to urinary implantation and inoculation with UTI89. Horizontal dashed lines represent the limit of detection for viable bacteria. Each symbol represents a mouse from at least two independent experiments with n = 5/group. The horizontal bars represent the median of each data set. *, P < 0.05; **, P < 0.0005; ***, P < 0.0005. ns, P > 0.05. Significance was evaluated by using the Mann-Whitney U test.
Mannoside treatment increases the efficiency of TMP-SMZ in preventing UPEC colonization.
Mannosides have been shown to potentiate the efficacy of TMP-SMZ when used in a combination treatment (8). This is due to the mechanism of action of mannosides in that they prevent bacterial invasion into bladder tissue and thus sequester bacteria to an extracellular niche. Since TMP-SMZ concentrates in the urine, this mechanism of action of mannosides results in bacterial exposure to increased concentrations of TMP-SMZ (8). Thus, the ability of mannosides to potentiate TMP-SMZ for the prevention of CAUTI was investigated by treating animals with 54 and 270 μg of TMP-SMZ/ml, respectively, in their drinking water for 3 days followed by treatment with saline or mannoside 6 (5 mg/kg) i.p. 30 min prior to implantation and bacterial inoculation. Treatment with mannoside or saline alone was also investigated. As shown in Fig. 4B, mannoside only treatment significantly reduced bladder colonization (P = 0.0114) in implanted animals but did not significantly prevent biofilm buildup on the catheter implant in vivo compared to saline-treated animals (P = 0.0547) (Fig. 4B). In previous studies, mannoside 6 was significantly more effective in preventing bladder colonization than TMP-SMZ at 6 hpi (8) however this was not the case in the presence of the catheter implant (Fig. 4B). However, in combination, mannoside potentiated the efficacy of TMP-SMZ resulting in a significant decrease in UPEC colonization of implants and bladders compared to treatment with antibiotic alone (P < 0.0005 in all cases) (Fig. 4B), as has been previously observed in the absence of catheters (8). These data establish the efficacy of combinatorial therapy for prevention of UPEC CAUTI in this model and suggest that further optimization of pharmacokinetic properties of the mannosides may improve the efficacy of mannosides in the presence of a catheter implant.
DISCUSSION
UPEC is the major etiological agent of CAUTI (22). However, the molecular mechanisms of urinary catheter and bladder colonization after urinary catheterization have not been elucidated. Studies in an optimized murine model of foreign body-associated UTI (18) show that urinary catheterization favors UPEC exploitation of the bladder extracellular milieu. We showed here that this occurs via type 1 pilus-dependent biofilm formation on the surface of silicone implants in the murine bladder. Thus, type 1 pili mediate implant and bladder colonization during CAUTI, providing definitive experimental evidence for previous reports postulating that type 1 pili may be required for UPEC persistence during CAUTIs (34). Interestingly, fimH-deficient UPEC strains have the ability to adhere and colonize the foreign body, albeit at significantly lower levels. This suggests the involvement of multiple biofilm determinants in this process (24) although mutant strains unable to express curli, S pili or flagella behaved similar to wild-type UTI89 in the murine model of CAUTI.
The absence of bacteriuria may not reflect the bacteriologic state of the bladder (51). QIRs can be established during an acute UTI and persist in the bladder tissue even upon resolution of bacteriuria. QIRs have been shown to be able to reemerge to cause recurrent UTI following damage to the uroepithelium (37, 52). Our findings indicate that urinary implantation of animals having resolved bacteriuria from a previous UTI, can present with recurrent bacteriuria with the original strain, strongly suggesting that tissue-associated reservoirs, which persist in the absence of bacteriuria, can provide a nidus for catheter colonization and lead to CAUTI. Thus, although CAUTI may be caused by introduction of bacteria into the urinary tract from the GI and vaginal tracts and periurethral areas (16), our murine model indicates that bacterial reservoirs existing within the urinary tract in the absence of bacteriuria may also serve as a nidus for CAUTI. These findings underscore the need for future epidemiological studies on the contribution of UPEC reservoirs in the establishment of rUTIs and CAUTIs.
In the presence of the silicone implant in the bladder lumen, IBC formation, a type 1 pilus-mediated process during UPEC pathogenesis, is significantly reduced compared to nonimplanted animals. After urinary catheterization, increased exfoliation or damage to the uroepithelium results in a denuded epithelium (9, 12, 17, 18, 28, 33, 44), a condition that does not efficiently support IBC formation, which is thought to occur only in terminally differentiated superficial umbrella cells. Thus, by day 5 after implantation of abacteriuric animals with a history of UTI, there is a significant reduction in bacterial bladder CFU compared to nonimplanted animals. A similar effect has been observed previously wherein denuding the uroepithelium of UPEC-infected mice at 14 dpi with protamine sulfate resulted in the clearance of QIRs and tissue-associated bacteria in all of the treated animals (37). Thus, urothelial exfoliation, either chemically or from urinary catheterization, can lead to the elimination of tissue-associated reservoirs that can otherwise serve as a nidus for rUTI. However, the tissue damage and exfoliation caused by implantation is a double-edged sword. Although it can result in immune-mediated clearance or exfoliation of infected cells, an alternative outcome is that a preexisting reservoir may gain a foothold in the urinary tract by colonizing the catheter, which can subsequently lead to CAUTI.
The identification of FimH as a critical virulence factor during UPEC CAUTI provides an avenue for the development of novel preventative measures against these infections. There has recently been an upsurge in recommendations and guidelines for management of CAUTIs and rUTIs in catheterized patients, with a particular focus on preventative measures (53), including the limited use of catheters, bacterial interference strategies, and even the prophylactic use of antibiotics prior and following catheter removal (7, 40, 56). However, the inappropriate use of antibiotics can exacerbate the development of antibiotic resistance (3, 29, 40). We have rationally designed small-molecule inhibitors of FimH called mannosides (8, 19, 20) that block FimH-mediated functions. In a murine model, we demonstrated that our mannosides were orally bioavailable (19) and highly efficacious for the treatment and prevention of chronic cystitis (8, 19). Thus, we investigated here their utility in preventing CAUTIs. We discovered that pretreatment with mannosides significantly prevented invasion and colonization of the bladder epithelium following UPEC infection of implanted bladders. The inability of mannoside treatment to eliminate UPEC from the implant, given that methyl mannose inhibits UPEC biofilm on catheter material in urine in vitro, may be due to the lack of urine flow in this implanted murine model. Urine flow through the catheter may enhance the efficacy of mannosides on implant clearance. In addition, improved dosing schedules or use of the newly optimized mannoside 8 or 10, which have better potency and improved pharmacokinetic properties (8), may improve the efficacy of mannoside in preventing biofilm formation on the catheter in vivo. Mannosides have been shown to potentiate the efficacy of TMP-SMZ and are able to convert clinically resistant strains to becoming susceptible (8). This activity is due to the mechanism of action of mannosides in preventing bacterial colonization and invasion of bladder tissue thus sequestering bacteria in the bladder lumen. TMP-SMZ is known to concentrate in the urine to levels above the MIC (8). Here, we found that mannosides were also able to potentiate the efficacy of TMP-SMZ in preventing CAUTI. Future research will be focused on improving pharmacokinetic properties and developing enhanced mannoside compounds (19) that are effective at disrupting established biofilms in vivo and exploring combination therapies with other classes of biofilm inhibitors (4, 45).
Urinary catheterization is a necessary medical procedure that causes major damage to the urinary tract. Pathogens, such as UPEC, take advantage of this compromised environment to exploit new and existing niches and establish severe infections. This report uncovers important molecular mechanisms underlying UPEC pathogenesis following urinary catheterization and raises important questions regarding the bacterial origins of urinary catheter colonization and subsequent CAUTI. In humans, removal of the contaminated urinary catheter is the preferred method for treatment of these CAUTI (57, 59). However, the presence of intracellular bacterial niches can serve as a nidus for recurrent UTI and CAUTI. If translated to application in humans, prophylaxis with mannosides could reduce the rates of CAUTI by preventing the ability of UPEC to colonize and invade the bladder and possibly by also preventing biofilm formation on the catheter material. In such instances, mannosides could potentially be used in accordance with the current guidelines for management of CAUTI and in combination with currently used antibiotics and other potential preventative measures (53) prior to urinary catheterization. This virulence-based combinatorial approach holds promise to be able to reduce the rate of rUTIs and CAUTIs originating from gastrointestinal, vaginal and urinary tract bacterial reservoirs, thus enhancing the efficacy of existing therapies.
Supplementary Material
ACKNOWLEDGMENTS
We thank Wandy Beatty for technical assistance with the CLSM, Osarenoma Olomu and Richard Chole for providing technical assistance with biofilm cultivation in the flow chamber system and peristaltic pumps (Watson Marlow 205S), Drew Schwartz for technical assistance with the IBC assays, and Kelly Wright for generating some of the mutants used in this study.
This study was funded by National Institutes of Health grants DK64540, DK51406, AI48689 (S.J.H.), and AI46433 (M.G.C.) and by an ASM Robert D. Watkins Graduate Research Fellowship Award (P.S.G.).
Footnotes
Published ahead of print 25 June 2012
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Alton G, Kjaergaard S, Etchison JR, Skovby F, Freeze HH. 1997. Oral ingestion of mannose elevates blood mannose levels: a first step toward a potential therapy for carbohydrate-deficient glycoprotein syndrome type I. Biochem. Mol. Med. 60:127–133 [DOI] [PubMed] [Google Scholar]
- 2. Anderson GG, et al. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105–107 [DOI] [PubMed] [Google Scholar]
- 3. Bi XC, et al. 2009. Pathogen incidence and antibiotic resistance patterns of catheter-associated urinary tract infection in children. J. Chemother. 21:661–665 [DOI] [PubMed] [Google Scholar]
- 4. Cegelski L, et al. 2009. Small-molecule inhibitors target Escherichia coli amyloid biogenesis and biofilm formation. Nat. Chem. Biol. 5:913–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen SL, et al. 2009. Positive selection identifies an in vivo role for FimH during urinary tract infection in addition to mannose binding. Proc. Natl. Acad. Sci. U. S. A. 106:22439–22444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Christensen BB, et al. 1999. Molecular tools for study of biofilm physiology. Methods Enzymol. 310:20–42 [DOI] [PubMed] [Google Scholar]
- 7. Conway LJ, Larson EL. 2012. Guidelines to prevent catheter-associated urinary tract infection: 1980 to 2010. Heart Lung 41:271–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cusumano CK, et al. 2011. Treatment and prevention of urinary tract infection with orally active FimH inhibitors. Sci. Transl. Med. 3:109–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Delnay KM, Stonehill WH, Goldman H, Jukkola AF, Dmochowski RR. 1999. Bladder histological changes associated with chronic indwelling urinary catheter. J. Urol. 161:1106–1109 [PubMed] [Google Scholar]
- 10. Dodson KW, et al. 2001. Structural basis of the interaction of the pyelonephritic Escherichia coli adhesin to its human kidney receptor. Cell 105:733–743 [DOI] [PubMed] [Google Scholar]
- 11. Ejernaes K. 2011. Bacterial characteristics of importance for recurrent urinary tract infections caused by Escherichia coli. Danish Med. Bull. 58:B4187. [PubMed] [Google Scholar]
- 12. Elliott TS, Reid L, Rao GG, Rigby RC, Woodhouse K. 1989. Bladder irrigation or irritation? Br. J. Urol. 64:391–394 [DOI] [PubMed] [Google Scholar]
- 13. Eto DS, Jones TA, Sundsbak JL, Mulvey MA. 2007. Integrin-mediated host cell invasion by type 1-piliated uropathogenic Escherichia coli. PLoS Pathog. 3:e100 doi:10.1371/journal.ppat.0030100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ferrieres L, Hancock V, Klemm P. 2007. Specific selection for virulent urinary tract infectious Escherichia coli strains during catheter-associated biofilm formation. FEMS Immunol. Med. Microbiol. 51:212–219 [DOI] [PubMed] [Google Scholar]
- 15. Firon N, Ashkenazi S, Mirelman D, Ofek I, Sharon N. 1987. Aromatic alpha-glycosides of mannose are powerful inhibitors of the adherence of type 1 fimbriated Escherichia coli to yeast and intestinal epithelial cells. Infect. Immun. 55:472–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garibaldi RA, Burke JP, Britt MR, Miller MA, Smith CB. 1980. Meatal colonization and catheter-associated bacteriuria. N. Engl. J. Med. 303:316–318 [DOI] [PubMed] [Google Scholar]
- 17. Goble NM, Clarke T, Hammonds JC. 1989. Histological changes in the urinary bladder secondary to urethral catheterisation. Br. J. Urol. 63:354–357 [DOI] [PubMed] [Google Scholar]
- 18. Guiton PS, Hung CS, Hancock LE, Caparon MG, Hultgren SJ. 2010. Enterococcal biofilm formation and virulence in an optimized murine model of foreign body-associated urinary tract infections. Infect. Immun. 78:4166–4175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Han Z, et al. 2012. Lead optimization studies on FimH antagonists: discovery of potent and orally bioavailable ortho-substituted biphenyl mannosides. J. Med. Chem. 55:3945–3959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Han Z, et al. 2010. Structure-based drug design and optimization of mannoside bacterial FimH antagonists. J. Med. Chem. 53:4779–4792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hannan TJ, Mysorekar IU, Hung CS, Isaacson-Schmid ML, Hultgren SJ. 2010. Early severe inflammatory responses to uropathogenic Escherichia coli predispose to chronic and recurrent urinary tract infection. PLoS Pathog. 6:e1001042 doi:10.1371/journal.ppat.1001042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hidron AI, et al. 2008. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the national health care safety network at the Centers for Disease Control and Prevention, 2006–2007. Infect. Control Hosp. Epidemiol. 29:996–1011 [DOI] [PubMed] [Google Scholar]
- 23. Hung CS, et al. 2002. Structural basis of tropism of Escherichia coli to the bladder during urinary tract infection. Mol. Microbiol. 44:903–915 [DOI] [PubMed] [Google Scholar]
- 24. Jacobsen SM, Stickler DJ, Mobley HL, Shirtliff ME. 2008. Complicated catheter-associated urinary tract infections due to Escherichia coli and Proteus mirabilis. Clin. Microbiol. Rev. 21:26–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Justice SS, et al. 2004. Differentiation and developmental pathways of uropathogenic Escherichia coli in urinary tract pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 101:1333–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Justice SS, Lauer SR, Hultgren SJ, Hunstad DA. 2006. Maturation of intracellular Escherichia coli communities requires SurA. Infect. Immun. 74:4793–4800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kucheria R, Dasgupta P, Sacks SH, Khan MS, Sheerin NS. 2005. Urinary tract infections: new insights into a common problem. Postgrad. Med. J. 81:83–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kurosaka Y, et al. 2001. A non-surgical rat model of foreign body-associated urinary tract infection with Pseudomonas aeruginosa. Microbiol. Immunol. 45:9–15 [DOI] [PubMed] [Google Scholar]
- 29. Magoha GA. 1997. Nosocomial infection of the urinary tract: pattern of antibiotic use and drug resistance. East African Med. J. 74:190–192 [PubMed] [Google Scholar]
- 30. Martinez JJ, Hultgren SJ. 2002. Requirement of Rho-family GTPases in the invasion of type 1-piliated uropathogenic Escherichia coli. Cell. Microbiol. 4:19–28 [DOI] [PubMed] [Google Scholar]
- 31. Martinez JJ, Mulvey MA, Schilling JD, Pinkner JS, Hultgren SJ. 2000. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 19:2803–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Martinez-Medina M, et al. 2009. Biofilm formation as a novel phenotypic feature of adherent-invasive Escherichia coli (AIEC). BMC Microbiol. 9:202 doi:10.1186/1471-2180-9-202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McTaggart LA, Rigby RC, Elliott TS. 1990. The pathogenesis of urinary tract infections associated with Escherichia coli, Staphylococcus saprophyticus, and S. epidermidis. J. Med. Microbiol. 32:135–141 [DOI] [PubMed] [Google Scholar]
- 34. Mobley HL, Chippendale GR, Tenney JH, Hull RA, Warren JW. 1987. Expression of type 1 fimbriae may be required for persistence of Escherichia coli in the catheterized urinary tract. J. Clin. Microbiol. 25:2253–2257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mulvey MA, et al. 1998. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282:1494–1497 [DOI] [PubMed] [Google Scholar]
- 36. Mulvey MA, Schilling JD, Hultgren SJ. 2001. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect. Immun. 69:4572–4579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mysorekar IU, Hultgren SJ. 2006. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc. Natl. Acad. Sci. U. S. A. 103:14170–14175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mysorekar IU, Mulvey MA, Hultgren SJ, Gordon JI. 2002. Molecular regulation of urothelial renewal and host defenses during infection with uropathogenic Escherichia coli. J. Biol. Chem. 277:7412–7419 [DOI] [PubMed] [Google Scholar]
- 39. Naves P, et al. 2008. Correlation between virulence factors and in vitro biofilm formation by Escherichia coli strains. Microb. Pathog. 45:86–91 [DOI] [PubMed] [Google Scholar]
- 40. Nazarko L. 2011. Should antibiotics be prescribed when urinary catheters are removed? Br. J. Community Nursing 16:374–380 [DOI] [PubMed] [Google Scholar]
- 41. Nicholson TF, Watts KM, Hunstad DA. 2009. OmpA of uropathogenic Escherichia coli promotes postinvasion pathogenesis of cystitis. Infect. Immun. 77:5245–5251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nielubowicz GR, Mobley HL. 2010. Host-pathogen interactions in urinary tract infection. Nat. Rev. Urol. 7:430–441 [DOI] [PubMed] [Google Scholar]
- 43. Parsons CL. 1986. Pathogenesis of urinary tract infections: bacterial adherence, bladder defense mechanisms. Urol. C. N. Am. 13:563–568 [PubMed] [Google Scholar]
- 44. Peychl L, Zalud R. 2008. Changes in the urinary bladder caused by short-term permanent catheter insertion. Casopis Lekaru Ceskych. 147:325–329 (In Czech.) [PubMed] [Google Scholar]
- 45. Pinkner JS, et al. 2006. Rationally designed small compounds inhibit pilus biogenesis in uropathogenic bacteria. Proc. Natl. Acad. Sci. U. S. A. 103:17897–17902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ronald LS, et al. 2008. Adaptive mutations in the signal peptide of the type 1 fimbrial adhesin of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 105:10937–10942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rosen DA, Hooton TM, Stamm WE, Humphrey PA, Hultgren SJ. 2007. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 4:e329 doi:10.1371/journal.pmed.0040329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rosen DA, et al. 2008. Molecular variations in Klebsiella pneumoniae and Escherichia coli FimH affect function and pathogenesis in the urinary tract. Infect. Immun. 76:3346–3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Saint S, Chenoweth CE. 2003. Biofilms and catheter-associated urinary tract infections. Infect. Dis. Clin. N. Am. 17:411–432 [DOI] [PubMed] [Google Scholar]
- 50. Sauer FG, et al. 1999. Structural basis of chaperone function and pilus biogenesis. Science 285:1058–1061 [DOI] [PubMed] [Google Scholar]
- 51. Schaeffer AJ, Schwan WR, Hultgren SJ, Duncan JL. 1987. Relationship of type 1 pilus expression in Escherichia coli to ascending urinary tract infections in mice. Infect. Immun. 55:373–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schilling JD, Lorenz RG, Hultgren SJ. 2002. Effect of trimethoprim-sulfamethoxazole on recurrent bacteriuria and bacterial persistence in mice infected with uropathogenic Escherichia coli. Infect. Immun. 70:7042–7049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Siddiq DM, Darouiche RO. 2012. New strategies to prevent catheter-associated urinary tract infections. Nat. Rev. Urol. 9:305–314 [DOI] [PubMed] [Google Scholar]
- 54. Song J, et al. 2009. TLR4-mediated expulsion of bacteria from infected bladder epithelial cells. Proc. Natl. Acad. Sci. U. S. A. 106:14966–14971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sun TT, Zhao H, Provet J, Aebi U, Wu XR. 1996. Formation of asymmetric unit membrane during urothelial differentiation. Mol. Biol. Rep. 23:3–11 [DOI] [PubMed] [Google Scholar]
- 56. Trautner BW. 2010. Management of catheter-associated urinary tract infection. Curr. Opin. Infect. Dis. 23:76–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Trautner BW, Darouiche RO. 2004. Role of biofilm in catheter-associated urinary tract infection. Am. J. Infect. Control 32:177–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Waksman G, Hultgren SJ. 2009. Structural biology of the chaperone-usher pathway of pilus biogenesis. Nat. Rev. Microbiol. 7:765–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Warren JW. 2001. Catheter-associated urinary tract infections. Int. J. Antimicrob. Agents 17:299–303 [DOI] [PubMed] [Google Scholar]
- 60. Weissman SJ, et al. 2007. Differential stability and trade-off effects of pathoadaptive mutations in the Escherichia coli FimH adhesin. Infect. Immun. 75:3548–3555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wellens A, et al. 2008. Intervening with urinary tract infections using anti-adhesives based on the crystal structure of the FimH-oligomannose-3 complex. PLoS One 3:e2040 doi:10.1371/journal.pone.0002040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wright KJ, Seed PC, Hultgren SJ. 2007. Development of intracellular bacterial communities of uropathogenic Escherichia coli depends on type 1 pili. Cell. Microbiol. 9:2230–2241 [DOI] [PubMed] [Google Scholar]
- 63. Wright KJ, Seed PC, Hultgren SJ. 2005. Uropathogenic Escherichia coli flagella aid in efficient urinary tract colonization. Infect. Immun. 73:7657–7668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yu J, Lin JH, Wu XR, Sun TT. 1994. Uroplakins Ia and Ib, two major differentiation products of bladder epithelium, belong to a family of four transmembrane domain (4TM) proteins. J. Cell Biol. 125:171–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zhou G, et al. 2001. Uroplakin Ia is the urothelial receptor for uropathogenic Escherichia coli: evidence from in vitro FimH binding. J. Cell Sci. 114:4095–4103 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.