Abstract
Tedizolid (TR-700, formerly torezolid) is the active moiety of the prodrug tedizolid phosphate (TR-701), a next-generation oxazolidinone, with high potency against Gram-positive species, including methicillin-resistant Staphylococcus aureus (MRSA). A recently completed randomized, double-blind phase 2 trial evaluated 200, 300, or 400 mg of oral tedizolid phosphate once daily for 5 to 7 days in patients with complicated skin and skin structure infections. This report examines the in vitro activity of tedizolid and Zyvox (linezolid) against Gram-positive pathogens isolated at baseline and describes the microbiological and clinical efficacy of tedizolid. Of 196 isolates tested, 81.6% were S. aureus, and of these, 76% were MRSA. The MIC50 and MIC90 of tedizolid against both methicillin-susceptible S. aureus (MSSA) and MRSA were 0.25 μg/ml, compared with a MIC50 of 1 μg/ml and MIC90 of 2 μg/ml for linezolid. For coagulase-negative staphylococci (n = 7), viridans group streptococci (n = 15), and beta-hemolytic streptococci (n = 3), the MICs ranged from 0.03 to 0.25 μg/ml for tedizolid and from 0.12 to 1 μg/ml for linezolid. The microbiological eradication rates at the test-of-cure visit (7 to 14 days posttreatment) in the microbiologically evaluable population (n = 133) were similar in all treatment groups, with overall eradication rates of 97.7% for all pathogens, 97.9% for MRSA, and 95.7% for MSSA. The clinical cure rates for MRSA and MSSA infections were 96.9% and 95.7%, respectively, across all dose groups. This study confirms the potent in vitro activity of tedizolid against pathogenic Gram-positive cocci, including MRSA, and its 4-fold-greater potency in comparison with linezolid. All dosages of tedizolid phosphate showed excellent microbiological and clinical efficacy against MRSA and MSSA.
INTRODUCTION
Acute bacterial skin and skin structure infections (ABSSSIs), previously termed complicated skin and skin structure infections (cSSSIs), are a frequent indication for antibiotic therapy and an increasing cause of hospitalization (11). A majority of ABSSSIs are caused by aerobic Gram-positive cocci, including Staphylococcus aureus, beta-hemolytic streptococci, enterococci, and certain coagulase-negative staphylococci (12, 26). First recognized in 1960, methicillin resistance in S. aureus has become widespread in the health care setting and in the community (4, 13, 15, 19, 24, 28, 29, 34).
With the spread of methicillin-resistant S. aureus (MRSA) in the community, the development of empirical antimicrobial therapeutic strategies for ABSSSIs has become more difficult. The effectiveness of clindamycin, one of the older agents available for treating MRSA, has been limited in use by high rates of resistance and the need to evaluate for inducible resistance (14, 30). The emergence of vancomycin-intermediate and vancomycin-resistant S. aureus strains has compromised the use of vancomycin, currently the mainstay of treatment for serious infections caused by Gram-positive pathogens (1, 16). Documented resistance to Zyvox (linezolid), the first member of the oxazolidinone class of antibiotics to be approved for complicated skin and skin structure infections (cSSSI) due to MRSA, is rare but has been reported among staphylococci and enterococci (10, 17, 18) and is associated with mutations in the 23S rRNA gene or the presence of the chloramphenicol-florfenicol resistance (cfr) methyltransferase gene (2, 23, 27). cfr methylation confers resistance to multiple classes of ribosome-targeting antibiotics and is worrisome due to its association with plasmids and, thus, the potential for more widespread dissemination. These findings underscore the eroding availability of therapeutic choices that can be used to manage infections caused by MRSA and highlight the need for new agents designed to address evolving mechanisms of resistance in Gram-positive pathogens.
Tedizolid (TR-700; Trius Therapeutics, Inc., San Diego, CA) is the active moiety of the prodrug tedizolid phosphate (TR-701), a novel once-daily oral or intravenous agent with attributes that distinguish it from other oxazolidinones. Tedizolid is being developed for treatment of serious infections caused by Gram-positive organisms, including MRSA. Tedizolid has potent activity against clinically important Gram-positive aerobic and anaerobic bacteria, with at least 4-fold greater in vitro activity than linezolid against staphylococci, including MRSA, streptococci, and enterococci (5, 32). Tedizolid also retains activity against vancomycin-resistant enterococci and linezolid-resistant MRSA strains harboring the cfr multidrug resistance gene (20, 33). Unlike linezolid, a bacteriostatic agent, tedizolid was bactericidal in vivo when tested in a mouse thigh infection model (21).
A phase 2 dose-ranging study evaluated the safety, efficacy, and tolerability of oral tedizolid phosphate at 200, 300, and 400 mg once daily for 5 to 7 days in the treatment of patients with cSSSIs (31). This report examines the in vitro activities of tedizolid and linezolid against Gram-positive pathogens isolated from patients in the study and describes the microbiological efficacy of tedizolid.
(This research was presented in part at the 49th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA, 2009, and at the 20th European Congress of Clinical Microbiology and Infectious Diseases, Vienna, Austria, 2010.)
MATERIALS AND METHODS
Study design.
The phase 2 dose-ranging, randomized, double-blind, noncomparative study of oral tedizolid phosphate in adults with cSSSI was conducted at 12 sites in the United States. Patients were randomized 1:1:1 to receive 200 mg, 300 mg, or 400 mg oral tedizolid phosphate once daily for 5 to 7 days. Treatment continued until all signs and symptoms of the cSSSI present at baseline either resolved or improved so that no further antimicrobial therapy was deemed necessary. Patients were evaluated at screening/day 1, days 2, 3, and 5 (if applicable), end-of-therapy visit, test-of-cure (TOC) visit (7 to 14 days posttreatment), and late-follow-up visit (21 to 28 days posttreatment). Microbiological samples were obtained from the site of infection at baseline and at any follow-up visits if medically indicated. Samples were collected using appropriate sampling methods, such as aspiration, biopsy, or deep swabbing (superficial swabs were not acceptable), and evaluated at a local laboratory. Isolates were forwarded to the central laboratory (Eurofins Medinet, Chantilly, VA) for confirmatory identification and susceptibility testing. The study was approved by Institutional Review Boards, and all patients provided informed consent before enrollment. The study was conducted in accordance with International Conference on Harmonization (ICH) and Food and Drug Administration (FDA) guidelines, good clinical practice, and the basic principles of the Declaration of Helsinki. The trial is registered at ClinicalTrials.gov (identifier: NCT00761215). The study was conducted from September 2008 to February 2009. The diagnosis and definition of cSSSI were consistent with FDA guidelines and clinical wisdom at the time the protocol was finalized and the study was conducted (6, 25). New draft guidelines for trials in patients with ABSSSI were introduced in 2010.
Study population.
The criteria for eligibility have been described previously (31). The study enrolled patients 18 to 75 years of age diagnosed with cSSSI caused by a suspected or confirmed Gram-positive pathogen. The infections studied were abscesses with at least 2 cm of surrounding induration or requiring incision and drainage, surgical or posttraumatic wounds, or deep cellulitis. All patients were required to have at least two local symptoms. If the lesion was <5 cm in its longest dimension, at least one objective systemic sign of infection was also required (fever of >38°C, white blood cell count of >10,000 cells/ml, or >10% immature neutrophils). The modified intent-to-treat (MITT) population of patients who received at least one dose of study drug primarily had abscesses (76.6%), and most of these patients had a surrounding induration of >4 cm. The infection sites were a median of 95.9 cm2 in area, and 47.9% of patients had one or more objective systemic signs of infection. Most patients (80.3%) had incision and drainage performed. All patients had at least two local symptoms of infection, e.g., discharge, erythema, fluctuance, swelling/induration, warmth, and/or tenderness.
Test organisms.
A total of 196 Gram-positive isolates were obtained at baseline from 188 patients in the MITT population. Of the baseline isolates collected and sent to the central reference laboratory for testing, 183 were Gram-positive cocci (160 S. aureus, 7 coagulase-negative staphylococci, 13 viridans group streptococci, and 3 beta-hemolytic streptococci). Of the 160 S. aureus isolates, 121 (76%) were identified as MRSA. An additional 3 MRSA isolates and 2 viridans group streptococcal isolates were identified at local laboratories; all isolates were included in susceptibility testing. Among the coagulase-negative staphylococci, S. epidermidis isolates were considered to be nonpathogenic skin contaminants and are not included in this report.
Antimicrobial susceptibility testing.
All isolates obtained during the trial period were centrally tested at Eurofins Medinet (Chantilly, VA) on dried commercial antibacterial panels (TREK Diagnostics, West Sussex, United Kingdom) against tedizolid (Trius Therapeutics, San Diego, CA) and linezolid (TREK Diagnostics, Cleveland, OH) for determination of MIC by broth microdilution according to Clinical and Laboratory Standards Institute (CLSI) guidelines M7-A8 and M100-S19 (7, 9). Cation-adjusted Mueller-Hinton broth (TREK Diagnostics, Cleveland, OH) was used for MIC testing. American Type Culture Collection (ATCC) strains of S. aureus (ATCC 29213) and S. pneumoniae (ATCC 49619) were used for quality control per CLSI specifications. MIC50s and MIC90s were determined.
Clindamycin resistance.
Isolates were tested for inducible clindamycin resistance using the double-disk diffusion (D-test) method, according to CLSI guideline M2-A10 (8).
Testing for PVL gene.
The multiplex PCR assay method described by McClure and colleagues in 2006 was used to detect the Panton-Valentine leukocidin (PVL) gene, encoding a toxin associated with highly virulent strains of MRSA and some strains of methicillin-susceptible S. aureus (MSSA) (22).
Microbiological response and clinical outcomes.
Two analysis populations defined for the study were used in the analyses presented here (31). The microbiological modified intent-to-treat (mMITT) population consisted of all randomized patients who had a diagnosis of cSSSI, had received at least one dose of tedizolid phosphate, and had at least one Gram-positive pathogen isolated from the primary site of infection. The microbiologically evaluable (ME) population consisted of patients with a diagnosis of cSSSI, with at least one Gram-positive pathogen isolated from the primary site of infection, and who were also clinically evaluable, i.e., received the minimum requirement of tedizolid phosphate, had an outcome assessment at the TOC visit, and had no confounding events or factors.
Microbiological responses were determined by the Evaluability Review Team based on culture data assessed at the baseline and TOC visits. Microbiological eradication was defined as absence of the original baseline pathogens in a TOC specimen (proven eradication) or absence of a source specimen to culture in patients assessed with a cure (presumed eradication). Microbiological response rates were summarized for each dose group.
Clinical response was assessed by the investigator and was categorized as clinical cure, clinical failure, or indeterminate, and the number and percentage of patients in each treatment group in each of these categories was reported. Clinical cure was defined as resolution or improvement of signs and symptoms of the cSSSI so that no further antibiotics (new or prolonged therapy) were required. Clinical failure was defined as follows: persistence, incomplete resolution, or worsening of signs and symptoms of infection that required further antibiotic therapy; development of new signs or symptoms; requirement for unplanned surgical intervention, additional antibiotic therapy, or therapy beyond 7 days; development of osteomyelitis; development of a treatment-limiting adverse event leading to discontinuation of the study drug; or death due to the cSSSI. An outcome was assessed as indeterminate when efficacy could not be evaluated because of treatment change before at least 2 doses of study medication, death occurred due to non-cSSSI cause, osteomyelitis was present at baseline, a Gram-negative organism that required treatment was isolated at baseline, or the patient was lost to follow-up. The clinical cure rate was defined as the number of patients with a clinical cure divided by the number of patients in the population.
RESULTS
In vitro activities of tedizolid and linezolid against isolated Gram-positive bacteria.
Of the 196 isolates sent to the central laboratory for testing, 81.6% were S. aureus, 6.6% were viridans group streptococci, and 3.6% were coagulase-negative staphylococci (Table 1). Of 163 S. aureus isolates, 124 were MRSA (76%) and 39 were methicillin-sensitive S. aureus (MSSA) (24%). Of the 124 MRSA isolates, 123 (99%) were PVL positive, and of the MSSA isolates, 30 (77%) were PVL positive.
Table 1.
Frequency of baseline pathogens among 196 isolates sent to the central laboratory
| Organism | No. | % |
|---|---|---|
| Staphylococcus aureus | 160 | 81.6 |
| Viridans group streptococci | 13 | 6.6 |
| Coagulase-negative staphylococci | 7 | 3.6 |
| Klebsiella pneumoniae | 5 | 2.6 |
| Corynebacterium spp. | 3 | 1.5 |
| Streptococcus agalactiae | 2 | 1.0 |
| Enterococcus avium | 1 | 0.5 |
| Micrococcus spp. | 1 | 0.5 |
| Morganella morganii | 1 | 0.5 |
| Proteus mirabilis | 1 | 0.5 |
| Pseudomonas aeruginosa | 1 | 0.5 |
| Streptococcus pyogenes | 1 | 0.5 |
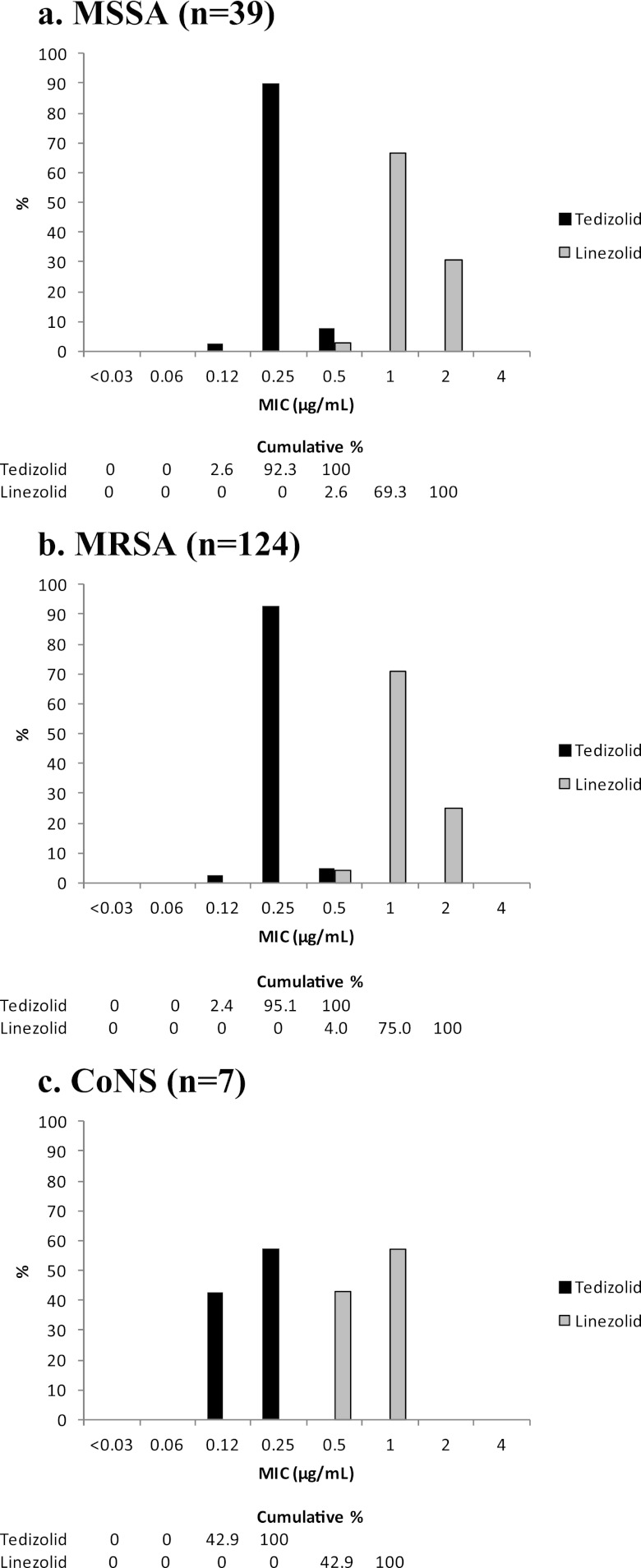
The MIC50 and MIC90 of tedizolid against both MSSA and MRSA were both 0.25 μg/ml, compared with a MIC50 of 1 μg/ml and a MIC90 of 2 μg/ml observed with linezolid (Table 2). Against both MSSA and MRSA, MICs of tedizolid were 4-fold lower than those of linezolid and ranged from 0.12 μg/ml to 0.5 μg/ml, compared with MICs for linezolid of 0.5 μg/ml to 2 μg/ml for both MSSA and MRSA (Fig. 1a and b). None of the 141 staphylococcal isolates tested for clindamycin resistance (isolates which tested as erythromycin resistant and clindamycin susceptible) were observed to have the D-shaped zone indicative of inducible clindamycin resistance.
Table 2.
Antimicrobial activities of tedizolid and linezolid against staphylococci
| Organism | Agent | Phenotypea | No. of isolates | MIC (μg/ml) |
% (No. with result/total no.) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Range | Mode | MIC50 | MIC90 | Susceptible | Intermediate | Resistant | ||||
| Staphylococcus aureus | Tedizolid | All | 163 | 0.12–0.5 | 0.25 | 0.25 | 0.25 | —b | — | — |
| MSSA | 39 | 0.12–0.5 | 0.25 | 0.25 | 0.25 | — | — | — | ||
| MRSA | 124 | 0.12–0.5 | 0.25 | 0.25 | 0.25 | — | — | — | ||
| Linezolid | All | 163 | 0.5–2 | 1 | 1 | 2 | 100 (163/163) | 0 | 0 | |
| MSSA | 39 | 0.5–2 | 1 | 1 | 2 | 100 (39/39) | 0 | 0 | ||
| MRSA | 124 | 0.5–2 | 1 | 1 | 2 | 100 (124/124) | 0 | 0 | ||
| Coagulase-negative staphylococci | Tedizolid | All | 7 | 0.12–0.25 | NAc | NA | NA | — | — | — |
| Linezolid | 0.5–1 | NA | NA | NA | 100 (7/7) | 0 | 0 | |||
MRSA, methicillin-resistant S. aureus; MSSA, methicillin-sensitive S. aureus.
—, CLSI MIC breakpoints not available for interpretation.
NA, not applicable: n < 10.
Fig 1.
MIC distributions of tedizolid and linezolid against staphylococci. CoNS, coagulase-negative staphylococci.
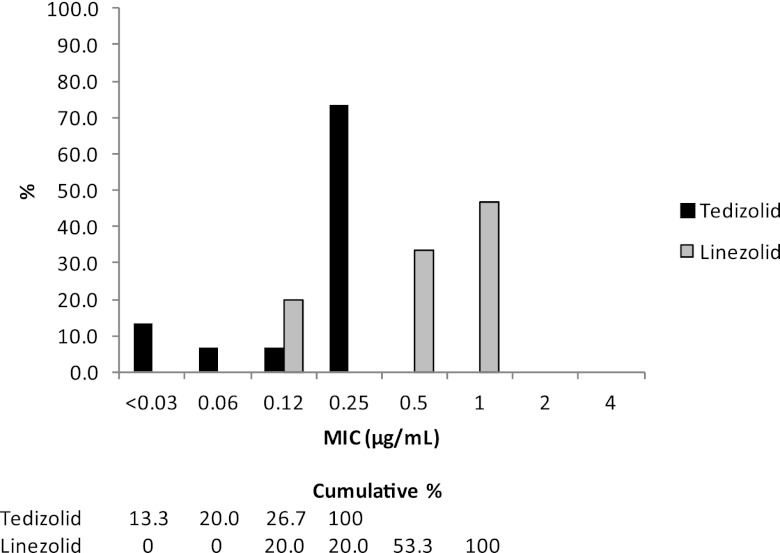
For tedizolid, all isolates of coagulase-negative staphylococci, viridans group streptococci, and beta-hemolytic streptococci had MICs of ≤0.25 μg/ml. Against coagulase-negative staphylococci, tedizolid MICs ranged from 0.12 μg/ml to 0.25 μg/ml, and linezolid MICs ranged from 0.5 μg/ml to 1 μg/ml (Table 2 and Fig. 1c). For viridans group streptococci, tedizolid had a MIC50 and MIC90 of 0.25 μg/ml, compared to the MIC50 of 0.5 μg/ml and MIC90 of 1 μg/ml with linezolid (Table 3). Tedizolid MICs ranged from 0.03 μg/ml to 0.25 μg/ml and linezolid MICs ranged from 0.12 μg/ml to 1 μg/ml against viridans group streptococci (Fig. 2). Three beta-hemolytic streptococci (2 S. agalactiae and 1 S. pyogenes) had tedizolid MICs of 0.25 μg/ml, 0.25 μg/ml, and 0.12 μg/ml, respectively, compared to MICs of 1 μg/ml, 0.5 μg/ml, and 0.5 μg/ml, respectively, for linezolid (Table 4).
Table 3.
Antimicrobial activities of tedizolid and linezolid against viridans group streptococci
| Agent | No. of isolates | MIC (μg/ml) |
% (No. with result/total no.) |
|||||
|---|---|---|---|---|---|---|---|---|
| Range | Mode | MIC50 | MIC90 | Susceptible | Intermediate | Resistant | ||
| Tedizolid | 15 | 0.03–0.25 | 0.25 | 0.25 | 0.25 | —a | — | — |
| Linezolid | 15 | 0.12–1 | 1 | 0.5 | 1 | 100 (15/15) | 0 | 0 |
—, CLSI MIC breakpoints not available for interpretation.
Fig 2.
MIC distributions of tedizolid and linezolid against viridans group streptococci (n = 15).
Table 4.
Antimicrobial activities of tedizolid and linezolid against 3 isolates of β-hemolytic streptococci
| Organism | No. of isolates | Tedizolid |
Linezolid |
||
|---|---|---|---|---|---|
| MIC (μg/ml) | CLSI Interpretation | MIC (μg/ml) | CLSI Interpretation | ||
| S. agalactiae | 2 | 0.25 | —a | 0.5–1 | Susceptible |
| S. pyogenes | 1 | 0.12 | — | 0.5 | Susceptible |
—, CLSI MIC breakpoints not available for interpretation.
Microbiological and clinical outcomes.
Among 154 treated patients in whom a Gram-positive pathogen was identified at baseline (mMITT population), S. aureus was isolated from the primary lesion in 139 patients (90.3%), with MRSA identified in 112 patients (80.6%) and MSSA in 27 patients (19.4%). A total of 21 treated patients were not included in the ME population because they were not clinically evaluable due to confounding events or factors, had no assessment at a TOC visit, or did not receive the minimum amount of study therapy.
The ME patient population consisted of 133 patients, 119 (89.5%) of whom had an S. aureus isolate (70.7% MRSA, 17.3% MSSA, and 1.5% unknown) at baseline. The majority of patients had only one species isolated, although seven patients had two and two patients had three species isolated. Of the 133 patients, 17 (12.8%) had cellulitis, 8 (6%) had an infected wound, and 108 (81.2%) had abscess. Most patients (83.5%) had incision and drainage procedures. Systemic signs of infection were observed in 59.4% of patients, and 48.9% had objective signs of infection, including 44.4% with elevated white blood cell counts of >10,000 per ml and 9.8% with fever of >38°C. Only 1 patient (0.8%) had >10% immature neutrophils. Three patients had positive blood cultures (MRSA).
Microbiological eradication rates at the TOC visit were similar in all tedizolid dose groups, with overall eradication rates of 97.7%, 97.9%, and 95.7% for all pathogens, MRSA, and MSSA, respectively (Table 5). Persistent infection was observed in 2 patients with MRSA and one with MSSA infection. The overall clinical cure rate at TOC was 96.2% (128/133) across all doses in the ME population. Of patients who failed therapy, three had MRSA, one had MSSA, and one had a coinfection of S. sanguinis and S. acidominimus at baseline. The clinical cure rate at TOC for patients with S. aureus in the ME population was 96.6% (115/119 patients), and the rates ranged from 91.7% to 100% across dose groups. For patients with MRSA or MSSA, the clinical cure rates were 96.9% and 95.7%, respectively, and were similar across all tedizolid dose groups (Table 6).
Table 5.
Microbiological eradication rates with tedizolid at the test-of-cure visit in the microbiologically evaluable population
| Pathogena | Microbiological eradication rate [% (no. of patients with eradication/total no. of patients)]b |
|||
|---|---|---|---|---|
| 200 mg tedizolid | 300 mg tedizolid | 400 mg tedizolid | All treatments | |
| All pathogens | 100 (43/43) | 93.2 (41/44) | 100 (46/46) | 97.7 (130/133) |
| MRSA isolates | 100 (32/32) | 92.6 (25/27) | 100 (35/35) | 97.9 (92/94) |
| MSSA isolates | 100 (7/7) | 88.9 (8/9) | 100 (7/7) | 95.7 (22/23) |
MRSA, methicillin-resistant S. aureus; MSSA, methicillin-sensitive S. aureus.
Includes proven or presumed eradication. The microbiologically evaluable population included 133 patients.
Table 6.
Clinical cure rates with tedizolid at the test-of-cure visit in patients with S. aureus infection in the microbiologically evaluable population
| Pathogena | Clinical cure rate [% (no. of patients with clinical cure/total no. of patients)]b |
|||
|---|---|---|---|---|
| 200 mg tedizolid | 300 mg tedizolid | 400 mg tedizolid | All treatments | |
| All S. aureus | 100 (39/39) | 91.7 (33/36) | 97.7 (43/44) | 96.6 (115/119) |
| MRSAc | 100 (32/32) | 92.6 (25/27) | 97.3 (36/37) | 96.9 (93/96) |
| MSSA | 100 (7/7) | 88.9 (8/9) | 100 (7/7) | 95.7 (22/23) |
MRSA, methicillin-resistant S. aureus; MSSA, methicillin-sensitive S. aureus.
The microbiologically evaluable population included 119 patients.
Includes two samples with local laboratory determination of MRSA.
DISCUSSION
The rapid spread of community-acquired MRSA strains with heightened virulence poses a challenge for therapy of cSSSIs (ABSSSIs), which are caused predominantly by S. aureus. There is a medical need for new intravenous and oral agents active against Gram-positive pathogens that can be used for both inpatient and outpatient therapy. Tedizolid is a next-generation oxazolidinone antibacterial agent being developed for treatment of ABSSIs.
A phase 2 study investigated the clinical and microbiological efficacy of oral tedizolid in patients with cSSSIs. In this report, we show that tedizolid had potent in vitro activity against baseline Gram-positive cocci and that this activity was 4- to 8-fold more potent than that of linezolid. The activity profile was not altered against MRSA, the most frequently encountered pathogen. Tedizolid demonstrated excellent microbiological and clinical efficacy at all dose levels, with both microbiological eradication rates and clinical cure rates approaching 100% for MRSA and MSSA infections.
The MIC50 and MIC90 of tedizolid for S. aureus isolates in this study were both 0.25 μg/ml, irrespective of methicillin resistance, compared with a MIC50 of 1 μg/ml and MIC90 of 2 μg/ml observed with linezolid. Similarly low MIC values for tedizolid have been reported by other investigators, as has the higher in vitro potency of tedizolid compared with linezolid for S. aureus clinical isolates from the United States and other countries (3, 5, 20, 32). Other reports have documented the potent activity of tedizolid against linezolid-resistant staphylococci, including cfr-positive MRSA strains, where the MIC differentials between tedizolid and linezolid were 8- to 32-fold (3, 33).
Tedizolid also showed strong in vitro activity against isolates of coagulase-negative staphylococci, viridans group streptococci, and beta-hemolytic streptococci, with MIC values ranging between 0.12 and 0.5 μg/ml. Based on MIC50/MIC90 values and MIC distributions, tedizolid was 4-fold more potent than linezolid against non-S. aureus Gram-positive isolates, confirming similar observations in other studies (5, 32).
The high rates of clinical and microbiological response with tedizolid phosphate in patients with cSSSIs mirrored the in vitro activity data. In this phase 2 study, S. aureus was responsible for approximately 90% of microbiologically documented infections, of which approximately 80% were caused by MRSA. Tedizolid phosphate demonstrated excellent microbiological efficacy (97.7% for all pathogens, 97.9% for MRSA, and 95.7% for MSSA) at all dose levels tested. Microbiological eradication rates were similar for S. aureus across all tedizolid dose groups, ranging from 92.6% to 100% for MRSA and 88.9% to 100% for MSSA.
The prevalence of the PVL gene was high among the S. aureus isolates from this study, with 99% of MRSA and 77% of MSSA being PVL positive. Given the complications and morbidity associated with the PVL toxin, the timely eradication of PVL-bearing strains with an effective antibiotic is of potential clinical significance. The high in vitro potency and clinical efficacy of tedizolid against MRSA and MSSA isolates, the vast majority of which were PVL positive, demonstrates its potential to improve the outcome of infections due to virulent PVL-producing S. aureus.
The overall clinical cure rate in microbiologically evaluable patients with MRSA infections was approximately 97% and was high at all dose levels. The 200-mg daily dose was found to be the lowest effective dose. Efficacy at the 200-mg-once-daily dose level also confirms the high intrinsic potency of the drug compared with linezolid, the only marketed oxazolidinone drug approved for the treatment of infections caused by specific Gram-positive bacteria. Phase 3 clinical trials with tedizolid phosphate are ongoing for the treatment of serious infections caused by Gram-positive organisms, including MRSA in ABSSSIs (formerly known as cSSSIs).
In conclusion, tedizolid phosphate had potent in vitro activity against baseline Gram-positive cocci, including MRSA isolates, isolated during a clinical trial in patients with cSSSIs. The potency of tedizolid was 4- to 8-fold higher than that of linezolid. All doses of tedizolid demonstrated high microbiologic and clinical efficacy in patients with cSSSIs, the majority of which were caused by PVL-positive MRSA.
ACKNOWLEDGMENTS
This work was supported by and conducted under the direction of Trius Therapeutics, Inc.
We thank Meher Dustoor and Sharon Dana for medical writing assistance in preparing the manuscript.
Footnotes
Published ahead of print 11 June 2012
REFERENCES
- 1. Appelbaum PC. 2007. Reduced glycopeptide susceptibility in methicillin-resistant Staphylococcus aureus (MRSA). Int. J. Antimicrob. Agents 30:398–408 [DOI] [PubMed] [Google Scholar]
- 2. Besier S, Ludwig A, Zander J, Brade V, Wichelhaus TA. 2008. Linezolid resistance in Staphylococcus aureus: gene dosage effect, stability, fitness costs, and cross-resistances. Antimicrob. Agents Chemother. 52:1570–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Betriu C, et al. 2010. Comparative activities of TR-700 (torezolid) against staphylococcal blood isolates collected in Spain. Antimicrob. Agents Chemother. 54:2212–2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boucher HW, Corey GR. 2008. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 46(Suppl 5):S344–S349 [DOI] [PubMed] [Google Scholar]
- 5. Brown SD, Traczewski MM. 2010. Comparative in vitro antimicrobial activities of torezolid (TR-700), the active moiety of a new oxazolidinone, torezolid phosphate (TR-701), determination of tentative disk diffusion interpretive criteria, and quality control ranges. Antimicrob. Agents Chemother. 54:2063–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chambers HF, Moellering RC, Jr, Kamitsuka P. 2008. Clinical decisions. Management of skin and soft-tissue infection. N. Engl. J. Med. 359:1063–1067 [DOI] [PubMed] [Google Scholar]
- 7. Clinical and Laboratory Standards Institute 2009. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M07-A08, 8th ed Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 8. Clinical and Laboratory Standards Institute 2009. Performance standards for antimicrobial disk susceptibility tests. Approved standard M02-A10, 10th ed Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 9. Clinical and Laboratory Standards Institute 2008. Performance standards for antimicrobial susceptibility testing. 18th informational supplement M100-S18. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 10. Draghi DC, Sheehan DJ, Hogan P, Sahm DF. 2005. In vitro activity of linezolid against key gram-positive organisms isolated in the United States: results of the LEADER 2004 surveillance program. Antimicrob. Agents Chemother. 49:5024–5032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Edelsberg J, et al. 2009. Trends in US hospital admissions for skin and soft tissue infections. Emerg. Infect. Dis. 15:1516–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fritsche TR, Sader HS, Jones RN. 2007. Potency and spectrum of garenoxacin tested against an international collection of skin and soft tissue infection pathogens: report from the SENTRY antimicrobial surveillance program (1999-2004). Diagn. Microbiol. Infect. Dis. 58:19–26 [DOI] [PubMed] [Google Scholar]
- 13. Gorak EJ, Yamada SM, Brown JD. 1999. Community-acquired methicillin-resistant Staphylococcus aureus in hospitalized adults and children without known risk factors. Clin. Infect. Dis. 29:797–800 [DOI] [PubMed] [Google Scholar]
- 14. Han LL, et al. 2007. High frequencies of clindamycin and tetracycline resistance in methicillin-resistant Staphylococcus aureus pulsed-field type USA300 isolates collected at a Boston ambulatory health center. J. Clin. Microbiol. 45:1350–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Herold BC, et al. 1998. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA 279:593–598 [DOI] [PubMed] [Google Scholar]
- 16. Jones RN. 2006. Microbiological features of vancomycin in the 21st century: minimum inhibitory concentration creep, bactericidal/static activity, and applied breakpoints to predict clinical outcomes or detect resistant strains. Clin. Infect. Dis. 42(Suppl 1):S13–S24 [DOI] [PubMed] [Google Scholar]
- 17. Jones RN, Fritsche TR, Sader HS, Ross JE. 2007. LEADER surveillance program results for 2006: an activity and spectrum analysis of linezolid using clinical isolates from the United States (50 medical centers). Diagn. Microbiol. Infect. Dis. 59:309–317 [DOI] [PubMed] [Google Scholar]
- 18. Jones RN, et al. 2009. Zyvox Annual Appraisal of Potency and Spectrum program: linezolid surveillance program results for 2008. Diagn. Microbiol. Infect. Dis. 65:404–413 [DOI] [PubMed] [Google Scholar]
- 19. King MD, et al. 2006. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Ann. Intern. Med. 144:309–317 [DOI] [PubMed] [Google Scholar]
- 20. Livermore DM, Mushtaq S, Warner M, Woodford N. 2009. Activity of oxazolidinone TR-700 against linezolid-susceptible and -resistant staphylococci and enterococci. J. Antimicrob. Chemother. 63:713–715 [DOI] [PubMed] [Google Scholar]
- 21. Louie A, Liu W, Kulawy R, Drusano GL. 2011. In vivo pharmacodynamics of torezolid phosphate (TR-701), a new oxazolidinone antibiotic, against methicillin-susceptible and methicillin-resistant Staphylococcus aureus strains in a mouse thigh infection model. Antimicrob. Agents Chemother. 55:3453–3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McClure JA, et al. 2006. Novel multiplex PCR assay for detection of the staphylococcal virulence marker Panton-Valentine leukocidin genes and simultaneous discrimination of methicillin-susceptible from -resistant staphylococci. J. Clin. Microbiol. 44:1141–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mendes RE, et al. 2008. First report of cfr-mediated resistance to linezolid in human staphylococcal clinical isolates recovered in the United States. Antimicrob. Agents Chemother. 52:2244–2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mera RM, et al. 2011. Increasing role of Staphylococcus aureus and community-acquired methicillin-resistant Staphylococcus aureus infections in the United States: a 10-year trend of replacement and expansion. Microb. Drug Resist. 17:321–328 [DOI] [PubMed] [Google Scholar]
- 25. Merlino JI, Malangoni MA. 2007. Complicated skin and soft-tissue infections: diagnostic approach and empiric treatment options. Cleve. Clin. J. Med. 74(Suppl 4):S21–S28 [DOI] [PubMed] [Google Scholar]
- 26. Moet GJ, Jones RN, Biedenbach DJ, Stilwell MG, Fritsche TR. 2007. Contemporary causes of skin and soft tissue infections in North America, Latin America, and Europe: report from the SENTRY Antimicrobial Surveillance Program (1998-2004). Diagn. Microbiol. Infect. Dis. 57:7–13 [DOI] [PubMed] [Google Scholar]
- 27. Morales G, et al. 2010. Resistance to linezolid is mediated by the cfr gene in the first report of an outbreak of linezolid-resistant Staphylococcus aureus. Clin. Infect. Dis. 50:821–825 [DOI] [PubMed] [Google Scholar]
- 28. Moran GJ, et al. 2006. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 355:666–674 [DOI] [PubMed] [Google Scholar]
- 29. National Nosocomial Infections Surveillance System 2004. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1992 through June 2004, issued October 2004. Am. J. Infect. Control 32:470–485 [DOI] [PubMed] [Google Scholar]
- 30. Patel M, Waites KB, Moser SA, Cloud GA, Hoesley CJ. 2006. Prevalence of inducible clindamycin resistance among community- and hospital-associated Staphylococcus aureus isolates. J. Clin. Microbiol. 44:2481–2484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Prokocimer P, et al. 2011. Phase 2, randomized, double-blind, dose-ranging study evaluating the safety, tolerability, population pharmacokinetics, and efficacy of oral torezolid phosphate in patients with complicated skin and skin structure infections. Antimicrob. Agents Chemother. 55:583–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schaadt R, Sweeney D, Shinabarger D, Zurenko G. 2009. In vitro activity of TR-700, the active ingredient of the antibacterial prodrug TR-701, a novel oxazolidinone antibacterial agent. Antimicrob. Agents Chemother. 53:3236–3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shaw KJ, et al. 2008. In vitro activity of TR-700, the antibacterial moiety of the prodrug TR-701, against linezolid-resistant strains. Antimicrob. Agents Chemother. 52:4442–4447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stryjewski ME, Chambers HF. 2008. Skin and soft-tissue infections caused by community-acquired methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 46(Suppl 5):S368–S377 [DOI] [PubMed] [Google Scholar]