Abstract
To compare tissue-based pharmacokinetics and efficacy of oral tenofovir disoproxyl fumarate (TDF) versus subcutaneous tenofovir (TFV), macaques were treated for 2 weeks starting 1 week after simian immunodeficiency virus inoculation. Despite lower plasma TFV levels in the oral TDF arm, similar TFV diphosphate levels and antiviral activities were measured in lymphoid cells of most tissues. In intestinal tissues, however, oral TDF resulted in higher active drug levels, associated with lower virus levels and better immune preservation.
TEXT
While our ability to manage HIV infection with chronic antiretroviral therapy represents a triumph of modern medicine, researchers are now on a quest to find strategies to completely cure or permanently control HIV infection in the absence of chronic antiretroviral drug treatment (7). Success is likely to depend on a better understanding of the dynamics of viral reservoirs, including residual virus replication and latency, and its interplay with pharmacokinetics and pharmacodynamics. Data suggest that levels of HIV and drugs in blood are not always representative of their levels in the lymphoid tissues, where most virus replication occurs (6).
One of the drugs most widely used to treat HIV infection is tenofovir (TFV). Following two phosphorylation steps, the pharmacologically active intracellular metabolite tenofovir diphosphate (TFV-DP) inhibits viral reverse transcription. Because TFV is hydrophilic, it has very poor oral bioavailability. Therefore, TFV is administered orally as the fumarate salt of its lipophilic and more cell-permeant disoproxyl prodrug (tenofovir disoproxyl fumarate [TDF]). TDF has >500-fold greater anti-HIV activity in vitro than TFV (23). As TDF is not observed in plasma at the earliest time points tested following administration to patients and has a reported half-life of less than 1 min (3, 17, 18), one might assume that it does not affect the loading of lymphoid cells or tissues with the active metabolite.
However, data from a small human study suggest that based on plasma drug levels, the oral administration of TDF had stronger antiviral effects than parenteral administration of TFV (3, 8). In addition, a pharmacokinetic study in macaques demonstrated previously that an oral TDF regimen, selected to give plasma exposures equivalent to those achieved with a subcutaneous TFV regimen, resulted in approximately 8-fold-higher levels of TFV-DP in peripheral blood mononuclear cells (PBMC). Because that study looked only at drug levels in blood, the present study was designed to compare TFV and TDF in simian immunodeficiency virus (SIV)-infected animals, including the measurement of drug and virus levels in lymphoid tissues. Special attention was given to the gut-associated lymphoid tissue (GALT), which, harboring the majority of the body's lymphoid tissue, is a major site of virus replication and pathogenesis (4). We hypothesized that, because of the administration route, oral TDF administration may result in preferential uploading of drug in GALT, relative to other lymphoid tissues and relative to parenteral TFV injection.
All 12 animals were healthy juvenile (41 to 43 months of age) male rhesus macaques (Macaca mulatta), from the HIV-2-, SIV-, type D retrovirus-, and simian T-cell lymphotropic virus type 1-free colony at the California National Primate Research Center (CNPRC). Animals were housed in accordance with American Association for Accreditation of Laboratory Animal Care standards. We strictly adhered to the Guide for the Care and Use of Laboratory Animals, prepared by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Resources, National Resource Council (21). For blood collections and oral drug administrations, animals were immobilized with ketamine HCl (Parke-Davis, Morris Plains, NJ) at a dose of 10 mg/kg of body weight, injected intramuscularly.
At time zero, all 12 animals were inoculated intravenously with 1 ml containing 103 50% tissue culture infectious doses (equivalent to 103 50% animal infectious doses) of virulent uncloned SIVmac251. The titer of this stock (designated 6/04), which was propagated in rhesus macaque PBMC, had been determined in vitro and in vivo (20). One week after SIV inoculation, animals were randomized to three treatment groups of four animals each. TFV and TDF solutions were prepared at 60 mg/ml and 8 mg/ml, respectively, as described previously (9), while placebo treatments consisted of sterile physiologic saline (0.9% NaCl). One group received TFV subcutaneously at 6 mg/kg, while the second group received TDF orally at 22 mg/kg (via intubation to ensure full drug uptake). Both TFV and TDF treatment groups received also placebo treatment via the other route (oral and subcutaneous, respectively). The third group (“placebo”) received placebo by both the oral and subcutaneous routes. All animals were dosed once daily, and samples were taken every few days, with 24-hour pharmacokinetic studies performed on day 7 (after the first dose) and day 20 (after the last dose). On day 21, all animals were euthanized for analysis of drug levels, virus levels, and immune markers in blood and tissues.
Previously described methods, including collagenase digestion techniques used for gut tissues, were used to separate plasma and isolate mononuclear cells from blood and tissue samples (1, 9, 24). The use of phosphate-buffered saline was avoided during sample preparation due to its negative effects on the analysis of TFV-DP (9). Mononuclear cells were treated with NH4Cl to remove contaminating red blood cells as described previously and then cryopreserved immediately at −70°C until further analysis. Plasma TFV and intracellular TFV-DP levels were measured as described previously (9).
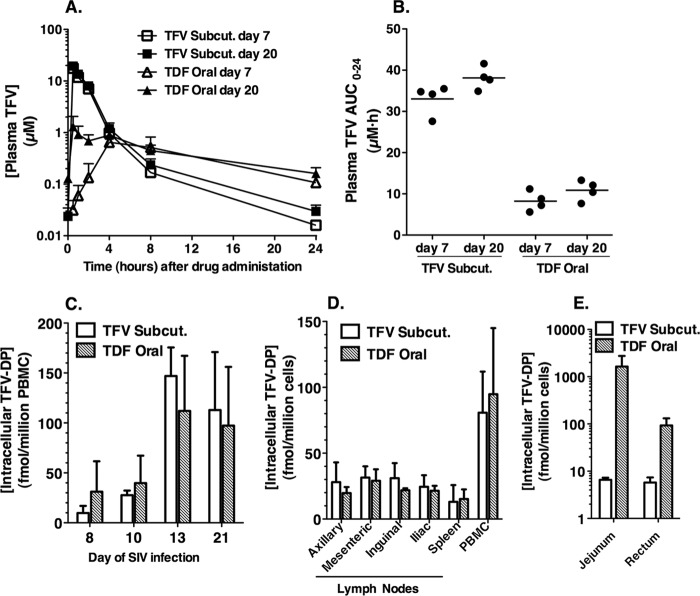
The drug regimens used in this study were calculated using repeated-dose pharmacokinetic modeling, using the data from our previous single-dose pharmacokinetic study in uninfected age-matched animals (9). We selected oral and subcutaneous doses aimed at achieving similar steady-state plasma exposures after repeated dosing, but with the hypothesis that the oral TDF regimen would provide higher intracellular levels of TFV-DP and thus higher antiviral activity than the subcutaneous regimen of 6 mg/kg (which is likely suboptimal in comparison to the subcutaneous 30 mg/kg induction regimen routinely used to suppress SIV replication in macaques [27]). However, despite this initial aim to achieve similar drug levels in plasma with the subcutaneous TFV and oral TDF regimens, marked differences in plasma pharmacokinetics were observed in the SIV-infected animals (Fig. 1A). The maximum concentration (Cmax) was higher and terminal elimination half-life shorter following subcutaneous TFV than after oral TDF. Slight accumulation was observed in plasma TFV exposures (area under the concentration-time curve from 0 to 24 h [AUC0-24]) between days 7 and 20 following administration of subcutaneous TFV (15% increase in mean AUC0-24) or oral TDF (32% increase in mean AUC0-24). The plasma TFV AUC0-24 values on day 20 following subcutaneous TFV daily versus oral TDF administration were 38.1 ± 2.8 versus 10.9 ± 2.4 μM · h, respectively (mean ± standard deviation [SD], n = 4; P < 0.001, two-tailed t test) (Fig. 1B). While these plasma TFV levels achieved in the subcutaneous TFV regimen were consistent with previous observations, the values for oral TDF were lower than predicted based on values obtained with uninfected animals (9). In hindsight, the most likely explanation is poor absorption of TDF associated with the gastrointestinal dysfunction that has been observed during acute infection with virulent SIV, even without outward signs of diarrhea or detectable opportunistic intestinal pathogens (15).
Fig 1.
Extracellular and intracellular TFV pharmacokinetics following subcutaneous TFV versus oral TDF administration. (A and B) Levels of TFV in plasma and values for area under the plasma concentration-versus-time curve (AUC0–24), respectively, for day 7 and day 20 of drug administration. (C) Accumulation of TFV-DP in PBMC collected on the respective study days, exactly 24 h after the drug dosing on the previous day. (D and E) Intracellular levels of the active metabolite TFV-DP in mononuclear cells from blood or tissues at the time of euthanasia on day 21. All values are means ± SD for samples collected from four animals, except for jejunum and rectum samples from the subcutaneous-tenofovir group, where samples were collected from two animals.
Despite this ∼3.5-fold-lower TFV plasma exposure (AUC0-24) following oral TDF relative to subcutaneous TFV, the intracellular levels of TFV-DP in PBMC turned out to be very similar in the two groups (Fig. 1C and D). Consistent with the data in the prior pharmacokinetic study, these results suggest that tenofovir disoproxil plays a direct role in drug delivery to lymphoid cells and tissues (9). Evidence of accumulation of TFV-DP was observed in PBMC samples from both treatment groups, particularly the first week of treatment. On day 21 (24 h after the last dosing), PBMC TFV-DP levels were an average of 105 fmol/million cells (i.e., 525 nM, assuming a volume of 0.2 pl/cell for PBMC) (Fig. 1D), which is more than 4-fold higher than the TFV-DP levels observed 24 h after the initial dosing on day 7 (P = 0.001, two-tailed t test) (Fig. 1C). These steady-state levels of TFV-DP in PBMC on day 21 resemble those observed in HIV-infected humans taking the oral TDF tablet daily (average, ∼100 fmol per million PBMC) (2, 13, 14, 22). At the time of euthanasia on day 21, TFV-DP levels in four different lymph nodes and spleen were also similar for both treatment regimens, averaging 23.8 fmol/million cells (Fig. 1D). An exception to this was the gastrointestinal tract, where levels of TFV-DP were 250-fold higher in jejunum and 16-fold higher in rectal tissue following oral TDF than after subcutaneous TFV administration (Fig. 1E). Higher TFV-DP levels in gut tissue than in other lymphoid tissues in uninfected macaques receiving oral TDF have also been reported (11).
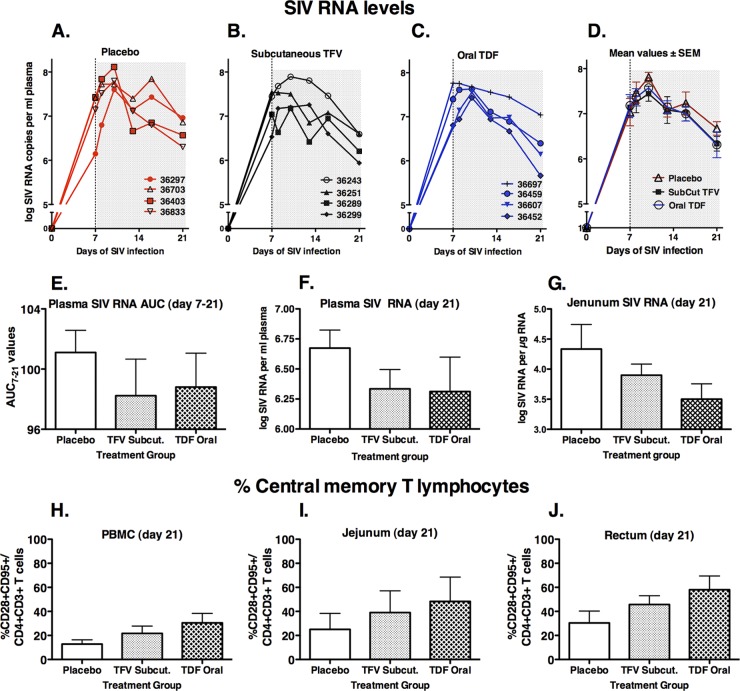
To monitor the effect of drug administration on virus replication, a real-time reverse transcription-PCR (RT-PCR) assay for SIV gag was used to quantitate SIV RNA levels in plasma and in total RNA extracted from tissues collected in RNAlater (Ambion, Austin, TX) and processed according to methods described previously (1, 5). As predicted, all animals had high peak viremia (>107 SIV RNA copies/ml plasma) by day 7 or 10 after SIVmac251 inoculation (Fig. 2A to D). The three groups had nearly identical SIV levels at day 7, when treatment was started. A decline in viremia was observed from day 10 onward, but the virological response in the two drug-treated groups was smaller than expected and remarkably similar in both treatment groups, which is consistent with the pharmacokinetic data (i.e., suboptimal drug levels early on and similar TFV-DP levels in the majority of tissues for the two drug-treated groups). At the time of euthanasia (day 21), the mean plasma SIV levels in the TDF- and TFV-treated groups were nearly identical and approximately 2-fold (0.35 log) lower than those in the placebo group, but due to considerable variability, this difference was statistically not significant (Fig. 2E and F). SIV RNA levels in tissues collected on day 21 were also highly variable, but the most interesting difference was observed in the jejunum, where mean SIV RNA levels in the subcutaneous TFV and oral TDF treatment groups were 3-fold and 7-fold lower than those in the placebo group, respectively (Fig. 2G).
Fig 2.
Virus levels and immune markers in blood and tissues. (A to D) Individual and mean plasma SIV levels for the three groups of SIV-infected macaques during the 3 weeks of observation. (E) Values of area under the SIV RNA plasma concentration-versus-time curve from day 7 to 21 (AUC7–21). (F and G) SIV RNA levels in plasma and jejunal tissue at time of euthanasia on day 21. (H to J) Frequency of CD28+ CD95+ central memory cells among the CD4+ T lymphocyte population. All values on graphs D through J are means ± standard errors of the means (SEM) for four animals per group. Viral RNA data were first normalized via log transformation prior to statistical analysis.
Because previous studies have demonstrated that tenofovir monotherapy selects for the emergence of K65R RT viral mutants with reduced in vitro susceptibility, sensitive real-time PCR methodology (with a detection limit of 0.4%) was used to quantitate K65R mutants (16, 26). At the time of euthanasia, K65R viral mutants were detected at a frequency of 0.8% in plasma and jejunum of TFV-treated animal 36243. The other tissues of this animal and all samples from the remaining animals had only wild-type sequence. Of all drug-treated animals, animal 36243 was the one with the highest peak plasma RNA levels (8 × 107 copies/ml on day 10). This observation of the earliest detection of K65R mutants in jejunal tissue is consistent with the role of gut-associated lymphoid tissue as a major target of virus replication and source of plasma viremia (4, 19). These findings also suggest the importance of obtaining suppressive drug levels in the tissues (6, 10).
At every blood collection, lymphocyte subpopulations were phenotyped for CD3, CD4, CD8, and CD20. In addition, at the time of euthanasia, additional markers were used to detect memory and effector cells (using CD28 and CD95) in cells from blood and tissues. Because of high variability, there were no clear biologically relevant differences in any of the lymphocyte markers, with the exception of a trend toward better preservation of central memory CD4+ cells in drug-treated animals, particularly in the gut-associated lymphoid tissues (oral TDF > subcutaneous TFV > placebo) (Fig. 2H to J). Because central memory cells are rapidly depleted during acute SIV infection (4, 19), this observation of preservation of memory CD4+ T cells is consistent with the higher TFV-DP levels and lower viral RNA levels in the gut with the oral TDF regimen than with the subcutaneous TFV regimen.
At the time of euthanasia, SIV-specific cell-mediated immune responses were evaluated in PBMC and lymphoid tissues (tonsil, spleen, jejunum, rectum, and four different lymph nodes). Intracellular cytokine production (interleukin 2 [IL-2], gamma interferon [IFN-γ], and tumor necrosis factor alpha [TNF-α]) and cell surface expression of the degranulation marker CD107 were assessed via multiparameter flow cytometry from fresh PBMC and lymphoid cells after stimulation with SIV antigens (aldrithiol-2 [AT-2]-inactivated whole SIVmac239; overlapping 15-mer peptides spanning the SIV gag p27 protein) according to methods reported previously (25). While all animals had some CD4+ and CD8+ cell-mediated immune responses, responses were nearly all monofunctional (i.e., one cytokine, mostly IFN-γ or TNF-α), and the magnitude was generally low and highly variable among tissues and among animals. There were no obvious biologically relevant differences between the treatment groups. These observations are consistent with an early, immature immune response during acute viremia with the highly virulent SIVmac251 that causes rapid immune dysfunction. As previous studies have demonstrated that antiviral immune responses play an important role in reducing viremia at the start of TVF therapy (27), these poor antiviral immune responses during acute infection likely contributed to the suboptimal virologic response in the two drug-treated groups.
In summary, despite the suboptimal virologic response due to lower-than-predicted absorption of the oral TDF regimen and immature antiviral immune responses, the present study is informative in several regards. First, it confirms in SIV-infected animals that administration of oral TDF more efficiently loads lymphoid cells with reduced plasma exposure. The advantage of oral prodrug administration in loading of relevant tissues was magnified in the gut, presumably due to direct intestinal exposure to a highly cell-permeant prodrug (11). These higher active-drug levels are relevant to the use of antiretroviral drugs like TDF in pre-exposure prophylaxis regimens against rectal virus transmission (11, 12). Second, this study demonstrates that drug levels measured in plasma or PBMC may not be reflective of intracellular drug levels in lymphoid tissues, and therefore, such correlations probably need to be established separately for every drug (2, 6). Tissue-dependent differences in active drug levels and availability of target cells for virus replication likely play an important role in the selection of drug-resistant viral mutants. Altogether, these observations provide further scientific impetus for the design and clinical development of other prodrugs of tenofovir (e.g., GS-7340 [18]) and/or other antiretroviral drugs that can further enhance selective targeting of the sites of viral replication and viral reservoirs, with the goal of persistently controlling replication, protecting from transmission in the context of pre-exposure prophylaxis, or ultimately curing infection with finite treatment programs.
ACKNOWLEDGMENTS
We thank Linda Hirst, Colony Services, Pathology, Veterinary, and Clinical Laboratory staff of the California National Primate Research Center, for expert technical assistance. The SIVmac239 gag p27 peptides were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. The AT-2 inactivated SIVmac239 was provided by J. Lifson (SAIC Frederick, Inc., National Cancer Institute, Frederick, MD). We thank the Quantitative Molecular Diagnostics Core of the AIDS Vaccine Program, SAIC Frederick, Inc., National Cancer Institute, Frederick, MD, for assistance with viral RNA and DNA load determinations.
This work was supported by Gilead Sciences, grant RR-00169 from the National Center for Research Resources (NCRR; a component of the National Institutes of Health [NIH]) to the California National Primate Research Center).
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH. D.B and A.S.R. are employees and shareholders and K.K.A.V.R. is a shareholder of Gilead Sciences.
Footnotes
Published ahead of print 9 July 2012
REFERENCES
- 1. Abel K, et al. 2006. Rapid virus dissemination in infant macaques after oral simian immunodeficiency virus exposure in the presence of local innate immune responses. J. Virol. 80:6357–6367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baheti G, Kiser JJ, Havens PL, Fletcher CV. 2011. Plasma and intracellular population pharmacokinetic analysis of tenofovir in HIV-1-infected patients. Antimicrob. Agents Chemother. 55:5294–5299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barditch-Crovo P, et al. 2001. Phase I/II trial of the pharmacokinetics, safety, and antiretroviral activity of tenofovir disoproxil fumarate in human immunodeficiency virus-infected adults. Antimicrob. Agents Chemother. 45:2733–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brenchley JM, Douek DC. 2008. HIV infection and the gastrointestinal immune system. Mucosal Immunol. 1:23–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cline AN, Bess JW, Piatak M, Jr., Lifson JD. 2005. Highly sensitive SIV plasma viral load assay: practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J. Med. Primatol. 34:303–312 [DOI] [PubMed] [Google Scholar]
- 6. Cohen J. 2011. HIV/AIDS research. Tissue says blood is misleading, confusing HIV cure efforts. Science 334:1614. [DOI] [PubMed] [Google Scholar]
- 7. Cohen J. 2011. The emerging race to cure HIV infections. Science 332:784–785, 787–789 [DOI] [PubMed] [Google Scholar]
- 8. Deeks SG, et al. 1998. Safety, pharmacokinetics and antiretroviral activity of intravenous 9-[2-(R)-(phosphonomethoxy)propyl]adenine, a novel anti-human immunodeficiency virus (HIV) therapy, in HIV-infected adults. Antimicrob. Agents Chemother. 42:2380–2384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Durand-Gasselin L, et al. 2009. Nucleotide analogue prodrug tenofovir disoproxil enhances lymphoid cell loading following oral administration in monkeys. Mol. Pharm. 6:1145–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Evering TH, et al. 2012. Absence of HIV-1 evolution in the gut-associated lymphoid tissue from patients on combination antiviral therapy initiated during primary infection. PLoS Pathog. 8:e1002506 doi:10.1371/journal.ppat.1002506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garcia-Lerma JG, et al. 2010. Intermittent prophylaxis with oral truvada protects macaques from rectal SHIV infection. Sci. Transl. Med. 2:14ra4 doi:10.1126/scitranslmed.3000391 [DOI] [PubMed] [Google Scholar]
- 12. Grant RM, et al. 2010. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N. Engl. J. Med. 363:2587–2599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hawkins T, et al. 2011. Intracellular nucleotide levels during coadministration of tenofovir disoproxil fumarate and didanosine in HIV-1-infected patients. Antimicrob. Agents Chemother. 55:1549–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hawkins T, et al. 2005. Intracellular pharmacokinetics of tenofovir diphosphate, carbovir triphosphate, and lamivudine triphosphate in patients receiving triple-nucleoside regimens. J. Acquir. Immune Defic. Syndr. 39:406–411 [DOI] [PubMed] [Google Scholar]
- 15. Heise C, Miller CJ, Lackner A, Dandekar S. 1994. Primary acute simian immunodeficiency virus infection of intestinal lymphoid tissue is associated with gastrointestinal dysfunction. J. Infect. Dis. 169:1116–1120 [DOI] [PubMed] [Google Scholar]
- 16. Johnson JA, Van Rompay KKA, Delwart E, Heneine W. 2006. A sensitive real-time PCR assay for the K65R drug resistance mutation in SIV reverse transcriptase. AIDS Res. Hum. Retroviruses 22:912–916 [DOI] [PubMed] [Google Scholar]
- 17. Kearney BP, Flaherty JF, Shah J. 2004. Tenofovir disoproxil fumarate: clinical pharmacology and pharmacokinetics. Clin. Pharmacokinet. 43:595–612 [DOI] [PubMed] [Google Scholar]
- 18. Lee WA, et al. 2005. Selective intracellular activation of a novel prodrug of the human immunodeficiency virus reverse transcriptase inhibitor tenofovir leads to preferential distribution and accumulation in lymphatic tissue. Antimicrob. Agents Chemother. 49:1898–1906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Q, et al. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434:1148–1152 [DOI] [PubMed] [Google Scholar]
- 20. Marthas ML, et al. 2011. Partial efficacy of a VSV-SIV/MVA-SIV vaccine regimen against oral SIV challenge in infant macaques. Vaccine 29:3124–3137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. National Research Council 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D. C [Google Scholar]
- 22. Pruvost A, et al. 2005. Measurement of intracellular didanosine and tenofovir phosphorylated metabolites and possible interaction of the two drugs in human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 49:1907–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Robbins BL, Srinivas RV, Kim C, Bischofberger N, Fridland A. 1998. Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob. Agents Chemother. 42:612–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shacklett BL, et al. 2003. Trafficking of human immunodeficiency virus type 1-specific CD8+ T cells to gut-associated lymphoid tissue during chronic infection. J. Virol. 77:5621–5631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Van Rompay KK, et al. 2008. Chronic administration of tenofovir to rhesus macaques from infancy through adulthood and pregnancy: summary of pharmacokinetics and biological and virological effects. Antimicrob. Agents Chemother. 52:3144–3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Van Rompay KK, et al. 2007. Sequential emergence and clinical implications of viral mutants with K70E and K65R mutation in reverse transcriptase during prolonged tenofovir monotherapy in rhesus macaques with chronic RT-SHIV infection. Retrovirology 4:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Van Rompay KKA, et al. 2004. CD8+ cell-mediated suppression of virulent simian immunodeficiency virus during tenofovir treatment. J. Virol. 78:5324–5337 [DOI] [PMC free article] [PubMed] [Google Scholar]