Abstract
There is an unmet need for an intravenous (i.v.) neuraminidase inhibitor, particularly for patients with severe influenza who cannot take oral medication. Two phase I pharmacokinetic and safety studies of i.v. oseltamivir were carried out in healthy volunteers. The first was an open-label, randomized, four-period, two-sequence, single-dose trial of 100 mg, 200 mg, and 400 mg oseltamivir i.v. over 2 h and a 75-mg oral dose of oseltamivir. The second was a double-blind, placebo-controlled, parallel-group, multiple-dose study in which participants were randomized to 100 mg or 200 mg oseltamivir or placebo (normal saline) i.v. over 2 h every 12 h for 5 days. Exposure to the active metabolite oseltamivir carboxylate (OC) after dosing achieved with 100 mg oseltamivir administered i.v. over 2 h was comparable to that achieved with 75 mg administered orally. Single i.v. doses of oseltamivir up to 400 mg were well tolerated with no new safety signals. Multiple-dose data confirmed good tolerability of 100 mg and 200 mg oseltamivir and showed efficacious OC exposures with 100 mg i.v. over 2 h twice daily for 5 days. These results support further exploration of i.v. oseltamivir as an influenza treatment option for patients unable to take oral medication.
INTRODUCTION
Oseltamivir (Tamiflu) is an established antiviral medication with an extensively studied efficacy and safety profile that is licensed for the treatment and prophylaxis of influenza in persons aged ≥1 year (5, 6). Oseltamivir has also been approved for use in infants aged <1 year during a pandemic influenza virus outbreak (6). When given within 48 h of the onset of symptoms, oseltamivir significantly reduces the severity and duration of symptoms and the frequency of secondary illness in persons with mild influenza (9, 10, 15, 21, 22) and reduces the risks of admission to intensive care and death in persons with serious disease (4, 11, 12, 14, 20).
The burden imposed by severe influenza virus infection and its complications is considerable (23). An estimated 5 to 10% of the world's population is affected by seasonal influenza each year, which results in 3 million to 5 million severe infections and up to half a million deaths (23). Persons with chronic medical conditions have a more than 30-fold increased risk of hospitalization and death (23). A study in 754 patients hospitalized with seasonal influenza showed comorbidities and serious complications to be common (61 to 77%), with 39 patients dying of pneumonia and respiratory failure or sepsis (12). Primary viral pneumonia is a known complication of influenza, and severe disease is most frequently seen in children aged <5 years, pregnant women, and those with underlying medical conditions (24). Complications and death have been observed in patients with pandemic influenza A virus (H1N1) infection, particularly pregnant women, the severely obese, and certain disadvantaged social groups (24). Interestingly, up to half of all patients who were hospitalized or died due to pandemic influenza A virus infection up to early 2010 had no reported coexisting medical conditions (24).
There is a need for prompt antiviral treatment in seriously ill patients, a group that includes individuals who are among those least able to tolerate, swallow, or absorb orally administered medication (17). Oseltamivir is given orally as a prodrug (oseltamivir phosphate) that is rapidly converted by hepatic esterases into the active metabolite, oseltamivir carboxylate (OC) (3). The pharmacokinetic (PK) profile of oseltamivir given enterally in critically ill adults with pandemic influenza (H1N1) has been found to be comparable to that in ambulatory patients (1), but nevertheless, there remains an unmet need for a parenteral formulation of the drug. Two phase I studies in healthy volunteers have therefore been carried out to characterize the pharmacokinetics, safety, and tolerability of oseltamivir and its active metabolite after single intravenous (i.v.) doses of the prodrug relative to those after a single oral dose, as well as those after multiple twice-daily dosing throughout a 5-day treatment period. Results of these studies in healthy volunteers have been used to support subsequent clinical studies in patients with influenza virus infection.
(This information has previously been presented at the following meetings: the XII International Symposium on Respiratory Viral Infections, 11 to 14 March 2010, Taipei, Taiwan, and the XIII International Symposium on Respiratory Viral Infections, 12 to 15 March 2011, Rome, Italy.)
MATERIALS AND METHODS
Study designs.
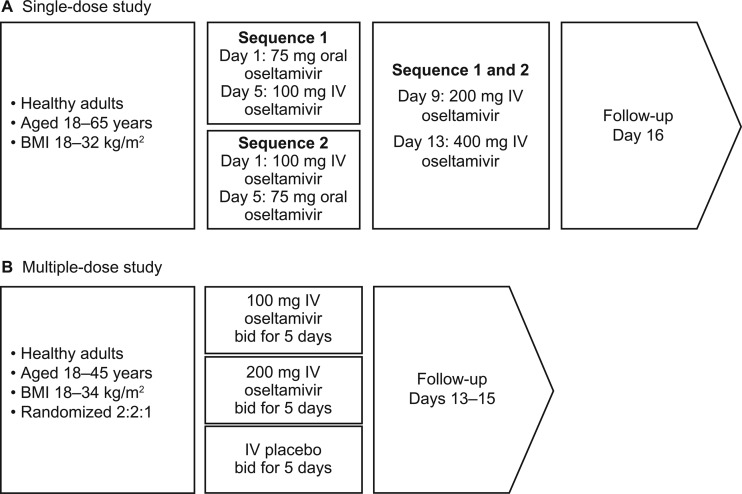
The single-ascending-dose study was an open-label, randomized, four-period, two-sequence, crossover trial of three i.v. doses of oseltamivir (100 mg, 200 mg, and 400 mg infused i.v. over 2 h) and a single oral dose of oseltamivir at 75 mg in healthy volunteers aged 18 to 65 years. The multiple-dose study was a randomized, double-blind, placebo-controlled, parallel-group study of oseltamivir (100 mg and 200 mg infused i.v. over 2 h) in healthy volunteers aged 18 to 45 years (Fig. 1).
Fig 1.
Treatment pathways in the single-dose (A) and multiple-dose (B) studies. bid, twice daily.
Inclusion criteria for the two studies were similar. Adult men and women aged ≥18 years who were in good physical health, as shown by medical examination and history, vital signs, 12-lead electrocardiogram (ECG), and laboratory tests, were recruited. Body mass index (BMI) ranges were 18 to 32 kg/m2 and 18 to 34 kg/m2 for men and women, respectively. Females of childbearing potential were required to have a history of effective contraceptive use, as specified by the protocol.
Volunteers were excluded if they had evidence of any clinically significant medical condition or clinically significant orthostatic hypotension; were positive for hepatitis B or C virus or HIV infection; had a history of organ transplantation, allergy, or hypersensitivity; had any clinically relevant abnormal laboratory results or a history of alcohol or drug abuse; had any major illness within 30 days of screening; smoked more than 10 cigarettes per day or the equivalent in other tobacco products; had received any medication (except acetaminophen) in the 7 days preceding the study or within six times the medication's elimination half-life (t1/2; whichever was longer); or had participated in another clinical trial or donated/lost >500 ml of blood at any time within the past 3 months.
No medications, vitamins, or herbal remedies were permitted, except for adverse event (AE) treatments and where a rationale for an exception was clearly stated. Substances prohibited at all times included probenecid (from 2 weeks before the study), corticosteroids or immunosuppressants, alcohol, caffeine-containing beverages (from 96 h prior to the first study dose), and foods containing poppy seeds (from 7 days prior to the first dose). Enrollees received standardized meals, with breakfast finished at least 2 h prior to dosing, and the evening meal was not to be given within 1 h before or after dosing.
The two studies were carried out in accordance with the provisions of the Declaration of Helsinki and applicable local laws. Full ethical committee approval was obtained. Both trials were monitored to ensure compliance with protocols and to verify data against source documents.
Initial dose selection in the single-dose study was based on simulations of prodrug and active metabolite profiles from a PK model developed using combined prodrug i.v. data in plasma and urine and metabolite data in plasma (study PP16361; summarized in reference 18). The PK model was an interim version of the sequential PK model published by Rayner et al. (18) containing all key elements, including prodrug nonrenal and renal clearance. Briefly, several i.v. dosing regimens at steady state were simulated, with the intention of reproducing metabolite plasma profiles equivalent to those seen with oral oseltamivir at 75 mg twice daily (study NP15717; summarized in reference 8). The simulations suggested an i.v. dosage of 100 mg over 2 h twice daily as being the most likely to give the required carboxylate exposures. This dose was also predicted to provide lower oseltamivir exposure than the 105 mg i.v. oseltamivir infused over 1 h that was well tolerated in a previous study in six healthy volunteers (study PP16361; summarized in reference 18); therefore, 100 mg i.v. infused over 2 h was deemed an appropriate starting dose for the single-dose study. Two, 2-fold dose escalations (to 200 mg and 400 mg infused over 2 h) were used to expand the clinical safety margin of i.v. oseltamivir.
In the single-dose study, participants were randomized to one of two treatment sequences, as shown in Fig. 1A. Each sequence consisted of four treatment periods of 1 day each, three washout/safety assessment periods (3 days each), and a follow-up evaluation. i.v. doses of oseltamivir were reconstituted in 2.1 ml of water and then further diluted to 50 ml with 0.9% sodium chloride. The study schedule included screening (days −28 to −2); predose safety screening on days −1, 4, 8, and 12; and follow-up on day 16. Admission to the study unit was on day 1 after a 4-h fast, and subjects stayed in the unit for the 16 days of the study. Vital signs, ECGs, body temperature, and laboratory parameters were measured before the first dose and at follow-up; laboratory tests were also carried out at 24 h, and vital signs and ECGs were assessed at 1, 2, 4, 8, 12, and 24 h on days 1, 5, 9, and 13 (i.e., day 1 of each dosing period).
In the multiple-dose study, participants were screened for up to 28 days, after which they were randomized 2:2:1 to oseltamivir at 100 mg or 200 mg or placebo i.v. over 2 h twice daily (0 and 12 h) for 5 days, with a follow-up 7 to 10 days after the final infusion (Fig. 1B). Infusions were given in a total volume of 50 or 100 ml, and participants were admitted to the study unit from the evening of day −2 and discharged approximately 12 h after the final infusion.
Study assessments.
Blood samples for PK assessment of oseltamivir and OC in the single-dose study were taken predose, at 30-min intervals to 4 h, and then at 5, 6, 8, 10, 12, and 24 h postdose. Urine samples were taken predose and at 0 to 2, 2 to 4, 4 to 8, 8 to 12, and 12 to 24 h postdose. Blood samples were taken at similar time intervals to 12 h after the morning infusion on days 1 and 5 of the multiple-dose study; single predose samples were taken on days 2 through 4 for assessment of trough (minimum) plasma concentrations (Cmin).
Plasma and urine samples were analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) for oseltamivir and OC (for the single-dose study, Bioanalytical Systems Ltd., Kenilworth, Warwickshire, United Kingdom; for the multiple-dose study, PRA International, Assen, The Netherlands). The assay range for plasma was 1.00 to 250 ng/ml for oseltamivir and 10.0 to 10,000 ng/ml for OC. For urine, the assay range was 5.00 to 1,000 ng/ml for oseltamivir and 30.0 to 30,000 ng/ml for OC. For each study, assay performance (accuracy and precision) was determined from the analysis of quality control (QC) samples. For plasma QCs in the single-dose study, accuracy ranged from 99.8 to 102.0% and 98.5 to 101.0% and precision ranged from 4.0 to 18.6% and 3.0 to 9.4% for oseltamivir and OC, respectively. For urine, accuracy ranged from 98.5 to 101.2% and 96.7 to 102.7% and precision ranged from 5.0 to 8.4% and 3.3 to 6.2% for oseltamivir and OC, respectively. For the multiple-dose study, accuracy ranged from 98.0 to 99.3% and 95.6 to 106.0% and precision ranged from 4.0 to 5.5% and 6.1 to 7.0% for oseltamivir and OC, respectively.
Primary PK parameters in the single-dose study were the area under the concentration-time curve (AUC) of OC from time zero to infinity (AUC0–∞) and the concentration of OC at 12 h (C12). Primary PK parameters in the multiple-dose study were AUC from 0 to 12 h (AUC0–12) and the maximum plasma concentration (Cmax) of OC on day 5. Additional PK parameters for oseltamivir and OC evaluated in the single- and/or multiple-dose study included AUC up to the last measurable concentration (AUC0–last), Cmax, time to Cmax (Tmax), apparent t1/2, volume of distribution (V), systemic clearance (CL), renal clearance (CLR), and Cmin.
AEs, clinical laboratory parameters, vital signs, and ECGs were monitored throughout the study. In the multiple-dose study, monitoring of the i.v. infusion site for pain/discomfort, extravasation, or phlebitis was performed with each administration of oseltamivir.
Statistical and analytical methods and sample size.
Noncompartmental analyses were applied using WinNonlin software (Pharsight Corporation, Mountain View, CA). PK data from both studies were summarized descriptively. As a validation of the PK modeling and simulation used to select doses, the exposures predicted by the PK model for both oseltamivir and OC for each of the three i.v. dose levels examined in the single-dose study were compared with observed exposures derived by noncompartmental analysis.
In the single-dose study, analysis of variance (ANOVA) was used to compare PK parameters with respect to treatment formulation. Data from both treatment sequences in periods 1 and 2 were used to calculate the bioavailability of OC following 100 mg i.v. oseltamivir over 2 h relative to the 75-mg oral dose using contrasts from a three-factorial ANOVA. Exploratory statistical analyses using corresponding ANOVAs were carried out for secondary PK characteristics and for data from all four study periods, where doses of 200 mg and 400 mg i.v. were compared with 75 mg orally. The relative bioavailability of the 100-mg i.v. and 75-mg oral doses was estimated on the basis of a prospectively defined lower confidence interval (CI) limit of 0.80 for both AUC0–∞ and C12; this was based on the premise that an acceptable i.v. regimen should provide OC exposures at least equivalent to those achieved with a 75-mg oral dose.
The sample size for the single-dose study was chosen to ensure adequate power for the bioavailability assessment: enrollment of nine subjects was expected to ensure with 80% power that the 90% CI for the ratio of the mean values for 75 mg orally relative to 100 mg i.v. (log scale) would be within 0.8 to 1.25 of the true mean, assuming that the expected ratio of means is 1.0 and the crossover root mean square error is 0.2 (log scale). The sample size for the multiple-dose study was selected on the basis of practical clinical judgment to provide an adequate number of subjects to characterize safety and multiple-dose pharmacokinetics.
RESULTS
Demographics.
In the single-dose study, 24 volunteers were enrolled from the 90 volunteers screened and were randomized to the two treatment sequences (12 volunteers in each one). In the multiple-dose study, an initial cohort of 50 volunteers was enrolled but was not included in the PK analysis because of a dosing error caused by the presence of residual normal saline in the i.v. tubing that interfered with accurate collection of the PK samples. These persons were retained for safety analysis, however. A second cohort of 50 subjects was enrolled, of which 1 was found not to satisfy the inclusion criteria (nonspecific T-wave changes) and did not receive the study drug. Thus, 49 subjects were available for the final PK analysis and 99 were available for the safety analysis. One volunteer was prematurely withdrawn from each treatment sequence in the single-dose study (one had ventricular extrasystoles and one withdrew consent for nonsafety reasons, both after treatment with 100 mg i.v.). In the multiple-dose study, there were no premature withdrawals.
In the single-dose study, 96% of subjects were male and 92% were white, with a mean age of 31.3 years. In the multiple-dose study, in the PK population, 80% of participants in the oseltamivir 200-mg group were male, whereas there were similar numbers of men and women in the other two groups (Table 1). There were also over twice as many white (n = 33) as black (n = 15) subjects. Mean ages across the treatment groups (28.1 to 30.2 years) were similar to the mean age in the single-dose study. In the multiple-dose study, in the 50 initial subjects (10 in the placebo group and 20 in each active-treatment group), most were male (85%, 80%, and 90% in the oseltamivir 200-mg and 100-mg and placebo groups, respectively), with similar proportions of white and black volunteers in the oseltamivir groups, although 70% in the placebo group were black. Mean ages across the treatment groups ranged from 28.6 to 31.1 years.
Table 1.
Demographics of participants in the single- and multiple-dose studies
| Parameter | Single-dose study (all periods; n = 24) | Multiple-dose study |
||
|---|---|---|---|---|
| Placebo (n = 10) | Oseltamivir 100 mg i.v. (n = 19a) | Oseltamivir 200 mg i.v. (n = 20) | ||
| No. (%) of volunteers by: | ||||
| Gender (male) | 23 (96) | 5 (50) | 9 (47) | 16 (80) |
| Race | ||||
| Caucasian | 22 (92) | 8 (80) | 15 (79) | 10 (50) |
| Black | 1 (4) | 2 (20) | 4 (21) | 9 (45) |
| Oriental | 1 (4) | |||
| American Indian/Alaska Native | 1 (5) | |||
| Age (yr) | ||||
| Mean ± SD | 31.3 ± 8.49 | 28.1 ± 6.19 | 28.3 ± 6.97 | 30.2 ± 7.73 |
| Median (range) | 33.0 (18–52) | 28.0 (19–36) | 27.0 (18–43) | 28.0 (19–44) |
| Body wt (kg) | ||||
| Mean ± SD | 74.70 ± 10.08 | 78.88 ± 11.85 | 75.22 ± 17.73 | 80.02 ± 12.84 |
| Median (range) | 72.20 (55.8–90.9) | 72.60 (66.5–98.6) | 72.20 (51.0–115.5) | 77.30 (54.5–105.4) |
| Ht (cm) | ||||
| Mean ± SD | 176.9 ± 7.17 | 170.5 ± 13.17 | 169.1 ± 9.27 | 174.8 ± 6.01 |
| Median (range) | 179.0 (158–191) | 170.5 (149–194) | 167.0 (157–184) | 175.5 (160–186) |
| BMI | ||||
| Mean ± SD | 23.83 ± 2.52 | 27.18 ± 3.25 | 26.10 ± 4.57 | 26.16 ± 3.85 |
| Median (range) | 24.10 (19.1–29.3) | 26.74 (24.10–33.72) | 25.55 (17.10–34.24) | 25.05 (20.29–33.49) |
| Estimated creatinine clearance | ||||
| Mean ± SD | 143.53 ± 23.58 | 143.26 ± 21.87 | 140.87 ± 31.61 | 137.90 ± 34.21 |
| Median (range) | 146.53 (101.2–185.7) | 132.22 (121.86–178.08) | 137.57 (90.03–202.21) | 130.98 (95.78–220.24) |
n = 18 for estimated creatinine clearance.
Pharmacokinetics. (i) Single-dose study.
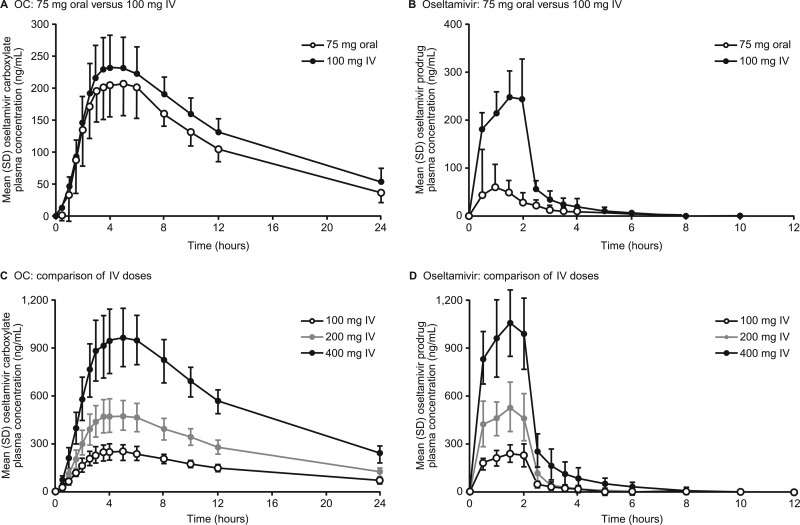
Mean plasma concentration-versus-time profiles of OC for the 100-mg i.v. and 75-mg oral doses are shown in Fig. 2, and PK data are summarized in Table 2. The mean Cmaxs for OC of 215 ng/ml and 240 ng/ml were attained after 5 h and 4 h (median Tmaxs) with the 75-mg oral and 100-mg i.v. doses, respectively. Concentrations decreased over parallel time courses (Fig. 2A). Mean exposure (AUC0–∞) to OC was marginally higher with 100 mg i.v. than with 75 mg orally (3,840 ng · h/ml versus 3,060 ng · h/ml), and mean C12 values were greater with i.v. than oral dosing (131 ng/ml versus 104 ng/ml) (Table 2). Exposure to the parent compound, oseltamivir, was higher with 100 mg i.v. than with 75 mg orally (Fig. 2B and Table 2) because of the avoidance of first-pass metabolism with i.v. dosing: the mean Cmax was 3.1 times higher (263 ng/ml versus 84.1 ng/ml) and AUC0–∞ was 3.9 times higher (540 ng · h/ml versus 140 ng · h/ml). The prodrug was cleared to below the lower limit of quantification after 12 h in approximately 85% of volunteers in both groups.
Fig 2.
Mean (standard deviation) plasma concentration-versus-time profiles of OC (A), oseltamivir after single i.v. 100-mg and single oral 75-mg doses of oseltamivir phosphate (B), OC after single i.v. doses of 100 mg, 200 mg, and 400 mg (C), and oseltamivir after single i.v. doses of 100 mg, 200 mg, and 400 mg (D).
Table 2.
PK parameters of oseltamivir (prodrug) and OC (active metabolite) in the single- and multiple-dose studiesa
| Study and oseltamivir regimen | Oseltamivir |
OC |
Accumulation ratiod | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cmax (ng/ml) | Tmax (h) | AUC0–∞ (ng · h/ml) | AUC0–12 (ng · h/ml) | AUC0–last (ng · h/ml) | t1/2 (h) | C12 (ng/ml) | AUC0–∞ (ng · h/ml) | AUC0–12 (ng · h /ml) | AUC0–last (ng · h /ml) | Cmax (ng/ml) | Cmin (ng/ml) | Tmax (h) | t1/2 (h) | V (ml) | CLR (liters/h) | ||
| Single-dose study | |||||||||||||||||
| 75 mg orally (n = 24) | 84.1 ± 77.7 (26.7–389) | 1.00 (0.50–1.50) | 140 ± 48.9 (80.4–418) | 138 ± 45.3 (80.2–373) | 136 ± 49.8 (76.8–411) | 1.7 ± 44.1 (0.921–4.83) | 104 ± 16.9 (75.8–143) | 3,060 ± 19.4 (2,240–4,520) | 1,750 ± 19.3 (1,320–2,660) | 2,600 ± 16.5 (2,050–3,750) | 215 ± 22.8 (140–338) | 5.00 (2.50–6.00) | 7.73 ± 29.4 (5.02–14.9) | 273,000 ± 22.8 (170,000–389,000) | 15.2 ± 17.4 (11.9–21.2) | ||
| 100 mg i.v. (n = 24) | 263 ± 23.8 (181–437) | 1.53 (1.00–2.00) | 540 ± 27.1 (382–1,100) | 538 ± 25.6 (382–1,040) | 537 ± 27.2 (380–1,100) | 1.54 ± 33.3 (0.956–3.62) | 131 ± 16.7 (95.1–175) | 3,840 ± 17.0 (2,640–5,070) | 2,030 ± 16.4 (1,490–2,780) | 3,140 ± 14.3 (2,340–4,050) | 240 ± 20.4 (173–363) | 4.00 (3.00–6.00) | 8.81 ± 22.1 (6.46–14.2) | 331,000 ± 17.7 (229,000–457,000) | 14.6 ± 13.2 (11.0–17.9) | ||
| 200 mg i.v. (n = 23) | 548 ± 28.2 (314–922) | 1.50 (0.50–2.00) | 1,150 ± 32.7 (690–2,640) | 1,140 ± 31.2 (688–2,520) | 1,140 ± 32.6 (687–2,620) | 1.76 ± 32.6 (1.08–4.07) | 265 ± 16.5 (193–365) | 7,860 ± 15.1 (5,770–10,200) | 4,070 ± 18.1 (3,090–5,760) | 6,340 ± 14.9 (4,860–8,620) | 478 ± 21.8 (370–755) | 4.00 (3.00–6.03) | 9.05 ± 20.5 (5.97–12.9) | 332,000 ± 20.4 (207,000–457,000) | 12.7 ± 23.1 (7.18–19.8) | ||
| 400 mg i.v. (n = 22) | 1,100 ± 20.3 (685–1,590) | 1.50 (0.50–2.00) | 2,360 ± 28.3 (1,440–4,830) | 2,350 ± 26.5 (1,430–4,540) | 2,360 ± 28.2 (1,430–4,800) | 1.82 ± 57.0 (1.23–6.45) | 551 ± 14.0 (412–716) | 16,200 ± 14.9 (11,700–21,800) | 8,420 ± 16.3 (6,460–11,500) | 13,100 ± 13.6 (10,100–17,100) | 977 ± 19.9 (738–1,540) | 5.00 (3.00–6.00) | 9.00 ± 20.0 (6.88–14.0) | 321,000 ± 18.2 (215,000–445,000) | 13.1 ± 22.3 (5.81–17.1) | ||
| Multiple-dose study | |||||||||||||||||
| Day 1 | |||||||||||||||||
| 100 mg i.v. (n = 19) | 279 ± 19.7 (193–368) | 2.00 (1–2.03) | 599 ± 21.9 (414–925) | 597 ± 21.8 (414–922) | 596 ± 22.0 (412–921) | 1.18 ± 27.1 (0.79–1.90) | 3,531 ± 21.2 (2,478–5,248) | 2,252 ± 16.8 (1,727–3,189) | 2,241 ± 16.8 (1,719–3,176) | 294 ± 22.2 (203–476) | 209 ± 43.6b | 4.00 (3–6) | 6.62 ± 31.4 (4.35–11.9) | ||||
| 200 mg i.v. (n = 20) | 495 ± 18.4 (377–727) | 2.00 (1–2.03) | 1,117 ± 20.5 (703–1,500) | 1,115 ± 20.5 (702–1,495) | 1,114 ± 20.5 (700–1,494) | 1.36 ± 24.0 (0.95–2.11) | 7,213 ± 25.4 (5,149–12,179) | 4,532 ± 16.3 (3,284–5,747) | 4,511 ± 16.3 (3,265–5,716) | 570.0 ± 16.7 (362–711) | 388 ± 95.4b | 3.50 (3–8) | 7.78 ± 23.3 (3.16–12.4) | ||||
| Day 5 | |||||||||||||||||
| 100 mg i.v. (n = 19) | 255 ± 32.3 (111–373) | 2.00 (1–2.02) | 555 ± 34.8 (245–930) | 553 ± 34.8 (243–927) | 551 ± 35.0 (240–926) | 1.5 ± 19.5 (0.85–2.01) | 4,086 ± 17.7 (3,074–5,862) | 4,066 ± 17.8 (3,042–5,828) | 482 ± 16.8 (375–667) | 262 ± 68.4c | 3.50 (2–6) | 6.85 ± 32.0 (5.58–11.9) | 1.83 ± 0.278 (1.35–2.62) | ||||
| 200 mg i.v. (n = 20) | 492 ± 14.4 (363–633) | 2.00 (1–2.05) | 1,133 ± 16.3 (776–1,575) | 1,129 ± 16.2 (775–1,566) | 1,129 ± 16.2 (773–1,566) | 1.83 ± 25.0 (0.98–2.82) | 7,848 ± 17.8 (6,205–10,609) | 7,815 ± 17.7 (6,178–10,559) | 945 ± 18.3 (728–1,340) | 502 ± 130.4c | 3.00 (2–6) | 7.88 ± 28.2 (3.95–13.1) | 1.74 ± 0.212 (1.38–2.34) | ||||
Data represent geometric mean ± percent coefficient of variation (range) for all parameters except Tmax, data for which are presented as median (range).
Day 2 predose; mean ± standard deviation.
Day 5 predose; mean ± standard deviation.
Day 5 AUC0–12/day 1 AUC0–12; mean ± standard deviation.
Using data from periods 1 and 2 only, the relative bioavailability of OC after 100 mg i.v. oseltamivir relative to that after 75 mg orally was estimated using a mixed-model analysis, by fitting a model containing fixed effects for treatment and period and a random-subject effect. The analyses indicated that the point estimate (90% CI) AUC0–∞ and C12 values were 1.25 (1.22 to 1.29) and 1.26 (1.21 to 1.31) times greater following i.v. dosing. As the lower limits of the 90% CIs for the mean i.v./oral ratios of OC AUC0–∞ and C12 were 0.8 for 100 mg i.v., the resulting exposures were deemed acceptable as OC exposures were at least equivalent to those achieved with 75 mg orally. Moreover, the 90% CI for the OC Cmax ratio (107.2 to 115.6) fell within regulatory bounds for bioequivalence (0.80 to 1.25). As shown in Fig. 2C and D, mean concentration-versus-time profiles for oseltamivir and OC at the three i.v. doses tested indicated linear and dose-proportional characteristics.
As predicted by the PK modeling, active metabolite exposures obtained after 100 mg i.v. over 2 h were broadly comparable to those after the registered 75-mg oral dose. Furthermore, as summarized in Table 3, the predicted exposures for both oseltamivir and OC for each of the three i.v. dose levels were similar to those observed in each case. These results indicated that the model performed adequately for the purposes of defining the i.v. doses for this study. While the study was not designed to examine the absolute bioavailability of OC, a mean estimate of 94.1% was determined following dose normalization, which is consistent with modest additional prodrug renal clearance prior to conversion to metabolite.
Table 3.
Comparison of observed and modeled mean oseltamivir and OC exposures following single-dose i.v. administration
| Dose regimen | Oseltamivir |
OC |
||||||
|---|---|---|---|---|---|---|---|---|
| AUC0–∞ (ng · h/ml) |
Cmax (ng/ml) |
AUC0–∞ (ng · h/ml) |
Cmax (ng/ml) |
|||||
| Observed | Predicted | Observed | Predicted | Observed | Predicted | Observed | Predicted | |
| 75 mg orallya | 152 (80.4–418) | 103 (26.7–389) | 3,120 (2,240–4,520) | 220 (140–338) | ||||
| 100 mg i.v. | 556 (382–1,100) | 539 | 270 (181–437) | 241 | 3,890 (2,640–5,070) | 2,740 | 244 (173–363) | 193 |
| 200 mg i.v. | 1,190 (690–2,640) | 1,079 | 568 (314–922) | 482 | 7,950 (770–10,200) | 5,480 | 488 (370–755) | 385 |
| 400 mg i.v. | 2,440 | 2,158 | 1,120 (685–1,590) | 965 | 16,400 (11,700–21,800) | 10,961 | 993 (738–1,540) | 770 |
Reference regimen.
The PK and safety (see later) data from the single-dose study led to the recommendation that the 100-mg and 200-mg i.v. doses should be carried forward to the multiple-dose study. The clinical safety margin provided by the 400-mg single dose supported these recommendations.
(ii) Multiple-dose study.
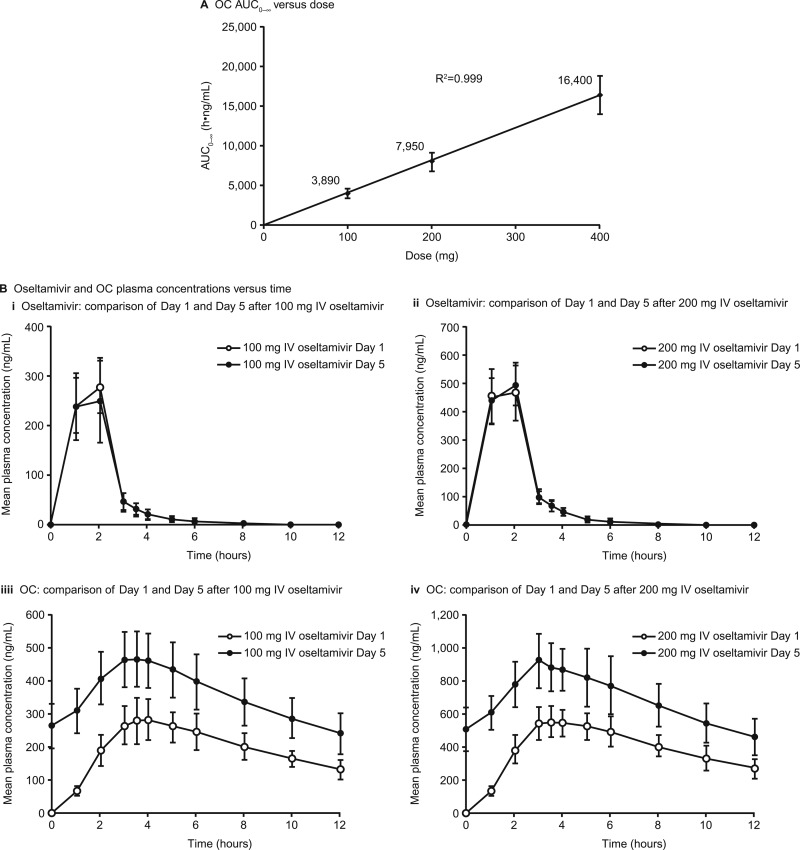
Single-dose (i.e., day 1) PK parameters reported in the multiple-dose study were similar to those in the single-dose study (Table 2). The pharmacokinetics of both oseltamivir and OC (Fig. 3A) were linear. The OC Tmax occurred after 3 to 4 h, which is similar to that with oral dosing. Plasma OC concentrations were higher on day 5 than on day 1 (Fig. 3B), which reflected an approximately 2-fold accumulation (Table 2, accumulation ratio), the same as that observed with oral dosing (8). Examination of trough concentrations of OC over the 5 days indicated that steady state was reached by day 3, consistent with the t1/2 of 7 to 8 h. Mean concentration-versus-time profiles of oseltamivir were similar on day 1 and day 5, reflecting the lack of accumulation of the parent compound due to the relatively short t1/2 of 1 to 2 h (Fig. 3B).
Fig 3.
(A) Plot of OC AUC0–∞ versus dose demonstrating linearity; (B) mean (standard deviation) plasma concentration-versus-time profiles of OC and oseltamivir after single and multiple doses of 100 mg and 200 mg from the multiple-dose study.
Safety. (i) Single-dose study.
In the single-dose study, all regimens were well tolerated (Table 4). Most AEs were of mild intensity and were related to infusion of the 400-mg dose: there were more AEs in sequence 1 (n = 14) than sequence 2 (n = 12), but with no evidence of a sequence effect. As all i.v. infusions were prepared to the same 50-ml volume, the higher rate of infusion site events with the 400-mg dose was considered related to the drug concentration. There were no serious AEs (SAEs). One subject who received 200 mg i.v. oseltamivir withdrew before study completion as a result of an AE (ventricular extrasystoles) that was considered of mild intensity and remotely related to treatment. There were no deaths or trends in laboratory parameters and no relevant ECG changes.
Table 4.
AEs reported in more than one person in any treatment group in the single- and multiple-dose studies
| AEa | No. (%) of subjects |
||||||
|---|---|---|---|---|---|---|---|
| Single-dose study with oseltamivirb |
Multiple-dose study |
||||||
| 75 mg orally (n = 24) | 100 mg i.v. (n = 24) | 200 mg i.v. (n = 23) | 400 mg i.v. (n = 22) | Placebo (n = 10) | Oseltamivir 100 mg i.v. (n = 19) | Oseltamivir 200 mg i.v. (n = 20) | |
| Infusion/injection site events | 13 (59) | 7 (70) | 17 (89) | 19 (95) | |||
| Venipuncture site events/pain | 1 (4) | 1 (4) | 2 (11) | 2 (10) | |||
| Dizziness | 1 (10) | 2 (11) | 1 (5) | ||||
| Headache | 1 (4) | 1 (5) | 1 (5) | 2 (10) | |||
| Abdominal pain | 1 (4) | 1 (5) | |||||
| Pruritus | 1 (10) | 2 (10) | |||||
Multiple occurrences of the same AE in one individual are counted only once.
Results for all periods.
(ii) Multiple-dose study.
In the multiple-dose study, all regimens were well tolerated and all AEs were of mild intensity in the 49 volunteers dosed correctly. The majority of subjects (44/49) reported at least one AE, with mild infusion/injection site events being the most common (Table 4). Infusion/injection site events were defined as the presence of sensory symptoms (pain, warmth, and cold) and/or physical findings (erythema, swelling, edema, induration, extravasation) associated with the i.v. infusion site. Mild infusion/injection site events were reported by 17/19 (89%), 19/20 (95%), and 7/10 (70%) of the persons in the 100-mg, 200-mg, and placebo arms, respectively. The vast majority of complaints (79%, 75%, and 30% in the 100-mg, 200-mg, and placebo arms, respectively) were related to mild pain/discomfort associated with the i.v. infusion (Table 4). There were no dose-related increases in reported AEs, no SAEs or withdrawals, and no relevant changes in vital signs, ECGs, or laboratory parameters.
As described above, the initial 50 subjects enrolled were judged as being ineligible for PK assessment due to dosing inaccuracies resulting from residual saline in the i.v. line. However, these subjects were estimated to have received approximately 70 to 80% of the planned dose; therefore, examination of safety data was still relevant, and indeed, the safety profile was similar to the profile for those subjects who were dosed as planned. Of these 50 subjects, 23 (46%) reported 41 AEs. In contrast to the 49 subjects described above, infusion/injection site events were reported by 3/20 (15%), 12/20 (60%), and 0/10 (0%) participants in the 100-mg, 200-mg, and placebo arms, respectively. Interestingly, the volume of the infusion was 50 ml in the first 50 subjects, and as a result of the apparent concentration-related increase in infusion/injection events, the infusion volume was increased to 100 ml for the second 49 subjects enrolled. However, the frequency of infusion/injection site events was higher in the second group of 49 subjects, despite the more dilute i.v. solution administered.
In the initial 50 subjects, there were more AEs with oseltamivir than with placebo, with one case each of dizziness and oropharyngeal pain in the latter group. All AEs were of mild intensity, except for three events in the 100-mg i.v. oseltamivir group (nausea, headache, and hyperhidrosis; all were of moderate intensity and possibly or remotely related to oseltamivir). There were no SAEs or treatment withdrawals and no clinically relevant changes in laboratory tests, vital signs, or ECGs.
DISCUSSION
Overall, dosing with oseltamivir at 100 mg i.v. over 2 h provides OC exposure (specifically, AUC, Cmax, and Cmin) at least as high as that achieved with the approved therapeutic oral dose of 75 mg. Consequently, i.v. oseltamivir administered at doses of 100 mg over 2 h twice daily for 5 days will provide OC exposures in the range previously shown by phase III studies of oral oseltamivir to be efficacious with a low probability of emergence of resistance (15, 21). In addition, OC exposures following i.v. doses of oseltamivir were well above the 50% inhibitory concentration (IC50) values reported for a variety of laboratory strains and clinical isolates of influenza A virus, including highly pathogenic influenza A virus H5N1 (2, 7, 13, 19). These strains exhibited IC50s of 0.01 to 69.2 nmol/liter for OC, while the day 5 Cmin reported for OC in the multiple-dose study (262 ng/ml) is equivalent to 935 nmol/liter.
Following i.v. administration of oseltamivir, both oseltamivir and OC demonstrated linear and dose-proportional PK characteristics. As expected, single-dose exposures in the multiple-dose study were consistent with those in the single-dose study. Upon repeat dosing, there was no accumulation of oseltamivir and an accumulation ratio of approximately 1.8 for OC, which are consistent with expectations for twice-daily dosing of a drug with a t1/2 of approximately 8 h and previous experience with oral administration of oseltamivir (8).
Earlier studies (8) have illustrated that after i.v. OC administration, the t1/2 of OC is only 1 to 2 h, whereas after i.v. administration of the parent compound, oseltamivir, the t1/2 of OC is comparable to that obtained by oral oseltamivir dosing (approximately 8 h), which permits twice-daily dosing. One possible explanation is that oseltamivir is converted to polar OC after delivery to hepatocytes, where it becomes trapped. OC then enters the plasma at a rate governed by permeability-limited release from these cells (8, 16). For this reason, oseltamivir, rather than OC, is being developed as an i.v. neuraminidase inhibitor, to allow twice-daily dosing. Despite the complexity of the PK behavior described, a sequential PK model was successfully applied to the selection of doses for the single-dose study, with good agreement between model predictions and exposures observed in the single-dose study.
Plasma concentrations of the parent compound, oseltamivir, are approximately 3 to 4 times higher when administered as a 2-h i.v. infusion than when administered orally because of the absence of the hepatic first-pass effect. In preclinical toxicology studies, the maximum concentration of the parent compound is the parameter most related to adverse findings. In the marmoset, repeated daily i.v. treatment with up to 50 mg/kg of body weight for 14 days did not reveal any treatment-related toxicological findings and provides a safety margin of >40-fold to oseltamivir at 200 mg/2 h i.v. twice daily in adults. It is, however, important that i.v. oseltamivir always be administered as a slow i.v. infusion and never as a bolus, in order to avoid very high oseltamivir plasma concentrations.
i.v. oseltamivir showed a good safety profile in both studies. The tolerability of i.v. oseltamivir was generally good, even at a dose as high as 400 mg. Infusion site events such as pain, swelling, and erythema, while frequent, were mild and did not interfere with treatment in any participant. Reports of mild infusion/injection site events, including pain, were initially thought to be related to the concentration of the injected solution since in the single-dose study and in the first 50 subjects in the multiple-dose study, the number of infusion/injection site events increased in a concentration-related manner: 15% reported pain with the 100-mg dose (2 mg/ml infusion), in comparison with 60% with the 200-mg dose (4-mg/ml infusion). However, in the second group of 49 subjects enrolled in the multiple-dose study, mild infusion/injection site events were reported by 89 to 95% of participants receiving active drug, despite the use of a more dilute drug solution (1 mg/ml for 100 mg and 2 mg/ml for 200 mg). The discrepancy in reports of infusion/injection site events may have resulted from a greater emphasis by clinical site staff on monitoring and solicitation of feedback on infusion-site AEs for the second enrollment of 49 subjects. This may also be reflected in the 70% of placebo subjects who experienced infusion/injection site events in the second group, in comparison with no placebo subjects in the first group. Furthermore, no such reports were received in the single-dose study in the 100-mg or 200-mg dose group. The mild pain that occurs with oseltamivir i.v. infusion is thought to be due to the acidic nature of the reconstituted solution (pH approximately 4). Following dilution with normal saline to a total volume of 100 ml, the pH remains acidic at approximately 4.8, which can cause local irritation.
Data from the present studies in healthy volunteers have been used to support an investigational new drug program in the United States to explore emergency use in case of a pandemic and a compassionate-use program in Europe and Australia and to aid the design of safety and efficacy studies in patients with influenza, including critically ill infants (NCT01053663), children (NCT01033734), and adolescents and adults who are unable to tolerate oral oseltamivir (NCT01050257). These studies are expected to report over the next 2 years, but interim findings are consistent with forecasts based on information available to date.
Conclusions.
The present findings confirm previous experimental observations and model predictions. Moreover, there were no new or unexpected safety signals. The multiple dosages used here support further investigation in patients with influenza, and studies are accordingly ongoing. The availability of oseltamivir for i.v. use will fulfill a medical need for a parenteral neuraminidase inhibitor for patients who are unable to tolerate or swallow oral medications and who currently have limited treatment options.
ACKNOWLEDGMENTS
This work was supported by funding from F. Hoffmann-La Roche, Ltd. Support for third-party writing assistance for the manuscript was provided by F. Hoffmann-La Roche, Ltd.
All authors are current or former employees of Roche. Barbara J. Brennan, Brian Davies, Sian Lennon-Chrimes, and Colombe Chappey also have stocks/stock options with Roche.
Footnotes
Published ahead of print 25 June 2012
REFERENCES
- 1. Ariano RE, et al. 2010. Enteric absorption and pharmacokinetics of oseltamivir in critically ill patients with pandemic (H1N1) influenza. CMAJ 182:357–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bantia S, et al. 2001. Comparison of the anti-influenza virus activity of RWJ-270201 with those of oseltamivir and zanamivir. Antimicrob. Agents Chemother. 45:1162–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davies BE. 2010. Pharmacokinetics of oseltamivir: an oral antiviral for the treatment and prophylaxis of influenza in diverse populations. J. Antimicrob. Chemother. 65(Suppl. 2):ii5–ii10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Farias JA, et al. 2010. Critically ill infants and children with influenza A (H1N1) in pediatric intensive care units in Argentina. Intensive Care Med. 36:1015–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. F. Hoffmann-La Roche, Ltd. 2011. Tamiflu prescribing information (PI). http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Search_Drug_Name Accessed 25 August 2011
- 6. F. Hoffmann-La Roche, Ltd. 2011. Tamiflu summary of product characteristics (SmPC). http://www.medicines.org.uk/emc/ Accessed 25 August 2011
- 7. Govorkova EA, Leneva IA, Goloubeva OG, Bush K, Webster RG. 2001. Comparison of efficacies of RWJ-270201, zanamivir, and oseltamivir against H5N1, H9N2, and other avian influenza viruses. Antimicrob. Agents Chemother. 45:2723–2732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. He G, Massarella J, Ward P. 1999. Clinical pharmacokinetics of the prodrug oseltamivir and its active metabolite Ro 64-0802. Clin. Pharmacokinet. 37:471–484 [DOI] [PubMed] [Google Scholar]
- 9. Heinonen S, et al. 2010. Early oseltamivir treatment of influenza in children 1–3 years of age: a randomized controlled trial. Clin. Infect. Dis. 51:887–894 [DOI] [PubMed] [Google Scholar]
- 10. Kaiser L, et al. 2003. Impact of oseltamivir treatment on influenza-related lower respiratory tract complications and hospitalizations. Arch. Intern. Med. 163:1667–1672 [DOI] [PubMed] [Google Scholar]
- 11. Kumar D, et al. 2010. Outcomes from pandemic influenza A H1N1 infection in recipients of solid-organ transplants: a multicentre cohort study. Lancet Infect. Dis. 10:521–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee N, et al. 2010. Outcomes of adults hospitalised with severe influenza. Thorax 65:510–515 [DOI] [PubMed] [Google Scholar]
- 13. Leneva IA, Roberts N, Govorkova EA, Goloubeva OG, Webster RG. 2000. The neuraminidase inhibitor GS4104 (oseltamivir phosphate) is efficacious against A/Hong Kong/156/97 (H5N1) and A/Hong Kong/1074/99 (H9N2) influenza viruses. Antiviral Res. 48:101–115 [DOI] [PubMed] [Google Scholar]
- 14. McGeer A, et al. 2007. Antiviral therapy and outcomes of influenza requiring hospitalization in Ontario, Canada. Clin. Infect. Dis. 45:1568–1575 [DOI] [PubMed] [Google Scholar]
- 15. Nicholson KG, et al. 2000. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Lancet 355:1845–1850 [DOI] [PubMed] [Google Scholar]
- 16. Parrott N, et al. 2011. Development of a physiologically based model for oseltamivir and simulation of pharmacokinetics in the very young. Clin. Pharmacokinet. 50:613–623 [DOI] [PubMed] [Google Scholar]
- 17. Power BM, Forbes AM, van Heerden PV, Ilett KF. 1998. Pharmacokinetics of drugs used in critically ill adults. Clin. Pharmacokinet. 34:25–56 [DOI] [PubMed] [Google Scholar]
- 18. Rayner CR, Chanu P, Gieschke R, Boak LM, Jonsson EN. 2008. Population pharmacokinetics of oseltamivir when coadministered with probenecid. J. Clin. Pharm. 48:935–947 [DOI] [PubMed] [Google Scholar]
- 19. Roberts NA, Wiltshire HR, Mendel DB, Webster RG. 2003. Oseltamivir carboxylate is effective against all subtypes of influenza neuraminidase, poster 135. Poster 3rd Annu. ASM Biodefense Res. Meet American Society for Microbiology, Washington, DC [Google Scholar]
- 20. Siston AM, et al. 2010. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA 303:1517–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Treanor JJ, et al. 2000. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. JAMA 283:1016–1024 [DOI] [PubMed] [Google Scholar]
- 22. Whitley RJ, et al. 2001. Oral oseltamivir treatment of influenza in children. Pediatr. Infect. Dis. J. 20:127–133 [DOI] [PubMed] [Google Scholar]
- 23. World Health Organization 2009. Influenza (seasonal). Fact sheet no. 211. April 2009. World Health Organization, Geneva, Switzerland: http://www.who.int/mediacentre/factsheets/fs211/en Accessed 25 August 2011 [Google Scholar]
- 24. Writing Committee of the WHO Consultation on Clinical Aspects of Pandemic (H1N1) 2009 Influenza et al 2009. Clinical aspects of pandemic influenza A (H1N1) virus infection. 2010. N. Engl. J. Med. 362:1708–1719 [DOI] [PubMed] [Google Scholar]