Abstract
We investigated the mechanisms leading to Pseudomonas aeruginosa pan-β-lactam resistance (PBLR) development during the treatment of nosocomial infections, with a particular focus on the modification of penicillin-binding protein (PBP) profiles and imipenem, ceftazidime, and ceftolozane (former CXA-101) PBP binding affinities. For this purpose, six clonally related pairs of sequential susceptible-PBLR isolates were studied. The presence of oprD, ampD, and dacB mutations was explored by PCR followed by sequencing and the expression of ampC and efflux pump genes by real-time reverse transcription-PCR. The fluorescent penicillin Bocillin FL was used to determine PBP profiles in membrane preparations from all pairs, and 50% inhibitory concentrations (IC50s) of ceftolozane, ceftazidime, and imipenem were analyzed in 3 of them. Although a certain increase was noted (0 to 5 2-fold dilutions), the MICs of ceftolozane were ≤4 μg/ml in all PBLR isolates. All 6 PBLR isolates lacked OprD and overexpressed ampC and one or several efflux pumps, particularly mexB and/or mexY. Additionally, 5 of them showed modified PBP profiles, including a modified pattern (n = 1) or diminished expression (n = 1) of PBP1a and a lack of PBP4 expression (n = 4), which correlated with AmpC overexpression driven by dacB mutation. Analysis of the essential PBP IC50s revealed significant variation of PBP1a/b binding affinities, both within each susceptible-PBLR pair and across the different pairs. Moreover, despite the absence of significant differences in gene expression or sequence, a clear tendency toward increased PBP2 (imipenem) and PBP3 (ceftazidime, ceftolozane, imipenem) IC50s was noted in PBLR isolates. Thus, our results suggest that in addition to AmpC, efflux pumps, and OprD, the modification of PBP patterns appears to play a role in the in vivo emergence of PBLR strains, which still conserve certain susceptibility to the new antipseudomonal cephalosporin ceftolozane.
INTRODUCTION
β-Lactam antibiotics, including antipseudomonal penicillins, cephalosporins, monobactams, and carbapenems, remain key components of our antimicrobial armamentarium for the treatment of life-threatening nosocomial infections by Pseudomonas aeruginosa (23). Nevertheless, resistance to these first-line antibiotics is increasing and frequently associated with multidrug resistance (MDR) phenotypes (4, 19). While the acquisition of potent exogenous β-lactamases such as class B carbapenemases (or metallo-β-lactamases [MBLs]) or extended-spectrum β-lactamases (ESBLs) through horizontal gene transfer is a growing threat, β-lactam resistance is still much more frequently caused by the selection of a complex repertoire of chromosomal mutations (19, 20, 31, 32). Particularly noteworthy among them are those leading to the repression or inactivation of the porin OprD, conferring resistance to carbapenems (8, 14, 30, 33), or those leading to the hyperproduction of the chromosomal cephalosporinase AmpC (4, 15, 24), causing resistance to penicillins, cephalosporins, and monobactams. Also, mutations leading to the upregulation of one of the several efflux pumps encoded in the P. aeruginosa genome, particularly MexAB-OprM and MexXY-OprM, may significantly contribute to β-lactam resistance phenotypes, in addition to reducing the activity of fluoroquinolones and aminoglycosides (4, 5, 22, 31). While the combination of these mechanisms leads to the emergence of resistance to all currently available β-lactams, some derivatives under clinical development, such as the new cephalosporin ceftolozane (formerly CXA-101), appear to be much less affected by them and thus represent a promising future approach for the treatment of P. aeruginosa infections (3, 16, 21, 25, 35).
Another potentially relevant resistance mechanism is the modification of the target of β-lactam antibiotics, the essential penicillin-binding proteins (PBPs), which are PBP1a, PBP1b, PBP2, and PBP3 (37). While the acquisition of modified PBPs showing low affinity for β-lactams is well known to be a major resistance mechanism in Gram-positive cocci, Haemophilus spp., and Neisseria spp., the role of PBPs in resistance has remained elusive, controversial, or ignored for most species of Gram-negative nosocomial pathogens (37).
Previous studies have demonstrated that Escherichia coli PBP2 mutants showing reduced affinity for imipenem can be selected in vitro upon antibiotic exposure (36), but the only current evidence of the natural occurrence of such mutants in Enterobacteriaceae is a single Proteus mirabilis clinical isolate (28). Particular attention has raised the potential role of PBPs in Acinetobacter baumannii imipenem resistance. Fernández-Cuenca et al. (10) demonstrated reduced expression of PBP2 in some imipenem-resistant clinical isolates, although a recent work did not find mutations in PBP-encoding genes that could be linked to resistance phenotypes (6). Likewise, there is very little information on the potential role of PBPs in P. aeruginosa β-lactam resistance; a previous work found no correlation between the expression of genes encoding PBP2 or PBP3 and carbapenem resistance (2), but results from two other studies suggest the possibility of a decreased expression in some resistant isolates (9, 13). Although not strictly related to target modification, recent studies have shown that nonessential PBPs may also play an important role in P. aeruginosa β-lactam resistance, since mutation of dacB, encoding the putative PBP4, triggers AmpC overexpression and resistance to penicillins, cephalosporins, and monobactams (24). Nevertheless, there are no current data correlating dacB mutations with a lack of PBP4 expression; it is also not known whether the absence of PBP4 could eventually modify the expression profiles of other PBPs or their relative binding affinities for β-lactam antibiotics.
In order to advance our knowledge in this field, we characterized clonally related pairs of P. aeruginosa clinical isolates that had developed pan-β-lactam resistance in vivo during treatment of infections in intensive care unit (ICU) patients. Along with characterizing classical β-lactam resistance mechanisms (expression of AmpC, OprD, and efflux pumps), we followed an integral approach to evaluate the involvement of PBPs in resistance phenotypes through the comparative analysis of the clonally related pairs of PBP profiles and the sequence and expression of PBP-encoding genes. Moreover, we comparatively evaluated in the clonally related pairs the PBP binding affinities of imipenem, ceftazidime, and ceftolozane, since this relevant information is currently available for wild-type strains but not for pan-β-lactam-resistant (PBLR) clinical isolates (25).
MATERIALS AND METHODS
Strains and susceptibility testing.
Six clonally related pairs of sequential P. aeruginosa isolates that developed resistance to all β-lactams during the treatment of nosocomial infections in ICU patients were used. The clonal relatedness had been previously assessed through pulsed-field gel electrophoresis (PFGE) (25). The MICs of ceftolozane, ceftazidime, cefepime, piperacillin-tazobactam, aztreonam, imipenem, meropenem, ciprofloxacin, and tobramycin were determined by standard CLSI broth microdilution (7) in the previous study (24). PAO1 was used as the control strain.
PCR amplification and sequencing of ampD, dacB, and oprD.
PCR amplification of ampD, dacB, and oprD was performed on whole DNA extracts (DNeasy tissue kit; Qiagen, Hilden, Germany) from both the susceptible and the PBLR isolates from each of the 6 pairs of P. aeruginosa strains using previously described conditions (14, 15, 24) and primers (Table 1). At least two independent PCR products for each isolate and gene were sequenced on both strands. The BigDye Terminator kit (PE-Applied Biosystems) was used for performing the sequencing reactions, and sequences were analyzed with the ABI Prism 3100 DNA sequencer (PE-Applied Biosystems).
Table 1.
Primers used in this work
| Primer | Primer sequence (5′—3′) | PCR product size (bp) | Use | Reference or source |
|---|---|---|---|---|
| ACrnaF | GGGCTGGCCTCGAAAGAGGAC | 246 | Quantification of ampC mRNA | 15 |
| ACrnaR | GCACCGAGTCGGGGAACTGCA | |||
| MexB-U | CAAGGGCGTCGGTGACTTCCAG | 273 | Quantification of mexB mRNA | 29 |
| MexB-L | ACCTGGGAACCGTCGGGATTGA | |||
| MexD-U | GGAGTTCGGCCAGGTAGTGCTG | 236 | Quantification of mexD mRNA | 29 |
| MexD-L | ACTGCATGTCCTCGGGGAAGAA | |||
| MexF-U | CGCCTGGTCACCGAGGAAGAGT | 254 | Quantification of mexF mRNA | 29 |
| MexF-L | TAGTCCATGGCTTGCGGGAAGC | |||
| MexY-Fa | TGGAAGTGCAGAACCGCCTG | 270 | Quantification of mexY mRNA | 29 |
| MexY-Ra | AGGTCAGCTTGGCCGGGTC | |||
| ponArnaF | GAAGCCGTGACCTGGGACAGC | 231 | Quantification of ponA mRNA | This work |
| ponArnaR | GGAGAAGCCGCCGACCAGCG | |||
| mrcBrnaF | CAACCTGGTGCTCGACGTGCTC | 217 | Quantification of mrcB mRNA | This work |
| mrcBrnaR | CGGATCGAAGCTGGTGAAGATGC | |||
| dacBrnaF | GGCCCGACCTACCAGTGGAAG | 217 | Quantification of dacB mRNA | This work |
| dacBrnaR | AACGGCTTGGTGTCGTCGCCG | |||
| PBP2rnaF | GTGACTCCATCGACCGGCCGC | 227 | Quantification of PBP2 mRNA | This work |
| PBP2rnaR | GTAGATCGCCGACTCCAGGCTC | |||
| PBP3rnaF | CGGCAGCTTGGTGATCATGGAC | 223 | Quantification of PBP3 mRNA | This work |
| PBP3rnaR | CGGGTAGACGTCGACGATATCG | |||
| dacCrnaF | CGCCTTCGCCGACATGATGAAC | 218 | Quantification of dacC mRNA | This work |
| dacCrnaR | AGCAGGTTGCGGTTCGGCTGC | |||
| PA-DEF | GTACGCCTGCTGGACGATG | 910 | ampD amplification and sequencing | 17 |
| PA-DER | GAGGGCAGATCCTCGACCAG | |||
| dacBF | CGACCATTCGGCGATATGAC | 1,400 | dacB amplification and sequencing | 24 |
| dacBR | CGCGTAATCCGAAGATCCATC | |||
| oprD-F | CGCCGACAAGAAGAACTAG | 1,413 | oprD amplification and sequencing | 16 |
| oprD-R | GTCGATTACAGGATCGACAG | |||
| ponAF | CGAAGGCCAGGCAAATGGC | 2,636 | ponA amplification and sequencing | This work |
| ponAR | CTCCCGTCGTCGCCAACG | |||
| ponAF2 | CCTGCAGGACGCGGATCG | ponA sequencing | This work | |
| ponAR2 | CTCAAGCACCTGGGCCAGC | |||
| ponAR3 | CGCTCGAGGATCCAGTTGC | |||
| mrcBF | CATTATGGCGGGAAGGGGTG | 2,551 | mrcB amplification and sequencing | This work |
| mrcBR | GCGACACACCATGGTGGTTC | |||
| mcrB-F2 | GAACCACCATGGTGTGTCGC | mrcB sequencing | This work | |
| mrcB-R2 | CGAGGCCGAGCTTGGCGG | |||
| PBP2F | GAGCAGCGCTGGTCGCTG | 2,135 | PBP2 amplification and sequencing | This work |
| PBP2R | GCAGGCGCTGCAACAGGC | |||
| PBP3F | GGCCGGTTGATTCTCGAGC | 1,921 | PBP3 amplification and sequencing | This work |
| PBP3R | GGTCAGCTCGCGGATCAGC |
Determination of the expression of AmpC and efflux pumps.
The expression of the genes encoding the four major P. aeruginosa efflux pumps, MexAB-OprM (mexB), MexCD-OprJ (mexD), MexEF-OprN (mexF), and MexXY-OprM (mexY), and AmpC (ampC) was determined by real-time reverse transcription-PCR (RT-PCR) for the 6 pairs of susceptible and PBLR isolates and PAO1 (as a control) following previously described protocols (15, 29). For the quantification of ampC induction the strains were incubated in the presence of 50 μg/ml of cefoxitin (15). Briefly, total RNA from logarithmic-phase-grown LB cultures was obtained with an RNeasy minikit (Qiagen, Hilden, Germany). Fifty nanograms of purified RNA was then used for one-step reverse transcription and real-time PCR using a QuantiTect SYBR green reverse transcription-PCR kit (Qiagen) in a SmartCycler II apparatus (Cepheid, Sunnyvale, CA). Previously described conditions and primers were used (15, 29). The rpsL housekeeping gene was used to normalize the expression levels, and results were always referenced against PAO1 basal expression. All RT-PCRs were performed in duplicate, and the mean values of mRNA expression resulting from three independent experiments were considered in all cases. Overexpression was considered when the corresponding mRNA level was at least 3-fold (mexB) or 10-fold (ampC, mexD, mexF, mexY) higher than that for PAO1 (4).
Complementation of AmpC hyperproduction phenotypes.
Plasmids pUCPAD (harboring the wild-type ampD gene) and pUCPADE (harboring the complete wild-type ampDE operon) were electroporated into the different PBLR strains or PAO1 (as a control) following previously described protocols (24). The complementation of AmpC hyperproduction phenotypes was then evaluated in selected transformants through the determination of β-lactam MICs and the quantification of ampC expression as described above.
OMP analysis.
A protocol adapted from those previously published (11, 27) was followed. Briefly, 200 ml of late-log-phase [optical density at 600 nm (OD600 nm) = 1] LB cultures was collected by centrifugation, washed, and suspended in 5 ml of 10 mM Tris-Mg (pH 7.3) buffer. Cells were then sonicated and centrifuged at 7,000 × g for 15 min. Membranes were isolated through ultracentrifugation at 100,000 × g for 1 h at 4°C. Pellets were suspended in 10 ml of 1% sarcosyl in 25 mM Tris-HCl (pH 8) buffer and incubated for 30 min at room temperature. Outer membrane proteins (OMPs) were collected afterward through ultracentrifugation at 70,000 × g for 40 min, suspended in the same buffer, and ultracentrifuged again. OMPs were then suspended in water, separated through SDS-PAGE [11% acrylamide-0.2% bisacrylamide-0.2% SDS-0.375 M (pH 8.8) Tris] and visualized through Coomassie blue staining.
Purification of PBPs.
Membranes containing the PBPs of each P. aeruginosa strain were obtained following described protocols (26, 38). Briefly, 500 ml late-log-phase (OD600 nm = 1) Luria-Bertani (LB) (Sigma-Aldrich, St. Louis, MO) cultures were collected by centrifugation (4,400 × g, 10 min) and then washed and suspended in 50 ml of 20 mM KH2PO4-140 mM NaCl (pH 7.5) (buffer A). Cells were then sonicated using a Digital Sonifier Unit Model S-450D (Branson Ultrasonics Corporation, Danbury, CT) at 20 W for three 30-s bursts (while immersed in an ice bath) and centrifuged at 12,000 × g for 10 min. Membranes containing the PBPs were isolated from the supernatant through two steps of ultracentrifugation at 150,000 × g for 1 h at 4°C using an Optima L-XP Series Preparative ultracentrifuge (Beckman Coulter Inc., Palo Alto, CA) and suspension in buffer A. The total protein content was measured through the Bradford method using the Quick Start Bradford Protein Assay kit with bovine serum albumin as a standard (Bio-Rad Laboratories, Hercules, CA) following the manufacturer's instructions. For analysis of the PBP profiles, membrane fractions were adjusted to 1 mg/ml, and the adjusted preparations (10 μl) were labeled with 25 μM Bocillin FL (38) and subsequently separated through 10% gel SDS-polyacrylamide gel electrophoresis (Bio-Rad Laboratories). Labeled PBPs were visualized (excitation at 488 nm and emission at 530 nm) using a Bio-Rad Molecular Imager FX Pro (Bio-Rad Laboratories). In order to evaluate the reproducibility and consistency, the profiles were determined on three independent occasions for each of the strains.
Determination of IC50s.
Following previously described protocols (26), 20 μl (final volume) of PBP-containing solution was incubated (30 min, 37°C) in the presence of growing concentrations of ceftolozane, ceftazidime, or imipenem (range of concentrations tested, 0.0156 to 2 μg/ml), and PBPs were labeled afterward with a 25 μM concentration of Bocillin FL. The reaction mixtures were then each denatured with 20 μl of SDS-denaturing solution at 100°C for 3 min. PBPs were then separated through 10% SDS polyacrylamide gel electrophoresis. The protein gels were rinsed in water immediately after electrophoresis. Labeled PBPs were visualized using a Bio-Rad Molecular Imager FX Pro (excitation at 488 nm and emission at 530 nm), and 50% inhibitory concentrations (IC50s) of ceftolozane, ceftazidime, and imipenem for the different PBPs were determined from triplicate independent experiments using the Quantity One software (Bio-Rad Laboratories, Hercules, CA) and compared using Student's t test. P values <0.05 were considered statistically significant.
Determination of the sequence and expression of PBP genes.
The genes encoding PBPs (PBP1a, PBP1b, PBP2, PBP3 or PBP4) showing modified patterns in any pan-β-lactam-resistant isolate were further analyzed through complete sequencing. PCR amplification was performed on genomic DNA extracts (DNeasy tissue kit; Qiagen, Hilden, Germany) of the susceptible and PBLR isolate of each pair using the primers described in Table 1. Two independent PCR products for each isolate and gene were sequenced on both strands. The BigDye Terminator kit (PE-Applied Biosystems) was used for performing the sequencing reactions that were analyzed with the ABI Prism 3100 DNA sequencer (PE-Applied Biosystems). The sequences were analyzed and compared within each susceptible-resistant pair and with the reference strain PAO1. The protocol described above was used for the quantification of the expression of PBP genes through real-time RT-PCR using the primers listed in Table 1.
RESULTS AND DISCUSSION
Involvement of OprD, AmpC, and efflux pumps in pan-β-lactam resistance development.
Results of the characterization of classical resistance mechanisms in the six isogenic pairs of sequential P. aeruginosa isolates that had developed resistance to all currently available antipseudomonal β-lactams (including penicillins, cephalosporins, monobactams, and carbapenems) during the treatment of nosocomial infections in ICU patients are shown in Table 2. The time lapse between the isolation of the susceptible and the PBLR isolates ranged from 14 to 52 days (average, 33.2 days). During this period all patients received one or several courses of treatment with antipseudomonal β-lactams, including carbapenems (imipenem), cephalosporins (ceftazidime or cefepime), and penicillin-β-lactamase inhibitor combinations (piperacillin-tazobactam); most of them were additionally treated with aminoglycosides (tobramycin) and/or fluoroquinolones (ciprofloxacin) (Table 2).
Table 2.
Characterization of isogenic susceptible-PBLR P. aeruginosa clinical isolates
| Patient | Isolatea | Treatment before emergence of resistanceb | MIC (μg/ml)b |
Resistance mutation(s)c |
Expression of resistance gened: |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CAZ | FEP | PTZ | ATM | IMP | MER | TOL | ampD | dacB | oprD | ampC basal | ampC inducede | mexB | mexF | mexD | mexY | |||
| 5 | 3-B7 | TOB, CIP, PTZ, IMP | 1 | 8 | 8 | 8 | 2 | 0.12 | 0.25 | WT | WT | WT | <5 | 199 | <2 | <5 | <5 | 8.3 |
| 3-F4 | 128 | 64 | 128 | 256 | 64 | 64 | 4 | D28G | G427D | T276A; W278X | 2,112 | 4,143 | <2 | <5 | 13 | 14 | ||
| 7 | 3-A2 | IMP, CIP, PTZ, TOB | 1 | 2 | 2 | 4 | 2 | 0.12 | 0.12 | WT | WT | WT | <5 | 1,218 | <2 | <5 | <5 | <5 |
| 3-D8 | 128 | 64 | 128 | 128 | 64 | 64 | 4 | WT | G366S; A394P | No OprDf | 1,351 | 2,846 | 26 | <5 | <5 | <5 | ||
| 8 | 1-H9 | PTZ, CAZ, TOB | 8 | 8 | 8 | 32 | 64 | 64 | 0.5 | WT | WT | ΔoprD | <5 | 250 | 4.0 | <5 | <5 | <5 |
| 2-A1 | 128 | 32 | 128 | 128 | 64 | 64 | 4 | Ins. 1 bp (C) in 481 | T428P | ΔoprD | 1,722 | 3,058 | 6.5 | <5 | <5 | <5 | ||
| 14 | 1-A10 | TOB, CIP, IMP,CAZ | 1 | 1 | 4 | 4 | 32 | 2 | 0.5 | WT | WT | W339X | <5 | 200 | <2 | <5 | <5 | <5 |
| 1-C5 | 32 | 16 | 128 | 32 | 64 | 64 | 1 | Q155X | WT | W339X | 317 | 2,267 | 14 | <5 | <5 | 21 | ||
| 16 | 2-G5 | FEP, TOB, IMP, CIP, CAZ | 1 | 4 | 4 | 16 | 1 | 0.12 | 0.5 | WT | WT | WT | <5 | 536 | <2 | <5 | <5 | <5 |
| 2-I4 | 128 | 128 | 128 | 64 | 64 | 64 | 2 | ΔampDE | M200I; del D201 | ΔoprD | 1,438 | 3,388 | <2 | <5 | <5 | 12 | ||
| 17 | 3-D9 | IMP, PTZ | 2 | 2 | 8 | 8 | 2 | 0.25 | 0.5 | WT | WT | WT | <5 | 443 | <2 | <5 | <5 | <5 |
| 3-F5 | 16 | 64 | 64 | 32 | 64 | 16 | 0.5 | V10G | WT | NoOprDf | 67 | 1,068 | <2 | <5 | <5 | <5 | ||
Resistance mechanisms for strains 2-A1, 1-C5, and 2-I4 had been previously partially characterized (17, 25).
TOB, tobramycin; CIP, ciprofloxacin; PTZ, piperacillin-tazobactam; IMP, imipenem; CAZ, ceftazidime; FEP, cefepime; ATM, aztreonam; MER, meropenem; TOL, ceftolozane.
WT, wild-type sequence.
Relative mRNA expression compared to wild-type PAO1. According to previous works (4), breakpoints used to define overexpression were ≥10-fold for ampC, mexF, mexD, and mexY (5 to 10 borderline) and ≥3-fold for mexB (2 to 3 borderline).
Induction experiments were carried out with 50 mg/ml cefoxitin.
Although these strains showed a wild-type oprD sequence, OprD was not expressed when outer membrane proteins (OMPs) were analyzed through SDS-PAGE.
All six PBLR isolates were OprD deficient, due to either a partial deletion of oprD, point mutations leading to a premature stop codon, or the lack of OprD expression in the absence of oprD mutations, each mechanism found in two of the strains. All the PBLR isolates additionally overexpressed ampC due to mutations in ampD (2 strains), dacB (1 strain), or both genes (3 strains). In order to denote the contribution of ampC overexpression to the PBLR profiles, and to confirm the underlying mechanisms, complementation studies were carried out using plasmids with cloned wild-type ampD (pUCPAD; complements ampD mutants) and the complete ampDE operon [pUCPADE; shown in a previous work to complement both ampD and dacB mutants (24)]. The MICs of the different β-lactams and ampC expression levels for the PBLR strains harboring these plasmids are shown in Table 3. Although a marked increase in β-lactam susceptibility was documented, particularly for pUCPADE complementations, the MICs for several β-lactams were still higher than those of the susceptible parent strain in most of the cases, suggesting the involvement of additional resistance mechanisms. Moreover, 2 of the strains overexpressed mexB, 2 mexY, and 1 both efflux pumps. These results were thus consistent with those of previous studies showing that P. aeruginosa pan-β-lactam resistance frequently results from combinations of mutations leading to OprD inactivation, AmpC hyperproduction, and efflux pump overexpression (4, 19). Regarding the activity of ceftolozane, although a certain increase in MICs was noted, ranging from 0 to 5 2-fold dilutions, they remained ≤4 μg/ml and thus within the susceptible category according to breakpoints suggested by pharmacokinetic/pharmacodymic (PK/PD) analysis (12). The greatest increases in ceftolozane MICs were documented for two strains showing extremely high ampC expression levels (>2,000-fold compared to that of PAO1) along with mexB and/or mexY overexpression (Table 2), while the lowest effect (no modification of the MICs) was documented for a strain showing only moderate (100-fold higher than that of PAO1) ampC overexpression. Thus, these results are consistent with previous data suggesting that ceftolozane is much less affected than currently available β-lactams by classical mutation-driven resistance mechanisms in P. aeruginosa (16, 25, 34).
Table 3.
Results of AmpC hyperproduction complementation studies in PBLR strainsa
| Strainb | Resistance mutation(s) |
Plasmid | MIC (μg/ml) |
ampC expressionc | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ampD | dacB | CAZ | FEP | PTZ | ATM | IMP | MER | TOL | |||
| PAO1 | WT | WT | 2 | 1 | 4 | 4 | 2 | 0.5 | 0.5 | 1 | |
| pUCPAD | 2 | 1 | 4 | 4 | 2 | 0.5 | 0.5 | ND | |||
| pUCPADE | 1 | 1 | 4 | 4 | 2 | 0.5 | 0.5 | ND | |||
| PAΔampD | ΔampD | WT | 8 | 4 | 32 | 8 | 2 | 2 | 0.5 | 48 | |
| pUCPAD | 1 | 1 | 2 | 2 | 2 | 0.5 | 0.5 | 1.5 | |||
| pUCPADE | 1 | 1 | 1 | 2 | 2 | 0.5 | 0.5 | 1.3 | |||
| PAΔdacB | WT | ΔdacB | 32 | 8 | 64 | 16 | 2 | 0.5 | 1 | 34 | |
| pUCPAD | 8 | 4 | 4 | 2 | 2 | 0.5 | 1 | 27 | |||
| pUCPADE | 1 | 1 | 2 | 2 | 1 | 0.5 | 0.5 | 2.5 | |||
| PAΔampD dacB | ΔampD | ΔdacB | 64 | 8 | 256 | 64 | 2 | 2 | 2 | 1,859 | |
| pUCPAD | 16 | 8 | 64 | 16 | 2 | 1 | 1 | 460 | |||
| pUCPADE | 4 | 1 | 4 | 8 | 1 | 0.5 | 1 | 5.2 | |||
| 3F4 | D28G | G427D | 256 | 32 | 256 | 512 | 32 | 32 | 4 | 2,112 | |
| pUCPAD | 128 | 32 | 128 | 64 | 32 | 32 | 2 | 64 | |||
| pUCPADE | 16 | 8 | 32 | 16 | 16 | 4 | 1 | 3.8 | |||
| 3D8 | WT | G366S; A394P | 128 | 32 | 256 | 256 | 32 | 32 | 4 | 1,351 | |
| pUCPAD | 16 | 16 | 32 | 32 | 32 | 16 | 2 | 504 | |||
| pUCPADE | 4 | 4 | 8 | 32 | 2 | 16 | 1 | 52 | |||
| 2A1 | Ins. 1 bp (C) in 481 | T428P | 256 | 64 | 256 | 128 | 32 | 32 | 4 | 1,722 | |
| pUCPAD | 32 | 8 | 64 | 32 | 32 | 16 | 2 | 419 | |||
| pUCPADE | 4 | 4 | 8 | 16 | 16 | 16 | 1 | 56 | |||
| 1C5 | Q155X | WT | 32 | 8 | 64 | 64 | 32 | 32 | 1 | 317 | |
| pUCPAD | 4 | 8 | 32 | 32 | 32 | 32 | 1 | 11 | |||
| pUCPADE | 4 | 8 | 16 | 16 | 16 | 32 | 1 | 4.5 | |||
| 2I4 | ΔampDE | M200I; del D201 | 128 | 32 | 256 | 128 | 32 | 16 | 4 | 1,438 | |
| pUCPAD | 16 | 8 | 32 | 16 | 16 | 8 | 1 | 466 | |||
| pUCPADE | 2 | 4 | 8 | 4 | 8 | 8 | 1 | 19 | |||
| 3F5 | V10G | WT | 16 | 16 | 64 | 16 | 32 | 16 | 1 | 67 | |
| pUCPAD | 16 | 16 | 64 | 16 | 32 | 8 | 1 | 14 | |||
| pUCPADE | 4 | 4 | 8 | 8 | 32 | 4 | 1 | 2.1 | |||
PBP expression profiles of susceptible-PBLR pairs.
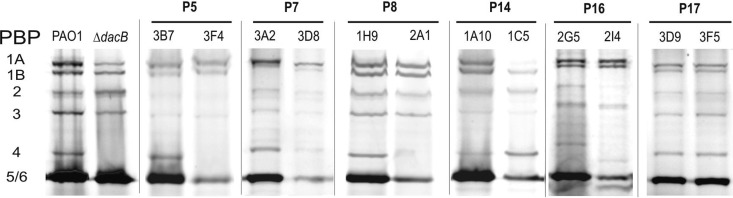
As shown in Fig. 1, five of the six susceptible-PBLR pairs showed modified PBP profiles. For four of the pairs (P5, P7, P8, and P16) the PBLR isolate lacked PBP4 expression. These four isolates were those showing acquired mutations in dacB (Table 2), leading to AmpC overexpression. Likewise, a lack of PBP4 expression was also observed for the control dacB knockout mutant of PAO1 (Fig. 1). Thus, this work expands previous findings (23), since it first correlates mutation of dacB with the lack of PBP4 expression. Interestingly, while all of the dacB mutations lead to the lack of expression of a functional PBP4, modified transcription was not observed in any of the cases (not shown). The PBLR isolate from one of these pairs (P7) additionally showed differences in migration of PBP1a and PBP1b, and the PBLR isolate of pair 14 did not express a functional PBP1a and apparently overexpressed PBP4 (Fig. 1). Interestingly, despite these evident modifications of PBP profiles, gene sequencing and transcription analysis revealed no differences between the susceptible and the PBLR isolates (not shown). When compared with PAO1, only silent nucleotide polymorphisms were detected, suggesting the occurrence of posttranscriptional events leading to modified periplasmic PBP expression patterns. In any case, the modification of PBP1a expression profiles could eventually have a relevant impact in β-lactam resistance. Of particular interest, a very recent study, using Stenotrophomonas malthopilia as a model organism, showed that the inactivation of PBP1a leads to the overexpression of L1 and L2 β-lactamases (18). Whether a similar effect occurs also in P. aeruginosa, and how it might be related to PBP4 activity, is under investigation in our laboratory.
Fig 1.
PBP profiles of the six clonally related pairs of sequential isolates. The first two lanes show the profile of PAO1 compared to its isogenic dacB mutant. For each pair, the lane on the left represents the profile of the susceptible isolate, while the right lane represents the PBLR isolate. The membrane fractions were adjusted to a 1 mg/ml total protein concentration and labeled with Bocillin FL (25 μM). Ten microliters of the labeled membrane preparations was separated by SDS-PAGE.
Additionally, as shown in Fig. 1, PBLR strains apparently expressed smaller amounts of PBP5/6 than their susceptible parent strains. Thus, although this PBP is apparently not involved in resistance (1), the expression of dacC, encoding PBP5/6, was monitored in all the strains. In all cases, the expression was found similar to that of PAO1 (mean, 0.94; range, 0.47 to 1.23, compared to PAO1). Moreover, no significant differences were observed between PBLR (mean, 0.89; range, 0.47 to 1.11) and susceptible (mean, 0.98; range, 0.72 to 1.23) strains.
Since previous studies have suggested a decreased expression of genes encoding PBP2 or PBP3 in some carbapenem-resistant P. aeruginosa isolates (9, 13), we also quantified the expression of these genes in 3 of the susceptible-PBLR pairs, and the results are shown in Table 4. While a slightly lower expression of the PBP3 gene was noted for the 3 strains compared to that of PAO1, we found no significant differences between the susceptible and PBLR isolates in each of the pairs. Similarly, a slightly higher expression of the PBP2 gene was noted in one of the strains, but again without significant differences between the susceptible and the PBLR isolates (Table 4). Moreover, sequencing of the genes encoding PBP2 and PBP3 revealed no differences between the susceptible and the PBLR pairs; besides the presence of silent nucleotide polymorphisms, all sequences were identical to that of wild-type PAO1, except for an L3V substitution in PBP3 found in both the susceptible and the PBLR isolates from one of the patients (Table 4). Thus, our results, first comparing clonally related susceptible-PBLR pairs, suggest that while a certain interstrain variability on the expression of PBP2/PBP3 genes exists, it seems not to be strongly linked to β-lactam resistance development.
Table 4.
Relative expression and sequence of PBP2 and PBP3 genes from 3 pairs of clonally related susceptible-PBLR sequential P. aeruginosa isolatesa
| Strain | PBP2 gene expressionb |
PBP2 sequencec | PBP3 gene expression |
PBP3 sequence | ||
|---|---|---|---|---|---|---|
| Susceptible | PBLR | Susceptible | PBLR | |||
| PAO1 | 1 | N/A | WT | 1 | N/A | WT |
| P8 | 2.69 ± 1.89 | 4.63 ± 1.43 | WT | 0.57 ± 0.04 | 0.60 ± 0.33 | WT |
| P14 | 0.99 ± 0.27 | 1.01 ± 0.73 | WT | 0.67 ± 0.31 | 0.49 ± 0.23 | L3V |
| P16 | 1.01 ± 0.73 | 1.95 ± 0.11 | WT | 0.33 ± 0.07 | 0.75 ± 0.27 | WT |
N/A, not applicable; WT, wild type.
Relative mRNA expression compared to that of wild-type PAO1.
Sequence compared to that of wild-type PAO1. In all cases, the susceptible and the PBLR isolates from each pair yielded an identical sequence.
PBP binding affinities of ceftazidime, ceftolozane, and imipenem in susceptible-PBLR pairs.
Table 5 shows the PBP IC50s of ceftazidime, ceftolozane, and imipenem for the above-described 3 susceptible-PBLR pairs. Interestingly, despite the absence of significant differences in gene expression or sequence, a clear tendency toward increased IC50s was noted when comparing the PBLR with the susceptible isolates for imipenem binding to PBP2 (0.30 ± 0.11 μg/ml vs 0.13 ± 0.02 μg/ml, P = 0.03) and for ceftazidime (0.19 ± 0.02 vs 0.12 ± 0.02, P = 0.004), ceftolozane (0.18 ± 0.13 vs 0.07 ± 0.02, P = 0.12), and imipenem (0.69 ± 0.12 vs 0.34 ± 0.06, P = 0.008) binding to PBP3. Additionally, strain-specific variations in PBP1a/b binding affinities of ceftazidime, ceftolozane, or imipenem were noted. In particular, the pair P14 (1A10-1C5) showed a significant increase of the binding affinity of PBP1b for the three antibiotics, likely due to the lack of PBP1a in the PBLR isolate (1C5). All together, these data suggest that the relative binding affinity of a given PBP for a given antipseudomonal agent is influenced by the relative abundance of each of the PBPs, which might be modulated by posttranscriptional events in PBLR isolates.
Table 5.
PBP IC50s of ceftazidime, ceftolozane, and imipenem for 3 pairs of susceptible-PBLR sequential P. aeruginosa isolates
| PBP | Isolate | IC50 for indicated isolate |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ceftazidime |
Ceftolozane |
Imipenem |
|||||||||||
| P8 | P14 | P16 | PAO1 | P8 | P14 | P16 | PAO1 | P8 | P14 | P16 | PAO1 | ||
| 1a | Susceptible | 0.08 ± 0.01 | 0.35 ± 0.15 | 0.35 ± 0.12 | 0.19 ± 0.10 | 0.37 ± 0.15 | 0.21 ± 0.16 | 0.12 ± 0.06 | 0.12 ± 0.04 | 0.66 ± 0.04 | 0.41 ± 0.26 | 0.08 ± 0.03 | 0.21 ± 0.06 |
| PBLR | 0.11 ± 0.02a | 0.29 ± 0.16 | N/A | 0.53 ± 0.34a | 0.21 ± 0.06 | N/A | 0.75 ± 0.15a | 0.19 ± 0.12a | N/A | ||||
| 1b | Susceptible | 1.48 ± 0.73 | 0.48 ± 0.17 | >2 | >2 | 1.19 ± 0.21 | 1.59 ± 0.34 | 0.15 ± 0.21 | 0.89 ± 0.24 | 0.15 ± 0.05 | 1.02 ± 0.05 | 0.14 ± 0.05 | 0.12 ± 0.05 |
| PBLR | >2 | 0.12 ± 0.01a | >2 | N/A | >2 | 0.09 ± 0.02a | 0.19 ± 0.12a | N/A | 0.24 ± 0.07 | 0.39 ± 0.15a | 0.11 ± 0.02 | N/A | |
| 2 | Susceptible | >2 | >2 | >2 | >2 | >2 | 1.89 ± 0.42 | >2 | 1.59 ± 0.42 | 0.10 ± 0.04 | 0.14 ± 0.03 | 0.15 ± 0.07 | 0.10 ± 0.04 |
| PBLR | >2 | >2 | >2 | N/A | >2 | 1.52 ± 0.37 | >2 | N/A | 0.21 ± 0.04a | 0.42 ± 0.13a | 0.28 ± 0.17 | N/A | |
| 3 | Susceptible | 0.13 ± 0.06 | 0.13 ± 0.04 | 0.09 ± 0.02 | 0.09 ± 0.02 | 0.09 ± 0.02 | 0.07 ± 0.01 | 0.06 ± 0.01 | 0.04 ± 0.01 | 0.41 ± 0.04 | 0.31 ± 0.14 | 0.41 ± 0.14 | 0.16 ± 0.04 |
| PBLR | 0.18 ± 0.04 | 0.19 ± 0.03 | 0.21 ± 0.20a | N/A | 0.33 ± 0.22a | 0.12 ± 0.01a | 0.09 ± 0.04 | N/A | 0.55 ± 0.14a | 0.77 ± 0.19a | 0.74 ± 0.26a | N/A | |
| 4 | Susceptible | 0.89 ± 0.09 | >2 | 1.88 ± 0.13 | 1.78 ± 0.53 | >2 | 1.72 ± 0.58 | 0.89 ± 0.18 | 0.21 ± 0.08 | 0.38 ± 0.21 | 0.06 ± 0.02 | 0.01 ± 0.005 | 0.03 ± 0.01 |
| PBLR | >2 | N/A | >2a | N/A | 0.03 ± 0.02 | N/A | |||||||
| 5/6 | Susceptible | >2 | >2 | >2 | >2 | >2 | >2 | >2 | >2 | 0.51 ± 0.34 | 0.70 ± 0.18 | 0.26 ± 0.08 | 0.40 ± 0.10 |
| PBLR | >2 | >2 | >2 | N/A | >2 | >2 | >2 | N/A | >2a | >2a | >2a | N/A | |
Statistically significant differences (Student's t test, P < 0.05) for the comparison of PBP IC50s for the susceptible and the PBLR sequential isolates.
Concluding remarks.
In this work we show that in addition to AmpC hyperproduction, inactivation of OprD, and overexpression of efflux pumps, modification of PBP patterns appears to play a role in the in vivo emergence of PBLR strains, which still conserve certain susceptibility to the new antipseudomonal cephalosporin ceftolozane. In particular, the correlation of dacB mutations (leading to AmpC overexpression) with a lack of PBP4 expression is demonstrated for the first time in this work. Additionally, posttranscriptional modifications of PBP1a/b expression patterns are observed in some PBLR isolates. Thus, these results suggest that altered patterns of PBP1a/b may also be involved in β-lactam resistance; therefore, future studies are needed to address this issue. Finally, this work investigated for the first time the binding affinities of antipseudomonal β-lactams to PBP extracts from clonally related susceptible and PBLR isolates, showing a clear tendency toward increased IC50s for PBP2 (imipenem) and/or PBP3 (ceftazidime, ceftolozane, and imipenem). While the underlying genetic or physiological drivers of these findings still need to be elucidated, as well as their specific impact in the resistance profiles, these results highlight the interest of testing β-lactam-resistant strains in the evaluation of the potency of new β-lactam molecules through the determination of their PBP binding affinities.
ACKNOWLEDGMENTS
This study was supported by a grant from Cubist Pharmaceuticals and by the Ministerio de Ciencia e Innovación of Spain, Instituto de Salud Carlos III, through the Spanish Network for the Research in Infectious Diseases (REIPI RD06/0008 and PS0900033), and by a grant from the Research Committee of Hospital Son Espases.
Footnotes
Published ahead of print 25 June 2012
REFERENCES
- 1. Alvarez-Ortega C, Wiegand I, Olivares J, Hancock RE, Martínez JL. 2010. Genetic determinants involved in the susceptibility of Pseudomonas aeruginosa to beta-lactam antibiotics. Antimicrob. Agents Chemother. 54:4159–4167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bratu S, Landman D, Gupta J, Quale J. 2007. Role of AmpD, OprF and penicillin-binding proteins in beta-lactam resistance in clinical isolates of Pseudomonas aeruginosa. J. Med. Microbiol. 56:809–814 [DOI] [PubMed] [Google Scholar]
- 3. Bulik CC, Christensen H, Nicolau DP. 2010. In vitro potency of CXA-101, a novel cephalosporin, against Pseudomonas aeruginosa displaying various resistance phenotypes, including multidrug resistance. Antimicrob. Agents Chemother. 54:557–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cabot G, et al. 2011. Overexpression of AmpC and efflux pumps in Pseudomonas aeruginosa isolates from bloodstream infections: prevalence and impact on resistance in a Spanish multicenter study. Antimicrob. Agents Chemother. 55:1906–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cavallo JD, Hocquet D, Plesiat P, Fabre R, Roussel-Delvallez M. 2007. Susceptibility of Pseudomonas aeruginosa to antimicrobials: a 2004 French multicentre hospital study. J. Antimicrob. Chemother. 59:1021–1024 [DOI] [PubMed] [Google Scholar]
- 6. Cayô R, et al. 2011. Analysis of genes encoding penicillin-binding proteins in clinical isolates of Acinetobacter baumannii. Antimicrob. Agents Chemother. 55:5907–5913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clinical and Laboratory Standards Institute 2008. Performance standards for antimicrobial susceptibility testing, vol 28, no. 3, 18th informational supplement. CLSI document M100–S18. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 8. El Amin N, et al. 2005. Carbapenem resistance mechanisms in Pseudomonas aeruginosa: alterations of porin OprD and efflux proteins do not fully explain resistance patterns observed in clinical isolates. APMIS 113:187–196 [DOI] [PubMed] [Google Scholar]
- 9. Farra A, Islam S, Strålfors A, Sörberg M, Wretlind B. 2008. Role of outer membrane protein OprD and penicillin-binding proteins in resistance of Pseudomonas aeruginosa to imipenem and meropenem. Int. J. Antimicrob. Agents. 31:427–433 [DOI] [PubMed] [Google Scholar]
- 10. Fernández-Cuenca F, et al. 2003. Relationship between beta-lactamase production, outer membrane protein and penicillin-binding protein profiles on the activity of carbapenems against clinical isolates of Acinetobacter baumannii. J. Antimicrob. Chemother. 51:565–574 [DOI] [PubMed] [Google Scholar]
- 11. Filip C, Fletcher G, Wulff JL, Earhart CF. 1973. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J. Bacteriol. 115:717–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ge Y, Whitehouse MJ, Friedland I, Talbot GH. 2010. Pharmacokinetics and safety of CXA-101, a new antipseudomonal cephalosporin, in healthy adult male and female subjects receiving single- and multiple-dose intravenous infusions. Antimicrob. Agents Chemother. 54:3427–3431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Giske CG, Buarø L, Sundsfjord A, Wretlind B. 2008. Alterations of porin, pumps, and penicillin-binding proteins in carbapenem resistant clinical isolates of Pseudomonas aeruginosa. Microb. Drug Resist. 14:23–30 [DOI] [PubMed] [Google Scholar]
- 14. Gutierrez O, et al. 2007. Molecular epidemiology and mechanisms of carbapenem resistance in Pseudomonas aeruginosa isolates from Spanish hospitals. Antimicrob. Agents Chemother. 51:4329–4335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Juan C, Moyá B, Pérez JL, Oliver A. 2006. Stepwise upregulation of the Pseudomonas aeruginosa chromosomal cephalosporinase conferring high-level beta-lactam resistance involves three AmpD homologues. Antimicrob. Agents Chemother. 50:1780–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Juan C, et al. 2010. Activity of a new antipseudomonal cephalosporin, CXA-101 (FR264205), against carbapenem-resistant and multidrug-resistant Pseudomonas aeruginosa clinical strains. Antimicrob. Agents Chemother. 54:846–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Juan C, et al. 2005. Molecular mechanisms of β-lactam resistance mediated by AmpC hyperproduction in Pseudomonas aeruginosa clinical strains. Antimicrob. Agents Chemother. 49:4733–4738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin CW, Lin HC, Huang YW, Chung TC, Yang TC. 2011. Inactivation of mrcA gene derepresses the basal-level expression of L1 and L2 β-lactamases in Stenotrophomonas maltophilia. J. Antimicrob. Chemother. 66:2033–2037 [DOI] [PubMed] [Google Scholar]
- 19. Lister PD, Wolter DJ, Hanson ND. 2009. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin. Microbiol. Rev. 22:582–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Livermore DM. 2002. Multiple mechanisms of antimicrobial resistance in Pseudomonas aeruginosa: our worst nightmare? Clin. Infect. Dis. 34:634–640 [DOI] [PubMed] [Google Scholar]
- 21. Livermore DM, Mushtaq S, Ge Y, Warner M. 2009. Activity of cephalosporin CXA-101 (FR264205) against Pseudomonas aeruginosa and Burkholderia cepacia group strains and isolates. Int. J. Antimicrob. Agents 34:402–406 [DOI] [PubMed] [Google Scholar]
- 22. Masuda N, et al. 2000. Substrate specificities of MexAB-OprM, MexCD-OprJ, and MexXY-OprM efflux pumps in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 44:3322–3327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mesaros N, et al. 2007. Pseudomonas aeruginosa: resistance and therapeutics options in the turn of the new millennium. Clin. Microbiol. Infect. 13:560–578 [DOI] [PubMed] [Google Scholar]
- 24. Moya B, et al. 2009. β-Lactam resistance response triggered by inactivation of a nonessential penicillin-binding protein. PLoS Pathog. 5:e1000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moya B, et al. 2010. Activity of a new cephalosporin, CXA-101 (FR264205), against beta-lactam-resistant Pseudomonas aeruginosa mutants selected in vitro and after antipseudomonal treatment of intensive care unit patients. Antimicrob. Agents Chemother. 54:1213–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moyá B, Zamorano L, Juan C, Ge Y, Oliver A. 2010. Affinity of the new cephalosporin CXA-101 to penicillin-binding proteins of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 54:3933–3937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mulet X, et al. 2011. Antagonistic interactions of Pseudomonas aeruginosa antibiotic resistance mechanisms in planktonic but not biofilm growth. Antimicrob. Agents Chemother. 55:4560–4568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Neuwirth C, Siebor E, Duez JM, Pechinot A, Kazmierczak A. 1995. Imipenem resistance in clinical isolates of Proteus mirabilis associated with alterations in penicillin binding proteins. J. Antimicrob. Chemother. 36:335–342 [DOI] [PubMed] [Google Scholar]
- 29. Oh H, Stenhoff S, Jalal S, Wretlind B. 2003. Role of efflux pumps and mutations in genes for topoisomerases II and IV in fluoroquinolone-resistant Pseudomonas aeruginosa strains. Microb. Drug Resist. 9:323–328 [DOI] [PubMed] [Google Scholar]
- 30. Pirnay JP, et al. 2002. Analysis of the Pseudomonas aeruginosa oprD gene from clinical and environmental isolates. Environ. Microbiol. 4:872–882 [DOI] [PubMed] [Google Scholar]
- 31. Poole K. 2004. Efflux-mediated multiresistance in Gram-negative bacteria. Clin. Microbiol. Infect. 10:12–26 [DOI] [PubMed] [Google Scholar]
- 32. Poole K. 2011. Pseudomonas aeruginosa: resistance to the max. Front. Microbiol. 2:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Quale J, Bratu S, Gupta J, Landman D. 2006. Interplay of efflux system, ampC, and oprD expression in carbapenem resistance of Pseudomonas aeruginosa clinical isolates. Antimicrob. Agents Chemother. 50:1633–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Riera E, et al. 2010. Anti-biofilm and resistance suppression activities of CXA-101 against chronic respiratory infection phenotypes of Pseudomonas aeruginosa strain PAO1. J. Antimicrob. Chemother. 65:1399–1404 [DOI] [PubMed] [Google Scholar]
- 35. Sader HS, Rhomberg PR, Farrell DJ, Jones RN. 2011. Antimicrobial activity of CXA-101, a novel cephalosporin tested in combination with tazobactam against Enterobacteriaceae, Pseudomonas aeruginosa, and Bacteroides fragilis strains having various resistance phenotypes. Antimicrob. Agents Chemother. 55:2390–2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sumita Y, Fukasawa M. 1995. Potent activity of meropenem against Escherichia coli arising from its simultaneous binding to penicillin-binding proteins 2 and 3. J. Antimicrob. Chemother. 36:53–64 [DOI] [PubMed] [Google Scholar]
- 37. Zapun A, Contreras-Martel C, Vernet T. 2008. Penicillin-binding proteins and β-lactam resistance. FEMS Microbiol. Rev. 32:361–385 [DOI] [PubMed] [Google Scholar]
- 38. Zhao G, Meier T, Kahi SD, Gee KR, Blaszczak LC. 1999. Bocillin FL, a sensitive and commercially available reagent for the detection of penicillin-binding proteins. Antimicrob. Agents Chemother. 43:1124–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]