Abstract
Voriconazole is a first-line agent in the treatment of many invasive fungal infections and is known to display highly variable pharmacokinetics. Previous studies of voriconazole therapeutic drug monitoring (TDM) have suggested concentration monitoring to be clinically useful but have been limited by small patient samples at a single institution. This multicenter retrospective study aimed to investigate relationships between voriconazole concentration and clinical outcomes and adverse events and to assess clinical factors and drug interactions that may affect voriconazole concentration. Medical records were reviewed for patients who received voriconazole and had at least 1 concentration measured at seven hospitals in Australia. The study included 201 patients with 783 voriconazole trough concentrations. Voriconazole concentrations of <1.7 mg/liter were associated with a significantly greater incidence of treatment failure (19/74 patients [26%]) than concentrations of ≥1.7 mg/liter (6/89 patients [7%]) (P < 0.01). Neurotoxic adverse events (visual and auditory hallucinations) occurred more frequently at voriconazole concentrations of >5 mg/liter (10/31 patients [32%]) than at concentrations of ≤5 mg/liter (2/170 patients [1.2%]) (P < 0.01). Multiple regression analysis of voriconazole concentration identified associations between increasing patient weight, oral administration of voriconazole, and coadministration of phenytoin or rifampin and significantly reduced concentrations, and associations between increasing patient age and coadministration of proton pump inhibitors and increased concentrations. Coadministration of glucocorticoids was found to significantly reduce voriconazole concentrations, inferring a previously unreported drug interaction between glucocorticoids and voriconazole.
INTRODUCTION
The triazole antifungal voriconazole is widely used in the treatment of invasive fungal infections (IFIs) due to its broad coverage of pathogenic yeasts and molds and evidence of superiority over amphotericin B in the primary treatment of invasive aspergillosis (12). Voriconazole is known to exhibit highly variable nonlinear pharmacokinetics and is metabolized primarily via CYP2C19 and, to a lesser extent, CYP3A4 and CYP2C9 (27). In agreement with studies of other azole antifungals, in vitro studies have found that the unbound drug area under the concentration-time curve divided by the MIC (fAUC/MIC) ratio is the pharmacokinetic/pharmacodynamic measure that is most predictive of voriconazole efficacy in murine models of candidiasis (1) and may be a useful metric in aspergillosis (17).
Therapeutic drug monitoring (TDM) is used to guide therapy for a number of clinically important medicines, both in improving response to therapy by individualizing dose regimens and in preventing drug-related adverse events. A number of studies have demonstrated a relationship between voriconazole plasma concentrations and clinical efficacy and toxicity (2, 21, 31), with a therapeutic concentration range between 1.0 and 5.5 mg/liter advocated to improve treatment outcome and minimize the risk of neurotoxic adverse events (22).
Despite this, previous studies of voriconazole TDM have generally been limited by small sample size and have typically only investigated patients from a single institution (14, 19, 22, 25, 28, 29, 32). Furthermore, while the CYP2C19 genotype has been identified as an important determinant of voriconazole pharmacokinetics in healthy volunteers (33), few studies have assessed the potential impacts of clinical factors and drug interactions on voriconazole concentration in patients receiving treatment with voriconazole. This study aimed to investigate relationships between voriconazole concentrations, clinical outcomes, and adverse events using a multicenter retrospective design. Furthermore, clinical factors and drug interactions that may affect voriconazole concentration were also investigated.
MATERIALS AND METHODS
Patient enrollment and data collection.
Patients aged 18 years or older who received voriconazole and had at least one voriconazole concentration measured during therapy at seven hospitals in Australia between December 2008 and May 2010 were eligible for inclusion. All voriconazole concentration data were collected from a central referral laboratory (SydPath, St. Vincent's Hospital, Sydney). A validated high-performance liquid chromatography (HPLC) assay was used to measure voriconazole concentrations (6). Patient medical records were individually reviewed using a standardized data collection template at each study site to collect demographic information and clinical data on outcomes of therapy and adverse events, as well as voriconazole dosing information and concomitant medications taken during voriconazole therapy. The study received multisite ethics approval from the Sydney Local Heath District Human Research Ethics Committee, Concord Repatriation General Hospital.
IFI classification and treatment outcome.
The 2008 guidelines from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group were used to classify IFI as proven, probable, or possible (8). Treatment success was assessed based on partial or complete improvement in clinical (symptoms of infection, fever) and radiological signs (computed tomography, high-resolution computed tomography, or magnetic resonance imaging findings) of infection. Treatment failure was defined as persistent or progressing IFI based on clinical and radiological signs or continuing positive cultures or death due to IFI after at least 7 days of therapy with voriconazole.
Statistical analysis.
Voriconazole dosing records for each patient were used to verify the time of voriconazole concentration sampling in relation to dose. As trough concentrations are recommended for voriconazole TDM (2) and to avoid the confounding effect of differing sampling times postdose, nontrough voriconazole concentrations (sampled >2 h before the next dose) were excluded from the analysis. In patients who received an intravenous or oral voriconazole loading dose, trough concentration measurements taken on day 2 of dosing or later were included in the analysis. In patients who did not receive a loading dose, trough concentration measurements taken on day 7 of dosing or later were included in the analysis.
The median voriconazole concentration was used to assess relationships between concentration and treatment outcome. The relationship between voriconazole concentration and treatment outcome was assessed both in the overall treatment population, including patients receiving voriconazole for the treatment of a proven, probable, or possible IFI or a localized fungal infection or for empirical antifungal therapy, and in a subset of patients receiving voriconazole for the treatment of a proven or probable IFI.
Receiver operating characteristic (ROC) curves were used to explore the relationship between voriconazole concentration and treatment outcome or reported adverse events. A multiple linear regression analysis was used to identify factors that contribute to the variability in voriconazole concentration. Elevations in results of liver function tests (LFTs; alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, gamma-glutamyl transpeptidase, and bilirubin) were assessed and graded according to Common Terminology Criteria for Adverse Events (CTCAE), version 4.03 (20). Univariate analyses were performed using the Mann-Whitney U test or Wilcoxon signed rank test as appropriate due to the nonnormality of voriconazole concentrations. Proportions were compared using the chi-squared or Fisher's exact test as appropriate. Statistical significance was defined by a P value of <0.05. Statistical analyses were performed with PASW Statistics 18 (SPSS, Inc., Chicago, IL).
RESULTS
Patient characteristics.
A total of 201 patients were included in the study. The majority of patients received voriconazole for the treatment of a known or presumed fungal infection (170/201 [85%]) versus prophylaxis against fungal infections (31/201 [15%]). Hematological malignancy was the most common underlying condition in patients receiving voriconazole (118/201 [59%]); among these patients, acute myeloid leukemia was the most common condition (n = 47). Demographic information, indications for therapy, underlying conditions, and sites of infection are included in Table 1.
Table 1.
Patient demographic and clinical characteristics and indications for voriconazole therapy
| Variable | No. of patients (n = 201) |
|---|---|
| Demographics | |
| Age (yr)a | 54 (18–88) |
| Sex, male/female | 116/85 |
| Weight (kg)a,b | 68 (38–113) |
| Indication for therapyc | |
| Proven IFI | 44 |
| Probable IFI | 23 |
| Possible IFI | 59 |
| Localized fungal infectiond | 17 |
| Empirical therapy | 27 |
| Antifungal prophylaxis | 31 |
| Underlying condition | |
| Hematological malignancy | 118 |
| Solid organ transplantation | 26 |
| Diabetes mellitus | 8 |
| Myelodysplastic syndrome | 7 |
| Other malignancy | 5 |
| Vasculitic condition | 5 |
| Other conditione | 18 |
| None | 14 |
| Site of infectionf | |
| Lungg | 104 |
| Brainh | 8 |
| Eyesi | 6 |
| Skin and soft tissue | 6 |
| Disseminated | 5 |
| Intra-abdominal | 4 |
| Invasive sinusitisj | 4 |
| Ear | 4 |
| Otherk | 9 |
| Unknown | 20 |
Median (range).
Weight was available for 187/201 patients.
Defined according to the 2008 EORTC/MSG guidelines (8).
Fungal cellulitis (6 patients), localized eye infection (5 patients), ear infection (4 patients), esophagitis (2 patients).
Chronic lung disease (3 patients), aplastic anemia (2 patients), IgG deficiency (1 patient), severe combined immunodeficiency (1 patient), HIV (1 patient), rheumatoid arthritis (1 patient), systemic lupus erythematosus (1 patient), sarcoidosis (1 patient), scleroderma, Caroli syndrome (1 patient), myasthenia gravis (1 patient), ulcerative colitis (1 patient), long-term corticosteroid use (1 patient), intravenous drug use (1 patient), recent brain surgery (1 patient).
Does not include patients taking voriconazole for antifungal prophylaxis.
Skin and soft tissue involvement (4 patients).
Disseminated infection in 4 patients.
Lung involvement (1 patient).
Lung involvement (1 patient).
Endocarditis (2 patients), liver (2 patients), bloodstream (2 patients), esophagitis (2 patients), osteomyelitis (1 patient).
Among patients receiving voriconazole for a proven or probable IFI (n = 67), Aspergillus was the most common fungal pathogen (38/67 [57%]), with A. fumigatus the most commonly identified species. Thirteen patients received voriconazole for treatment of Scedosporium (9 S. apiospermum, 2 S. prolificans, and 2 species not identified), and 10 patients were treated for infections due to Candida (3 C. glabrata, 2 C. tropicalis, 2 C. glabrata and C. tropicalis, 1 C. albicans, and 2 species not identified). The yeast Cryptococcus was identified for five patients (two C. gattii, one C. neoformans, and two species not identified), with less common fungal pathogens identified for two patients (Bipolaris sp. and Fusarium sp./Paecilomyces lilacinus). Two patients were treated with voriconazole for unidentified invasive mold infections.
Voriconazole therapy.
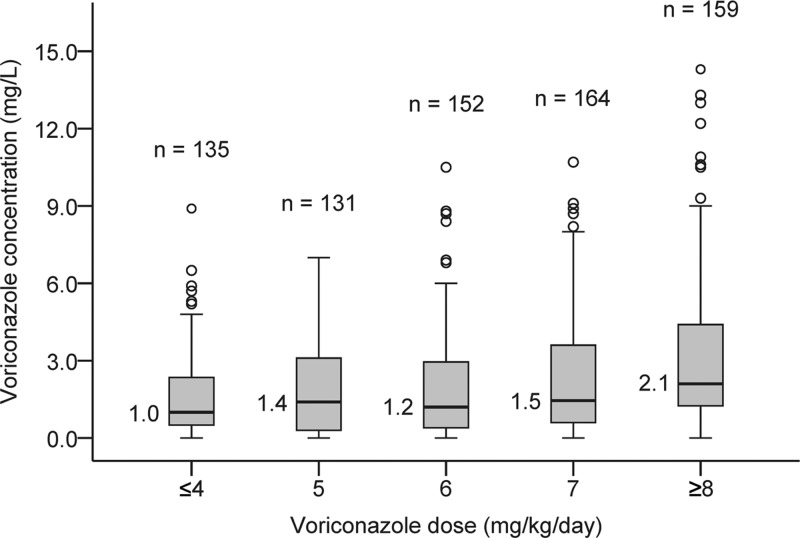
A total of 783 voriconazole trough concentrations from 201 patients were included in the analysis. The median voriconazole concentration was 1.4 mg/liter (range, 0 to 14.3 mg/liter). Voriconazole was administered orally to 48% of patients (97/201), with 76 patients receiving both intravenous and oral voriconazole during treatment; 28 patients received only intravenous voriconazole. The median maintenance dose of voriconazole was 6.1 mg/kg of body weight/day (range, 2.4 to 17.4 mg/kg/day). The median number of trough concentrations measured per patient was 2 (range, 1 to 22). The relationship between voriconazole daily dose and trough concentration was highly variable (Fig. 1).
Fig 1.
Boxplot of relationship between voriconazole daily dose and voriconazole trough concentration. The box line and corresponding value represent the median voriconazole trough concentration within the dose group; the lower and upper box ends represent the lower and upper quartile, respectively. The lower and upper whiskers represent the minimum and maximum concentrations within 1.5 times the interquartile range (box length) from the lower and upper quartile, respectively. Open circles represent concentrations >1.5 times interquartile range above the upper quartile.
Voriconazole concentration and treatment outcome.
Among patients receiving voriconazole for the treatment of a suspected or confirmed fungal infection (n = 170), treatment outcome was evaluable for 163 patients. Twenty-five patients failed therapy (15.3%); the median voriconazole concentration was significantly lower in patients failing therapy (0.9 mg/liter) than in those treated successfully (2.1 mg/liter) (P < 0.05). A higher rate of treatment failure was observed in patients with proven or probable IFI (14/67 [20.9%]); the voriconazole concentration was lower in cases of treatment failure than in cases of treatment success (0.9 versus 2.0 mg/liter) (P < 0.05). ROC curve analysis indicated that voriconazole concentration was a significant predictor of treatment success in both the treatment population and the proven or probable IFI subset; a voriconazole concentration of ≥1.7 mg/liter minimized the incidence of treatment failure (Fig. 2A and B). The incidence of treatment failure is described in Table 2. No patient receiving voriconazole as antifungal prophylaxis developed a breakthrough IFI in this study.
Fig 2.

Receiver operating characteristic (ROC) curves for predicting treatment success for all treatment patients (A) and for patients with proven or probable IFI (B) or neurotoxic adverse events (C) from voriconazole trough concentrations. The true-positive rate represents the proportion of true positives that are correctly classified as positive. The false-positive rate represents the proportion of true negatives that are incorrectly classified as positive. True-positive rate = true positives/(true positives + false negatives); false-positive rate = false positives/(false positives + true negatives). 95% CI, 95% confidence interval.
Table 2.
Incidence of treatment failure and visual or auditory hallucinations below and above voriconazole concentration limits identified from ROC analysis
| Incident | No. of patients with incident/total no. with indicated concn (%) | P valuea | |
|---|---|---|---|
| Treatment failure | <1.7 mg/liter | ≥1.7 mg/liter | |
| All treatment patients (n = 163)b | 19/74 (26) | 6/89 (7) | <0.01 |
| Proven or probable IFI (n = 67) | 12/34 (35) | 2/33 (6) | <0.01 |
| Visual/auditory hallucinations | ≤5 mg/liter | >5 mg/liter | |
| All patients (n = 201) | 2/170 (1.2) | 10/31 (32) | <0.01 |
Fisher's exact or chi-squared test as appropriate.
All patients receiving voriconazole for treatment of a known or suspected fungal infection (n = 170); treatment outcome was evaluable in 163/170 patients.
Voriconazole concentration and adverse events.
Neurotoxic adverse events characterized by visual or auditory hallucinations were reported in 21 patients (10.5%) during voriconazole therapy. The median number of days from time of voriconazole initiation to onset of hallucinations was 4 days (range, 1 to 52 days). Of the 21 patients experiencing neurotoxic adverse events, 11/21 (52%) were receiving intravenous voriconazole at the time of onset, with 10/21 (48%) administered oral voriconazole. Trough voriconazole concentrations were measured at the time of this adverse event in 12 patients and were significantly higher than median voriconazole concentrations in patients not experiencing hallucinations (median, 6.5 versus 1.6 mg/liter; P < 0.01). ROC curve analysis indicated that voriconazole concentration was a significant predictor of neurotoxic adverse events, and a voriconazole concentration of ≤5 mg/liter was found to minimize the incidence of neurotoxic adverse events (Fig. 2C). The incidence of visual or auditory hallucinations is described in Table 2. All occurrences of neurotoxicity resolved following voriconazole cessation or dose reduction.
Data on liver function tests measured on one or more occasions during voriconazole therapy were available for 86% of patients (173/201); data on baseline LFTs prior to voriconazole dosing were available for 46% of patients (93/201). At baseline, 68% of patients (63/93) had elevated LFTs meeting the criteria of CTCAE grade 1 or higher. During voriconazole therapy, 87% of patients (151/173) had CTCAE grade 1 LFT elevation, with 60% meeting grade 2 criteria, 41% grade 3, and 11% grade 4. The voriconazole concentration in patients with elevated LFTs (using CTCAE grade 1, 2, 3, or 4 cutoff values [20]) was not significantly different from that in patients without LFT elevation.
Factors affecting voriconazole concentration.
A multiple linear regression analysis identified a number of clinical factors and drug interactions associated with a significant change in voriconazole concentration (Table 3). Increasing patient age, increasing daily dose, and concomitant administration with any proton pump inhibitor (omeprazole, pantoprazole, esomeprazole, or rabeprazole) were associated with significantly increased voriconazole concentrations; factors associated with reduced voriconazole concentrations included oral administration of voriconazole (compared to intravenous administration), increasing patient weight, coadministration with rifampin or phenytoin, and coadministration with a glucocorticoid (prednisone/prednisolone, methylprednisolone, or dexamethasone). Evidence for an interaction between voriconazole and glucocorticoids has not previously been reported; therefore, a univariate analysis of the effect of glucocorticoids on voriconazole concentration was performed (Table 4). The analysis of data from this study suggests that all glucocorticoids significantly reduce voriconazole concentration, with coadministration of methylprednisolone and dexamethasone reducing voriconazole concentration to a greater extent than prednisone or prednisolone.
Table 3.
Factors associated with a significant change in voriconazole concentration identified from multiple linear regression analysisa
| Model term | Coefficient | 95% Confidence interval |
P value | |
|---|---|---|---|---|
| Lower | Upper | |||
| Oral administrationb | −1.348 | −1.741 | −0.955 | <0.01 |
| Age (yr)c | 0.026 | 0.017 | 0.036 | <0.01 |
| Weight (kg) | −0.028 | −0.038 | −0.018 | <0.01 |
| Daily dose (mg) | 0.005 | 0.003 | 0.006 | <0.01 |
| Concomitant medication | ||||
| CYP2C19 inducerd | −2.367 | −3.181 | −1.553 | <0.01 |
| Prednisone/prednisolone | −1.012 | −1.346 | −0.678 | <0.01 |
| Methylprednisolone | −1.833 | −2.445 | −1.221 | <0.01 |
| Dexamethasone | −1.245 | −1.991 | −0.500 | <0.01 |
| Omeprazole | 1.141 | 0.575 | 1.706 | <0.01 |
| Pantoprazole | 0.685 | 0.330 | 1.041 | <0.01 |
| Esomeprazole | 1.009 | 0.192 | 1.826 | <0.05 |
| Rabeprazole | 1.414 | 0.800 | 2.028 | <0.01 |
R2 = 0.24; n = 736 concentration measurements.
Compared to intravenous administration.
Age at time of first voriconazole concentration measurement.
Phenytoin or rifampin.
Table 4.
Univariate analysis of the effect of glucocorticoid coadministration on voriconazole concentration
| Concomitant medication | Voriconazole concn (mg/liter)a when parameter was: |
P valueb | |
|---|---|---|---|
| Present | Absent | ||
| Prednisone or prednisolone | 1.25 (2.2) | 1.60 (3.1) | <0.01 |
| Methylprednisolone | 1.00 (1.5) | 1.50 (2.8) | <0.01 |
| Dexamethasone | 0.40 (1.2) | 1.50 (2.8) | <0.01 |
Median (interquartile range).
Mann-Whitney U test.
DISCUSSION
This study is the largest multicenter investigation of voriconazole TDM to date and presents significant evidence of the relationships between voriconazole concentration and clinical efficacy and toxicity, as well as identifying a number of important clinical factors and drug interactions that predict voriconazole exposure in patients.
In this study, voriconazole concentrations below 1.7 mg/liter were associated with significantly higher rates of treatment failure (Fig. 2A and B). This difference was observed both in the overall treatment population (including patients treated empirically and for localized fungal infections) and in patients with proven or probable IFIs, where higher rates of treatment failure were observed (Table 2).
Neurotoxic adverse events were relatively common in patients treated with voriconazole (approximately 10%) and usually occurred within several days of commencing voriconazole. The voriconazole concentrations measured at the time of this adverse event in 12 patients were significantly higher than the concentrations in patients without hallucinations; the majority of neurotoxic adverse events occurred at concentrations above 5 mg/liter (Fig. 2C and Table 2).
A number of smaller studies of voriconazole TDM have recommended lower and upper concentration limits for voriconazole, with the goal of maximizing treatment success and minimizing drug-related toxicity (Table 5). Pascual and colleagues prospectively studied 52 patients receiving voriconazole for the treatment of known or suspected IFI (22). In this cohort, the treatment success rate was significantly higher at voriconazole concentrations of >1 mg/liter (88%) than in patients with concentrations of ≤1 mg/liter (54%). All patients experiencing neurotoxicity in this study had voriconazole concentrations above 5.5 mg/liter (22). A study of children receiving voriconazole for the treatment of IFI identified a similar efficacy target of >1 mg/liter (21).
Table 5.
Comparison of recommended lower and upper target voriconazole concentration limits from voriconazole TDM studies
| Study | Sample sizea,b | Indication for voriconazole | Concn (mg/liter)a |
|
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| Troke et al. (31) | 401 (NR) | Treatment | ≥2 (trough/MIC) | ≤5 (trough/MIC)c |
| Present study | 201d (7) | Treatment | ≥1.7 | ≤5 |
| Pascual et al. (22) | 52 (1) | Treatment | >1 | ≤5.5 |
| Neely et al. (21) | 46 (1) | Treatment | >1 | NR |
| Ueda et al. (32) | 34 (1) | Treatment | >2 | <6 |
| Smith et al. (25) | 28 (1) | Treatment | >2.05e | NR |
| Imhof et al. (14) | 26 (1) | Treatment | NR | <4 |
| Miyakis et al. (19) | 25 (1) | Treatment | >2.2 | NR |
| Mitsani et al. (18) | 93 (1) | Prophylaxis | >1.5 | NR |
| Trifilio et al. (30) | 71 (1) | Prophylaxis | >2 | NR |
NR, not reported.
Number of patients (number of hospitals).
This limit was based on an association with reduced treatment response rates above 5 mg/liter; no association with toxicity was observed.
n = 201 for upper concentration limit (toxicity); patients receiving voriconazole for prophylaxis were not included in assessment of the lower concentration limit (n = 170).
This recommendation was based on randomly timed voriconazole samples rather than trough sampling.
Ueda et al. retrospectively investigated voriconazole TDM in a cohort of 34 patients with hematological diseases (32). This study identified greater treatment success at voriconazole concentrations above 2 mg/liter in patients without refractory hematological diseases, as well as identifying a greater incidence of LFT elevation at voriconazole concentrations above 6 mg/liter (32).
Other studies of voriconazole concentration-effect relationships in patients receiving treatment for IFIs have identified concentrations of >2.05 mg/liter (random sampling) (25) and >2.2 mg/liter (trough sampling) (19) as potential lower concentration limits for voriconazole therapy. Simulations from a pooled analysis of random voriconazole concentrations from 9 clinical trials identified a trough/MIC ratio of 2 to 5 as being associated with the highest probability of response (31). The lack of MIC data in this patient cohort precluded the investigation of trough/MIC ratios, although the clinical utility of this metric may be limited by its reliance on a fungal MIC measurement being available. While treatment failures were not identified for concentrations between 1.7 and 2 mg/liter in the present study, in the context of previous studies (Table 5), aiming for voriconazole trough concentrations of at least 2 mg/liter appears to be a rational recommendation.
Reports of neurotoxicity with voriconazole have generally occurred at high voriconazole concentrations. Imhof et al. reported high voriconazole concentrations (>5 mg/liter) for four of six cases of neurotoxic adverse events with voriconazole (14); a further case report of confusion and hallucination on voriconazole reported a concentration of 8.96 mg/liter (4). In addition to neurotoxicity, other common adverse events with voriconazole include temporary visual disturbances and elevations in hepatic enzyme concentrations (15). Both of these adverse events have been reported more frequently at higher voriconazole concentrations (26), although a significant relationship between voriconazole concentration and LFT elevation was not observed in this study, which may reflect the high incidence of elevated LFTs prior to commencing voriconazole in this study or limitations in the available LFT data. Based on the low frequency of neurotoxic adverse events reported at concentrations of ≤5 mg/liter in the present study and by other authors (14, 22), voriconazole trough concentrations should be maintained below 5 mg/liter.
As has been reported by other authors (22), the relationship between voriconazole dose and concentration was highly variable in this study (Fig. 1). A multiple linear regression analysis of voriconazole concentrations identified a number of drug interactions, as well as demographic and clinical factors, that predict changes in voriconazole concentrations (Table 3).
Most notably, the use of a systemic glucocorticoid, including prednisone or prednisolone, methylprednisolone, or dexamethasone, was associated with significantly reduced voriconazole concentrations, suggesting a previously unreported drug interaction. An in vitro study has identified glucocorticoid receptor binding sites in the promoter region of the CYP2C19 gene and demonstrated upregulation of CYP2C19 in response to dexamethasone, supporting an inductive effect of glucocorticoids on CYP2C19 (5). An in vivo study has also demonstrated an inductive effect of a 12- to 15-day course of prednisone on the metabolism of cyclophosphamide, a substrate of both CYP2C19 and CYP2C9 (10). In addition, CYP3A4, which glucocorticoids have been shown to induce at higher doses (7), contributes to the metabolism of voriconazole (27). Taken together, a glucocorticoid-mediated induction of CYP2C19 (and possibly CYP3A4), resulting in increased voriconazole metabolism, appears to be a plausible mechanism for this interaction.
While the magnitude of this interaction appears to be less than those observed with other known inducers of CYP2C19, such as rifampin and phenytoin (Table 3), the clinical implications for voriconazole therapy may be significant. In the present study, 47% of patients had at least one voriconazole concentration measured while taking a glucocorticoid, suggesting that the use of glucocorticoids in patients taking voriconazole is common. Differences in the degrees of interaction observed with different glucocorticoids are also apparent. In both the multiple regression and univariate analysis (Tables 3 and 4), coadministration of dexamethasone and methylprednisolone reduced voriconazole concentrations to a greater extent than prednisone or prednisolone; these results correlate with the higher glucocorticoid receptor potency observed with dexamethasone and methylprednisolone (9).
Voriconazole is reported to have nearly complete oral bioavailability of 96% in healthy volunteers (23), although lower bioavailability has recently been reported in patients (13). Oral administration of voriconazole was associated with significantly lower voriconazole concentrations than intravenous voriconazole in this study (Table 3). A study of voriconazole's absolute oral bioavailability in healthy volunteers determined a mean value of 82.6%; bioavailability appeared to differ based on the CYP2C19 genotype (mean of 75.2% [62.9 to 87.4] in CYP2C19 extensive metabolizers compared to 94.4% [78.8 to 109.9] in poor metabolizers) (24). Although these differences did not reach statistical significance, the observed trend toward lower bioavailability in CYP2C19 extensive metabolizers suggests that CYP2C19 expression in the gut wall may lower voriconazole exposure (24). While studies of absolute oral bioavailability in patients are lacking, our data support the presence of a small but considerable first-pass effect for voriconazole, which may vary based on CYP2C19 genotype.
A significant increase in voriconazole concentrations was observed with all proton pump inhibitors when coadministered with voriconazole (Table 3). Previous studies have demonstrated increased voriconazole exposure with omeprazole (34) due to an inhibition of CYP2C19; however, there have been few reports regarding the interaction of voriconazole with other proton pump inhibitors, such as pantoprazole, rabeprazole, and esomeprazole. While pantoprazole and rabeprazole are generally expected to have a lower drug interaction potential than omeprazole (3), all proton pump inhibitors are known to competitively inhibit CYP2C19 activity in vitro (16). A previous report by Heinz et al. demonstrated higher voriconazole concentrations when coadministered with pantoprazole (11). The lower regression coefficient determined for pantoprazole (Table 3) suggests a reduced (but still significant) interaction with voriconazole compared with those of other proton pump inhibitors, in agreement with in vitro findings that pantoprazole is the weakest inhibitor of CYP2C19 (16). Increasing patient age was also found to be a significant predictor of increased voriconazole concentrations; this finding is consistent with a previous pooled analysis that found approximately 80 to 90% higher voriconazole concentrations in patients aged >65 years compared to the concentrations in younger patients (23).
The results of this study infer a number of novel clinical implications for voriconazole therapy. In addition to the known CYP2C19 inducers rifampin and phenytoin, clinicians should be aware of the potential for reduced voriconazole exposure and subsequent treatment failure in patients administered systemic glucocorticoids. While previous reports had identified a significant interaction between omeprazole and voriconazole, we have found that all proton pump inhibitors may lead to increased voriconazole concentrations and consequent greater risk of neurotoxic adverse events. In the context of previous studies, these results support a narrow therapeutic range for voriconazole trough concentrations of between 2 and 5 mg/liter. In light of the highly variable pharmacokinetics observed with voriconazole, the established concentration-efficacy and -toxicity relationships, and the significant drug interactions, therapeutic drug monitoring is fundamental to the optimal use of voriconazole.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the support of the medical records and pharmacy departments at Concord Repatriation General Hospital, St. Vincent's Hospital, Royal North Shore Hospital, Royal Prince Alfred Hospital, Westmead Hospital, St. George Hospital, and Prince of Wales Hospital.
M.J.D. is supported by an Australian Postgraduate Award.
M.J.D., J.E.R., K.N., L.G.P., and A.J.M. declare no conflicts of interest. S.C.-A.C. has been a member of the Antifungal Advisory Boards of Pfizer Australia, Merck, and Gilead Sciences, Inc.
Footnotes
Published ahead of print 2 July 2012
REFERENCES
- 1. Andes D, Marchillo K, Stamstad T, Conklin R. 2003. In vivo pharmacokinetics and pharmacodynamics of a new triazole, voriconazole, in a murine candidiasis model. Antimicrob. Agents Chemother. 47:3165–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andes D, Pascual A, Marchetti O. 2009. Antifungal therapeutic drug monitoring: established and emerging indications. Antimicrob. Agents Chemother. 53:24–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blume H, Donath F, Warnke A, Schug BS. 2006. Pharmacokinetic drug interaction profiles of proton pump inhibitors. Drug Saf. 29:769–784 [DOI] [PubMed] [Google Scholar]
- 4. Boyd AE, et al. 2004. Adverse reactions to voriconazole. Clin. Infect. Dis. 39:1241–1244 [DOI] [PubMed] [Google Scholar]
- 5. Chen Y, Ferguson SS, Negishi M, Goldstein JA. 2003. Identification of constitutive androstane receptor and glucocorticoid receptor binding sites in the CYP2C19 promoter. Mol. Pharmacol. 64:316–324 [DOI] [PubMed] [Google Scholar]
- 6. Chhun S, et al. 2007. Simultaneous quantification of voriconazole and posaconazole in human plasma by high-performance liquid chromatography with ultra-violet detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 852:223–228 [DOI] [PubMed] [Google Scholar]
- 7. Czock D, Keller F, Rasche FM, Häussler U. 2005. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin. Pharmacokinet. 44:61–98 [DOI] [PubMed] [Google Scholar]
- 8. De Pauw B, et al. 2008. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 46:1813–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Derendorf H, et al. 1993. Receptor-based pharmacokinetic-pharmacodynamic analysis of corticosteroids. J. Clin. Pharmacol. 33:115–123 [DOI] [PubMed] [Google Scholar]
- 10. Faber OK, Mouridsen HT, Skovsted L. 1974. The biotransformation of cyclophosphamide in man: influence of prednisone. Acta Pharmacol. Toxicol. (Copenh.) 35:195–200 [DOI] [PubMed] [Google Scholar]
- 11. Heinz WJ, et al. 2007. Comparison of plasma trough concentrations of voriconazole in patients with or without comedication of ranitidine or pantoprazole. Clin. Microbiol. Infect. 13(Suppl 1):S357 [Google Scholar]
- 12. Herbrecht R, et al. 2002. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 347:408–415 [DOI] [PubMed] [Google Scholar]
- 13. Hope WW. 2012. Population pharmacokinetics of voriconazole in adults. Antimicrob. Agents Chemother. 56:526–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Imhof A, Schaer DJ, Schanz U, Schwarz U. 2006. Neurological adverse events to voriconazole: evidence for therapeutic drug monitoring. Swiss Med. Wkly. 136:739–742 [DOI] [PubMed] [Google Scholar]
- 15. Johnson LB, Kauffman CA. 2003. Voriconazole: a new triazole antifungal agent. Clin. Infect. Dis. 36:630–637 [DOI] [PubMed] [Google Scholar]
- 16. Li X-Q, Andersson TB, Ahlström M, Weidolf L. 2004. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab. Dispos. 32:821–827 [DOI] [PubMed] [Google Scholar]
- 17. Mavridou E, Bruggemann RJM, Melchers WJG, Verweij PE, Mouton JW. 2010. Impact of cyp51A mutations on the pharmacokinetic and pharmacodynamic properties of voriconazole in a murine model of disseminated aspergillosis. Antimicrob. Agents Chemother. 54:4758–4764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mitsani D, et al. 2012. Prospective, observational study of voriconazole therapeutic drug monitoring among lung transplant recipients receiving prophylaxis: factors impacting levels of and associations between serum troughs, efficacy, and toxicity. Antimicrob. Agents Chemother. 56:2371–2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miyakis S, van Hal SJ, Ray J, Marriott D. 2010. Voriconazole concentrations and outcome of invasive fungal infections. Clin. Microbiol. Infect. 16:927–933 [DOI] [PubMed] [Google Scholar]
- 20. National Cancer Institute, National Institutes of Health 14 June 2010. Common terminology criteria for adverse events (CTCAE), version 4.03. NIH publication no. 09-5410. National Cancer Institute, National Institutes of Health, U.S. Department of Health and Human Services, Bethesda, MD: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf [Google Scholar]
- 21. Neely M, Rushing T, Kovacs A, Jelliffe R, Hoffman J. 2010. Voriconazole pharmacokinetics and pharmacodynamics in children. Clin. Infect. Dis. 50:27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pascual A, et al. 2008. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin. Infect. Dis. 46:201–211 [DOI] [PubMed] [Google Scholar]
- 23. Pfizer, Inc 2011. Vfend prescribing information. Pfizer, New York, NY: http://labeling.pfizer.com/ShowLabeling.aspx?id=618 [Google Scholar]
- 24. Scholz I, et al. 2009. Pharmacokinetics, metabolism and bioavailability of the triazole antifungal agent voriconazole in relation to CYP2C19 genotype. Br. J. Clin. Pharmacol. 68:906–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smith J, et al. 2006. Voriconazole therapeutic drug monitoring. Antimicrob. Agents Chemother. 50:1570–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tan K, Brayshaw N, Tomaszewski K, Troke P, Wood N. 2006. Investigation of the potential relationships between plasma voriconazole concentrations and visual adverse events or liver function test abnormalities. J. Clin. Pharmacol. 46:235–243 [DOI] [PubMed] [Google Scholar]
- 27. Theuretzbacher U, Ihle F, Derendorf H. 2006. Pharmacokinetic/pharmacodynamic profile of voriconazole. Clin. Pharmacokinet. 45:649–663 [DOI] [PubMed] [Google Scholar]
- 28. Trifilio S, et al. 2005. Voriconazole therapeutic drug monitoring in allogeneic hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 35:509–513 [DOI] [PubMed] [Google Scholar]
- 29. Trifilio S, et al. 2007. Monitoring plasma voriconazole levels may be necessary to avoid subtherapeutic levels in hematopoietic stem cell transplant recipients. Cancer 109:1532–1535 [DOI] [PubMed] [Google Scholar]
- 30. Trifilio S, et al. 2007. Breakthrough fungal infections after allogeneic hematopoietic stem cell transplantation in patients on prophylactic voriconazole. Bone Marrow Transplant. 40:451–456 [DOI] [PubMed] [Google Scholar]
- 31. Troke PF, Hockey HP, Hope WW. 2011. Observational study of the clinical efficacy of voriconazole and its relationship to plasma concentrations in patients. Antimicrob. Agents Chemother. 55:4782–4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ueda K, et al. 2009. Monitoring trough concentration of voriconazole is important to ensure successful antifungal therapy and to avoid hepatic damage in patients with hematological disorders. Int. J. Hematol. 89:592–599 [DOI] [PubMed] [Google Scholar]
- 33. Weiss J, et al. 2009. CYP2C19 genotype is a major factor contributing to the highly variable pharmacokinetics of voriconazole. J. Clin. Pharmacol. 49:196–204 [DOI] [PubMed] [Google Scholar]
- 34. Wood N, et al. 2003. Effect of omeprazole on the steady-state pharmacokinetics of voriconazole. Br. J. Clin. Pharmacol. 56(Suppl 1):56–61 [DOI] [PMC free article] [PubMed] [Google Scholar]