Abstract
Echinocandins are frontline agents against invasive candidiasis (IC), but predictors for echinocandin therapeutic failure have not been well defined. Mutations in Candida FKS genes, which encode the enzyme targeted by echinocandins, result in elevated MICs and have been linked to therapeutic failures. In this study, echinocandin MICs by broth microdilution and FKS1 and FKS2 mutations among C. glabrata isolates recovered from patients with IC at our center were correlated retrospectively with echinocandin therapeutic responses. Thirty-five patients with candidemia and 4 with intra-abdominal abscesses were included, 92% (36/39) of whom received caspofungin. Twenty-six percent (10) and 74% (29) failed and responded to echinocandin therapy, respectively. Caspofungin, anidulafungin, and micafungin MICs ranged from 0.5 to 8, 0.03 to 1, and 0.015 to 0.5 μg/ml, respectively. FKS mutations were detected in 18% (7/39) of C. glabrata isolates (FKS1, n = 2; FKS2, n = 5). Median caspofungin and anidulafungin MICs were higher for patients who failed therapy (P = 0.04 and 0.006, respectively). By receiver operating characteristic (ROC) analyses, MIC cutoffs that best predicted failure were >0.5 (caspofungin), >0.06 (anidulafungin), and >0.03 μg/ml (micafungin), for which sensitivity/specificity were 60%/86%, 50%/97%, and 40%/90%, respectively. Sensitivity/specificity of an FKS mutation in predicting failure were 60%/97%. By univariate analysis, recent gastrointestinal surgery, prior echinocandin exposure, anidulafungin MIC of >0.06 μg/ml, caspofungin MIC of >0.5 μg/ml, and an FKS mutation were significantly associated with failure. The presence of an FKS mutation was the only independent risk factor by multivariate analysis (P = 0.002). In conclusion, detection of C. glabrata FKS mutations was superior to MICs in predicting echinocandin therapeutic responses among patients with IC.
INTRODUCTION
Candida glabrata has emerged as the most common cause of invasive candidiasis (IC) at many centers in the United States, likely as a result of widespread use of azole antifungal agents and the growing populations of immunosuppressed and other high-risk hosts (12). The echinocandin antifungals (caspofungin, anidulafungin, and micafungin) inhibit β-1,3-d-glucan synthase, an enzyme that synthesizes a major component of the Candida cell wall. These agents have become a preferred front-line therapy against C. glabrata and other Candida species that may demonstrate decreased susceptibility to azoles (2, 23). In recent years, echinocandin therapeutic failures and breakthrough Candida infections have been increasingly reported (7, 8, 11, 13, 17–19, 30, 34). As such, the development of rapid and accurate methods for detecting echinocandin-resistant Candida isolates is a priority.
The role of in vitro echinocandin susceptibility testing of clinical Candida isolates in directing therapy is not established. Echinocandin MICs against Candida isolates infrequently exceed the breakpoints for resistance, as originally proposed by the Clinical and Laboratory Standards Institute (CLSI) (29). This observation has led to concerns about the sensitivity of the CLSI-recommended methodology in identifying isolates that are unlikely to respond to echinocandin therapy (4). In response to these concerns, CLSI revised the echinocandin interpretive breakpoints by considering factors such as relative differences in susceptibility among Candida spp., epidemiological MIC cutoff values (ECVs), molecular mechanisms of resistance, β-1,3-d-glucan synthase enzyme kinetics, pharmacokinetic and pharmacodynamic data, and published clinical data linking MICs with therapeutic outcomes (28). Using this approach, CLSI has proposed lower resistance breakpoints of >0.25 μg/ml for caspofungin and anidulafungin and >0.12 μg/ml for micafungin against C. glabrata. At present, the impact of these revised breakpoints on the identification of resistant isolates and their utility in predicting the response to echinocandin therapy are unknown.
In C. glabrata, β-1,3-d-glucan synthase is encoded by three related genes (FKS1, FKS2, and FKS3) (15). FKS1 and FKS2 mutations are responsible for reduced echinocandin susceptibility in C. glabrata, and they have been linked to the failure of echinocandin therapy in case reports and small series (7, 8, 13, 14, 34). The limited clinical data are supported by in vitro kinetic analyses demonstrating that β-1,3-d-glucan synthase encoded by FKS mutant C. glabrata isolates is less sensitive to echinocandins (15). As such, identification of mutations in FKS1 and FKS2 may be more sensitive than antifungal susceptibility testing in detecting echinocandin-resistant isolates. To date, the prevalence of FKS mutations appears to be low, but a relatively small number of Candida isolates have been screened in the United States (36) and worldwide (6). In order to determine if FKS genotype testing has a role in the management of IC, studies that characterize a substantial number of isolates associated with both successful and unsuccessful therapy are needed.
In this study, we performed a retrospective evaluation of IC caused by C. glabrata among patients treated with an echinocandin at our institution. We sought to correlate therapeutic responses with echinocandin MICs and the presence of FKS mutations and to identify risk factors predisposing patients to FKS mutations and echinocandin therapeutic failure.
MATERIALS AND METHODS
Isolates.
To be considered for inclusion in the study, patients with IC due to C. glabrata must have been treated with an echinocandin for ≥3 days following the diagnosis, or they must have developed breakthrough IC due to C. glabrata while receiving ≥3 days of an echinocandin for preventive or empirical therapy. In all cases of candidemia, vascular catheters had to be removed within 48 h of starting treatment with an echinocandin. In cases of intra-abdominal abscesses, drainage had to be performed. Exclusion criteria included treatment with an antifungal other than an echinocandin (n = 25), death prior to receiving 3 days of antifungal therapy (n = 12), retention of a vascular catheter for >48 h after the start of antifungal therapy (n = 9), or lack of medical records (n = 5). C. glabrata isolates were selected from −80°C stock in the biorepository at the University of Pittsburgh Mycology Research Unit. Prior to testing, isolates were subcultured onto Sabouraud dextrose agar plates, grown at 35°C for 24 to 48 h, and subcultured again for 24 h. The study was approved by the University of Pittsburgh Institutional Review Board.
Echinocandin susceptibility testing.
Echinocandin MICs were determined in duplicate according to the method described in CLSI document M27-A3 (9) using a 50% turbidity endpoint at 24 h in RPMI 1640 (buffered to a pH of 7.0 with morpholinepropanesulfonic acid [MOPS]). Standard powders of caspofungin (Merck, Rahway, NJ), anidulafungin (Pfizer, New York, NY), and micafungin (Astellas Pharma, Japan) were obtained from the manufacturers. For each agent, the range of concentrations tested was 0.015 to 16 μg/ml. Candida krusei ATCC 6258 and Candida parapsilosis ATCC 22019 were used as quality controls. The quality control strains were incorporated in each set of experiments, and the MICs of each echinocandin agent were within the expected range (10). For C. krusei ATCC 6258, acceptable CLSI MIC ranges for caspofungin, anidulafungin, and micafungin were 0.12 to1 μg/ml, 0.03 to 0.12 μg/ml, and 0.12 to 0.5 μg/ml, respectively. For C. parapsilosis ATCC 22019, the corresponding MIC ranges were 0.25 to 1 μg/ml, 0.25 to 2 μg/ml, and 0.5 to 2 μg/ml, respectively.
Determination of FKS mutations.
C. glabrata genomic DNA was extracted from yeast cells grown overnight in YPD broth (2% yeast extract, 4% peptone, 4% dextrose) and purified using the Wizard genomic DNA purification kit (Promega, Wisconsin). Hot spots 1 and 2 of FKS1 and FKS2 were amplified using PCR, as previously described (15). Standard Sanger DNA sequencing of purified PCR amplicons was performed using a 3130 DNA analyzer (Applied Biosystems, Carlsbad, CA). DNA sequences were analyzed with the software program Sequence Scanner (Applied Biosystems), and the corresponding amino acid sequences were compared to C. glabrata databases (http://www.ncbi.nlm.nih.gov/blast/).
Definitions.
Therapeutic failure was assessed at day 14 and defined using consensus definitions of outcomes for antifungal therapy (33) or as a breakthrough infection while receiving an echinocandin for ≥3 days as preventive or empirical therapy. CLSI breakpoint MICs and recently proposed ECVs were used to identify isolates as echinocandin susceptible, intermediate, or resistant (26, 28). In separate analyses, isolates classified as intermediate were considered to be resistant or susceptible. Correlations between MICs and therapeutic outcomes were made for the specific agent used to treat each patient. In addition, MICs for each echinocandin were assessed as proxies for the class, regardless of which specific agent was used to treat a given patient.
Statistical analysis.
Statistical analyses were performed using the PASW Statistics 18 software program (SPSS Inc., Chicago, IL). MICs were logarithmically transformed to approximate normal distribution. Differences in MICs between echinocandins were compared using the Wilcoxon rank sum test. To compare sensitivity and specificity between variables (echinocandin MIC or FKS mutations), we used the McNemar χ2 test (35). Receiver operating characteristic (ROC) analysis was conducted to determine optimal cutoffs for specific echinocandin MICs. Comparisons between two groups (stratified by FKS mutations and therapeutic outcome) were performed by Wilcoxon rank sum test for continuous variables and chi-squared or Fisher's exact tests for categorical variables. To evaluate independent predictors of echinocandin failure, we performed multivariate analysis, including variables that were significant by univariate analysis. Significance was defined as a P value ≤0.05 (two-tailed).
RESULTS
Thirty-nine C. glabrata isolates from patients with IC who were treated with caspofungin (92%; 36/39), anidulafungin (2%; 1/39) or micafungin (5%; 2/39) for at least 3 days were included. Ninety percent (35/39) and 10% (4/39) of patients had candidemia and intra-abdominal abscesses, respectively. Ten percent (4/39) of patients developed breakthrough IC while receiving an echinocandin, and an additional 10% (4/39) developed it while receiving an azole. Demographics and clinical characteristics are presented in Table 1.
Table 1.
Patient demographics, clinical characteristics, and risk factors for FKS mutations
| Characteristic | Value for group |
P valuec | ||
|---|---|---|---|---|
| All patients (n = 39) | FKS mutation (n = 7) | No FKS mutation (n = 32) | ||
| No. (%) female | 31 (80) | 6 (86) | 25 (78) | NS (1.00) |
| No. (%) of race | ||||
| White | 31 (80) | 6 (86) | 25 (78) | NS (1.00) |
| Black | 6 (15) | 1 (14) | 5 (16) | |
| Other | 2 (5) | 0 | 2 (6) | |
| Median age in yrs (range) | 59 (22–88) | 63 (38–86) | 59 (22–88) | NS (0.78) |
| No. (%) with type of IC | ||||
| Candidemia | 35 (90) | 5 (71) | 30 (94) | NS (0.14) |
| Abscess | 4 (10) | 2 (29) | 2 (6) | |
| No. (%) with underlying condition | ||||
| Transplant | 14 (36) | 3 (43) | 11 (34) | NS (0.69) |
| Malignancy | 4 (10) | 0 | 4 (13) | NS (1.00) |
| GI diseasea | 14 (36) | 4 (57) | 10 (31) | NS (0.23) |
| Otherb | 7 (18) | 0 | 7 (22) | NS (0.31) |
| No. (%) with GI surgery within 30 days of IC | 19 (49) | 7 (100) | 12 (38) | 0.003 |
| No. (%) with TPN within 30 days of IC | 14 (36) | 6 (86) | 8 (25) | 0.005 |
| No. (%) with prior echinocandin exposure | 13 (33) | 7 (100) | 6 (19) | 0.0001 |
| No. (%) with prior azole exposure | 22 (56) | 5 (71) | 17 (53) | NS (0.44) |
| Median days of prior echinocandin exposure (range) | 0 (0–117) | 64 (3–117) | 0 (0–20) | <0.0001 |
| Median days of prior azole exposure (range) | 6 (0–238) | 34 (0–100) | 5 (0–238) | NS (0.09) |
| No. (%) with breakthrough IC | ||||
| Echinocandin | 4 (10) | 4 (57) | 0 | 0.0004 |
| Azole | 4 (10) | 0 | 4 (12) | NS (1.00) |
GI disease includes short gut syndrome (n = 6), superior mesenteric artery syndrome (n = 2), abdominal fistula (n = 2), Crohn's disease (n = 1), diverticulitis (n = 1), necrotizing pancreatitis (n = 1), and liver cirrhosis (n = 1).
Other underlying diseases include cardiovascular disease (n = 4), scleroderma (n = 1), and subarachnoid hemorrhage (n = 1); one patient had no significant past medical history.
NS, not significant.
In vitro echinocandin susceptibility testing.
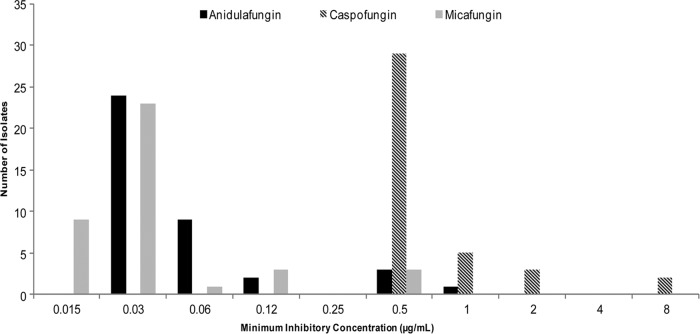
Caspofungin MICs ranged from 0.5 to 8 μg/ml; the MIC50 and MIC90 were 0.5 and 1 μg/ml, respectively. The corresponding values for anidulafungin were 0.03 to 1 μg/ml, 0.03, and 0.5 μg/ml, respectively. For micafungin, values were 0.015 to 0.5 μg/ml, 0.03, and 0.12 μg/ml, respectively. The distribution of echinocandin MICs is presented in Fig. 1. Caspofungin, anidulafungin, and micafungin MICs against C. krusei ATCC 6258 and C. parapsilosis ATCC 22019 were within the acceptable ranges.
Fig 1.
Distribution of caspofungin, anidulafungin, and micafungin MICs.
Overall, caspofungin MICs were higher than those of anidulafungin and micafungin (P < 0.0001 for both). Using the revised CLSI breakpoints for C. glabrata, 0% (0/39), 90% (35/39), and 85% (33/39) of isolates were in vitro susceptible to caspofungin, anidulafungin, and micafungin, respectively. Eight percent (3/39) were intermediate to micafungin, and none of the isolates were intermediate to caspofungin or anidulafungin. One hundred percent (39/39), 10% (4/39), and 8% (3/39) of isolates were resistant to caspofungin, anidulafungin, and micafungin, respectively.
Presence of FKS mutations.
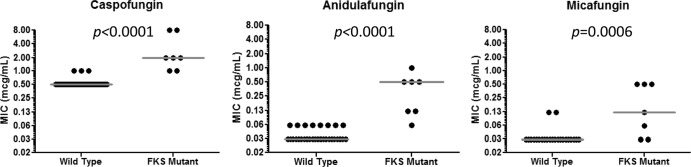
FKS mutations were detected in 18% (7/39) of isolates (candidemia, n = 5; intra-abdominal abscess, n = 2). As detailed in Table 2, mutations occurred at hot spots in FKS1 or FKS2 (n = 2 or 5, respectively). Echinocandin MICs were higher in isolates harboring mutations than in wild-type strains (Fig. 2). Factors significantly associated with FKS mutations included gastrointestinal (GI) surgery or receipt of total parenteral nutrition (TPN) within 30 days of IC, prior echinocandin exposure, prolonged duration of prior echinocandin exposure, and breakthrough IC during echinocandin therapy (Table 1). As determined by ROC analyses, the MIC cutoffs that best predicted FKS mutations were >0.5, >0.06, and >0.03 μg/ml for caspofungin, anidulafungin, and micafungin, respectively (data not shown). At these cutoffs, the sensitivity and specificity of caspofungin MICs for predicting FKS mutations were 100% (7/7) and 91% (29/32), respectively. The corresponding values for anidulafungin and micafungin MICs were 86% (6/7) and 100% (32/32) and 71% (5/7) and 94% (30/32), respectively.
Table 2.
FKS mutations and corresponding echinocandin MICs
| Isolate | Mutation |
MIC (μg/ml) of drug |
|||||
|---|---|---|---|---|---|---|---|
|
FKS1 |
FKS2 |
Caspofungin | Anidulafungin | Micafungin | |||
| Hot spot 1 | Hot spot 2 | Hot spot 1 | Hot spot 2 | ||||
| 309 | D632H | None | None | None | 2 | 0.5 | 0.5 |
| 1 | D632Y | None | None | None | 2 | 0.5 | 0.5 |
| 102 | None | None | F659del | None | 8 | 1 | 0.5 |
| 190 | None | None | F659del | None | 8 | 0.5 | 0.12 |
| 35 | None | None | F659L | None | 1 | 0.12 | 0.06 |
| 129 | None | None | F659L | None | 1 | 0.12 | 0.03 |
| 187 | None | None | F659L | None | 2 | 0.06 | 0.03 |
Fig 2.
Distribution of caspofungin, anidulafungin, and micafungin MICs according to FKS mutations.
Correlation between echinocandin MICs or presence of FKS mutations and therapeutic outcomes.
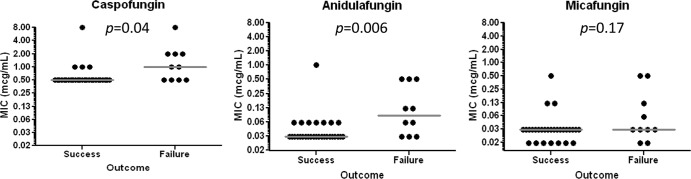
Twenty-six percent (10/39) and 74% (29/39) of patients experienced failure and success of echinocandin therapy, respectively. Median caspofungin and anidulafungin MICs were higher in cases of therapeutic failure (P = 0.04 and 0.006, respectively), but micafungin MICs were not (P = 0.17) (Fig. 3). ROC analyses identified the optimal cutoffs for therapeutic failure to be >0.5 μg/ml for caspofungin, >0.06 μg/ml for anidulafungin, and >0.03 μg/ml for micafungin. The sensitivity and specificity of CLSI breakpoints, ECVs, ROC cutoffs, and FKS mutations in predicting therapeutic failure are presented in Table 3. For CLSI breakpoints, ECVs, and ROC cutoffs, the sensitivity and specificity were identical whether outcomes were correlated with the MIC of the specific echinocandin used to treat each patient or the MIC of each echinocandin was used as a proxy for the class.
Fig 3.
Distribution of caspofungin, anidulafungin, and micafungin MICs according to clinical outcome.
Table 3.
Performance of echinocandin breakpoints and FKS mutations in predicting therapeutic failure
| Variablea | % sensitivityb | % specificityc | Likelihood ratio |
|---|---|---|---|
| CLSI breakpoints (intermediate isolates considered to be resistantd) | |||
| Caspofungin BP > 0.12 μg/ml | 100 (10/10) | 0 (0/29) | NAe |
| Anidulafungin BP > 0.12 μg/ml | 30 (3/10) | 97 (28/29) | 8.7 |
| Micafungin BP > 0.06 μg/ml | 30 (3/10) | 90 (26/29) | 2.9 |
| CLSI breakpoints (intermediate isolates considered to be sensitived) | |||
| Caspofungin BP > 0.25 μg/ml | 100 (10/10) | 0 (0/29) | NA |
| Anidulafungin BP > 0.25 μg/ml | 30 (3/10) | 97 (28/29) | 8.7 |
| Micafungin BP > 0.12 μg/ml | 20 (2/10) | 97 (28/29) | 5.8 |
| Epidemiologic cutoff values | |||
| Caspofungin BP > 0.12 μg/ml | 100 (10/10) | 0 (0/29) | NA |
| Anidulafungin BP > 0.25 μg/ml | 30 (3/10) | 97 (28/29) | 8.7 |
| Micafungin BP > 0.03 μg/ml | 40 (4/10) | 90 (26/29) | 3.9 |
| Cutoffs determined by ROC | |||
| Caspofungin MIC > 0.5 μg/ml | 60 (6/10) | 86 (25/29) | 4.3 |
| Anidulafungin BP > 0.06 μg/ml | 50 (5/10) | 97 (28/29) | 14.5 |
| Micafungin BP > 0.03 μg/ml | 40 (4/10) | 90 (26/29) | 3.9 |
| FKS genotype | |||
| Presence of mutation | 60 (6/10) | 97 (28/29) | 17.4 |
BP, breakpoint.
Sensitivity is defined as the number of tests positive for the individual variable listed in the first column, divided by the number of patients who failed echinocandin therapy (n = 10) × 100%.
Specificity is defined as the number of tests negative for the individual variable listed in the first column, divided by the number of patients in whom echinocandin therapy was successful (n = 29) × 100%.
CLSI breakpoints define C. glabrata strains as susceptible (≤0.12 μg/ml for caspofungin and anidulafungin and ≤0.06 μg/ml for micafungin), intermediate (0.25 μg/ml for caspofungin and anidulafungin and 0.12 μg/ml for micafungin), or resistant (>0.25 μg/ml for caspofungin and anidulafungin and >0.12 μg/ml for micafungin).
NA, not applicable.
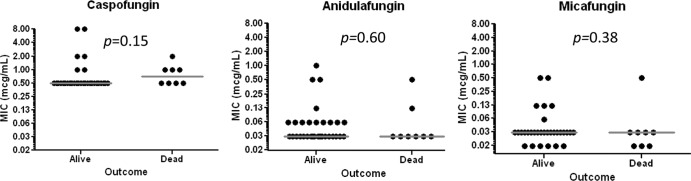
The 30-day mortality rates for patients with and without FKS mutations were 29% (2/7) and 19% (6/32), respectively (P = 0.62). Severity of illness did not differ between patients who died (median acute physiology score [APS] = 9.5; range, 5 to 15) or survived (median APS = 8; range, 2 to 14; P = 0.24). Likewise, the median APS for patients with FKS mutations (9; range, 6 to 13) was similar to that for patients without mutations (8; range, 2 to 15; P = 0.51). The median caspofungin, anidulafungin, and micafungin MICs against isolates associated with 30-day mortality were 0.75, 0.03, and 0.03 μg/ml, respectively, which were not significantly different from median MICs against isolates associated with survival (0.5, 0.03, and 0.03 μg/ml, respectively) (Fig. 4).
Fig 4.
Distribution of caspofungin, anidulafungin, and micafungin MICs according to patient survival at 30 days.
Risk factors for failure of echinocandin therapy.
By univariate analysis, recent GI surgery, prior echinocandin exposure, caspofungin MICs of >0.5 μg/ml, anidulafungin MICs of >0.06 μg/ml, and presence of an FKS mutation were significantly associated with therapeutic failure (Table 4). The presence of an FKS mutation was the only independent risk factor for therapeutic failure, as demonstrated by multivariate logistic regression analysis using factors identified by univariate analysis (Table 4).
Table 4.
Risk factors for echinocandin failure
| Variable | % successa | % failureb | Univariate analysis P value | Multivariate analysis P valuef | Odds ratio (95% CI) |
|---|---|---|---|---|---|
| GI surgery within 30 days | 40% (11/29) | 80% (8/10) | 0.031 | NS (0.58) | |
| TPN within 30 days | 31% (9/29) | 50% (5/10) | NS (0.44) | ||
| Immunosuppressionc | 31% (9/29) | 50% (5/10) | NS (0.44) | ||
| Previous echinocandin exposure | 21% (6/29) | 70% (7/10) | 0.008 | NS (0.73) | |
| Previous azole exposure | 52% (15/29) | 70% (7/10) | NS (0.47) | ||
| Median APS (range) | 8 (2–14) | 9 (5–15) | NS (0.27) | ||
| Echinocandin MICd | |||||
| Caspofungin, >0.5 μg/ml | 14% (4/29) | 60% (6/10) | 0.009 | NS (0.49) | |
| Anidulafungin, >0.06 μg/ml | 3% (1/29) | 50% (5/10) | 0.002 | NS (0.66) | |
| Micafungin, >0.03 μg/ml | 10% (3/29) | 40% (4/10) | 0.06 | NS (0.39) | |
| Presence of FKS mutation | 3% (1/29) | 60% (6/10) | 0.0004 | 0.002 | 41.7 (3.96–445.7) |
| Initiation of antifungal within 48 he | 36% (9/25) | 33% (2/6) | NS (1.00) | ||
| Initiation of antifungal within 72 he | 44% (11/25) | 50% (3/6) | NS (1.00) | ||
| Initiation of antifungal within 120 he | 92% (23/25) | 67% (4/6) | NS (0.16) | ||
| Initiation of echinocandin within 48 he | 17% (5/29) | 17% (1/6) | NS (1.00) | ||
| Initiation of echinocandin within 72 he | 31% (9/29) | 33% (2/6) | NS (1.00) | ||
| Initiation of echinocandin within 120 he | 79% (23/29) | 67% (4/6) | NS (0.60) |
Results are no. of successful treatments/no. treated (n = 29) (does not apply for median APS).
Results are no. of treatment failures/no. treated (n = 10) (does not apply for median APS).
Immunosuppression was defined as a history of bone marrow or solid organ transplantation, or receipt of immunosuppressive therapy.
Caspofungin, anidulafungin, and micafungin MICs were each considered individually in multivariate analyses.
Patients with azole or echinocandin breakthrough IC were excluded from this analysis (n = 4 each).
NS, not significant.
DISCUSSION
We have demonstrated a correlation between the presence of FKS mutations and echinocandin therapeutic failure among patients with IC due to C. glabrata. Patients infected with C. glabrata isolates harboring FKS mutations were more likely to fail echinocandin therapy (86%; 6/7) than those infected with isolates without FKS mutations (12%; 4/32; P = 0.0004). By univariate analysis, the presence of a mutation within hot spot regions of FKS1 or FKS2, a caspofungin MIC of >0.5 μg/ml, an anidulafungin MIC of >0.06 μg/ml, prior echinocandin exposure, and GI surgery within 30 days of developing IC were significant risk factors for therapeutic failure. By multiple regression analysis, the presence of an FKS mutation was the only independent risk factor for failure. The sensitivity and specificity of FKS mutations in predicting therapeutic failure were 60% (6/10) and 97% (28/29), respectively. These findings reiterate that resistance is not the sole determinant of responses to antifungal therapy among patients with IC, since factors such as host immune function, underlying diseases, intravenous catheter removal, adjunctive surgical interventions, and pharmacokinetic parameters also play significant roles (31, 32). As such, it is unsurprising that some of our patients failed to respond to an echinocandin despite being infected with C. glabrata isolates with wild-type FKS alleles and others responded despite being infected with FKS mutant isolates.
Echinocandin MICs correlated with therapeutic outcomes to a lesser degree than FKS mutations. Median caspofungin and anidulafungin MICs were significantly higher in cases of therapeutic failure than in cases of success, and it was possible to establish cutoff values for echinocandin resistance. By ROC analyses, the optimal breakpoints for caspofungin, anidulafungin, and micafungin resistance were >0.5, >0.06 and >0.03 μg/ml, respectively. The sensitivity and specificity of our caspofungin breakpoint for predicting therapeutic failure were 60% (6/10) and 86% (25/29), respectively. The anidulafungin and micafungin breakpoints were somewhat less sensitive (50% and 40%, respectively) but more specific (97% and 90%, respectively), although the differences between agents were not statistically significant. The likelihood ratio for therapeutic failure with anidulafungin MICs of >0.06 μg/ml was particularly strong, approaching that of an FKS mutation (14.5 versus 17.4). Nevertheless, no MICs for any of the echinocandins were independently associated with therapeutic failure by multiple regression analyses.
It is important to note that the caspofungin breakpoint suggested by our data was higher than that proposed by CLSI (28), which reflected the fact that our caspofungin MICs, in general, were higher than those used by CLSI. In fact, 100% (39/39) of caspofungin MICs in our study were above the proposed CLSI breakpoint (28) and ECV (27). Our caspofungin MICs were higher than those in some reports (27, 36) but corroborated the findings of other studies (1, 16, 21, 22). The reasons for the discrepancies between studies are not immediately apparent, but the variability of caspofungin susceptibility testing is well recognized and may reflect differences in lot-to-lot potency of pure drug or choice of solvent (3, 4). Unfortunately, the currently recommended control strains are insensitive at detecting caspofungin MIC variability (4). Our anidulafungin and micafungin MIC distributions and breakpoints, on the other hand, were more consistent with those of previous reports. Of note, an anidulafungin breakpoint of >0.06 μg/ml has been proposed as a surrogate for resistance to the echinocandin class (5). Our findings support this breakpoint, but the data are too limited to draw conclusions about the value of anidulafungin MIC as a surrogate or the role of echinocandin MICs in guiding therapeutic decisions.
To date, studies attempting to correlate in vitro echinocandin resistance and putative breakpoint MICs to the clinical outcomes of IC have yielded conflicting results. Our finding that caspofungin MICs correlated with the response to therapy is consistent with results in a previous study evaluating 17 Candida isolates recovered from patients receiving ≥3 doses of micafungin. In the earlier study, FKS hot spot mutations were found in 5 C. glabrata and 2 C. tropicalis breakthrough isolates, each of which exhibited caspofungin MICs of >2 μg/ml (30). As in our study, this report included immunocompromised hosts who received prolonged echinocandin therapy (median duration, 33 days) (30). In contrast, a relationship between caspofungin MICs and treatment outcomes was not apparent in a study of patients treated with caspofungin for IC (114 patients) or oropharyngeal candidiasis (292 patients) (16). In fact, patients infected with Candida isolates against which MICs were highest (>2 μg/ml) had better outcomes than patients infected with isolates for which MICs were lower (<1 μg/ml) (16). There are several possible explanations for the disparate results between this study and ours. Most importantly, the previous study evaluated outcomes across types of infection and different species, which likely limited the ability to establish correlations. For example, echinocandin MICs against C. parapsilosis are typically higher than those against other species, but C. parapsilosis is well recognized to be associated with good clinical outcomes (2, 20). Along related lines, the earlier study included only 15 C. glabrata isolates, against which caspofungin MICs fell within a narrow, 2-fold dilution range (0.5 or 1 μg/ml). In our study, on the other hand, caspofungin MICs ranged from 0.5 to 8 μg/ml. Other notable differences in our study were that patients were more likely to be immunosuppressed and to have received prior echinocandin therapy, vascular catheters had to be removed and foci of infection drained, and patients were not selected from participants in prospective, randomized trials.
Our findings that FKS mutations directly correlated with echinocandin MICs and that MICs that best predicted the presence of FKS mutations also best predicted therapeutic failure are consistent with numerous smaller reports of various Candida spp., in which amino acid substitutions in FKS gene products were linked with elevated echinocandin MICs and therapeutic failure. To our knowledge, this is the first report associating F659L with in vitro echinocandin resistance and therapeutic failure among C. glabrata isolates, since previous substitutions at this site were reported only for serine, valine, or tyrosine (15). Based on our limited data, F659L appears to be a less prominent mutation, since echinocandin MICs were lower than those for other mutations. Our two isolates with FKS1 mutations had the amino acid substitutions D632Y and -H. Although D632G, -E, and -Y were linked to echinocandin resistance, D632H has not been reported previously. As studies continue to chronicle FKS mutations, we will likely continue to encounter novel mutation patterns. Future studies of enzyme kinetics will be needed to clearly establish which specific mutations are responsible for echinocandin resistance.
We must acknowledge that the data presented in this study are from a single center, the number of FKS mutants is small, and the vast majority of patients were treated with caspofungin. In light of the last point, cutoff anidulafungin and micafungin MICs can only be extrapolated from our data. Nevertheless, our data support the conclusion that the FKS genotype is a better indicator of echinocandin therapeutic failure than MIC alone and suggest that alternatives to traditional phenotypic testing for resistance are warranted for C. glabrata. Similar studies are needed to validate genotypic testing of other Candida species. FKS mutations are the only known mechanism for reduced echinocandin susceptibility, and as such they provide an ideal platform for molecular assays to rapidly detect drug resistance (24, 25). However, until laboratory-based approaches like in vitro echinocandin susceptibility testing using reliable interpretive breakpoints and FKS genotyping are validated, such data should be used cautiously in the management of individual patients. Along these lines, clinicians should recognize the clinical scenarios in which the risk for echinocandin resistance is elevated, including in the settings of ongoing or prior echinocandin therapy, TPN administration, or recent GI surgery.
ACKNOWLEDGMENTS
We thank Lloyd Clarke for his assistance in data retrieval.
This project was supported by the National Institutes of Health through grants KL2 RR024154 and KL2TR000146, awarded to R.K.S., and by investigator-initiated grants to R.K.S. from Astellas and Merck.
R.K.S. has received research funding from Astellas and Merck. M.H.N. has received research funding from Viracor-IBT Laboratories and grant support from Pfizer, Merck, CSL Behring, and Biotherapies for Life. C.J.C. has received grant support from Pfizer, Merck, and AstraZeneca. For all other authors, there are no conflicts of interest to report.
Footnotes
Published ahead of print 2 July 2012
REFERENCES
- 1. Alexander BD, et al. 2007. Comparative evaluation of Etest and Sensititre YeastOne panels against the Clinical and Laboratory Standards Institute M27-A2 reference broth microdilution method for testing Candida susceptibility to seven antifungal agents. J. Clin. Microbiol. 45:698–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andes DR, et al. 2012. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin. Infect. Dis. 54:1110–1122 [DOI] [PubMed] [Google Scholar]
- 3. Arendrup MC, et al. 2009. Breakthrough Aspergillus fumigatus and Candida albicans double infection during caspofungin treatment: laboratory characteristics and implication for susceptibility testing. Antimicrob. Agents Chemother. 53:1185–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arendrup MC, et al. 2010. Echinocandin susceptibility testing of Candida species: comparison of EUCAST EDef 7.1, CLSI M27-A3, Etest, disk diffusion, and agar dilution methods with RPMI and isosensitest media. Antimicrob. Agents Chemother. 54:426–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arendrup MC, et al. 2011. EUCAST technical note on anidulafungin. Clin. Microbiol. Infect. 17:E18–E20 [DOI] [PubMed] [Google Scholar]
- 6. Castanheira M, et al. 2010. Low prevalence of fks1 hot spot 1 mutations in a worldwide collection of Candida strains. Antimicrob. Agents Chemother. 54:2655–2659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chapeland-Leclerc F, et al. 2010. Acquisition of flucytosine, azole, and caspofungin resistance in Candida glabrata bloodstream isolates serially obtained from a hematopoietic stem cell transplant recipient. Antimicrob. Agents Chemother. 54:1360–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cleary JD, Garcia-Effron G, Chapman SW, Perlin DS. 2008. Reduced Candida glabrata susceptibility secondary to an FKS1 mutation developed during candidemia treatment. Antimicrob. Agents Chemother. 52:2263–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A3, 3rd ed Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 10. Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts. Third Informational Supplement, vol 28 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 11. Costa-de-Oliveira S, et al. 2011. FKS2 mutations associated with decreased echinocandin susceptibility of Candida glabrata following anidulafungin therapy. Antimicrob. Agents Chemother. 55:1312–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fidel PL, Jr, Vazquez JA, Sobel JD. 1999. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin. Microbiol. Rev. 12:80–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garcia-Effron G, et al. 2010. Novel FKS mutations associated with echinocandin resistance in Candida species. Antimicrob. Agents Chemother. 54:2225–2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garcia-Effron G, Katiyar SK, Park S, Edlind TD, Perlin DS. 2008. A naturally occurring proline-to-alanine amino acid change in Fks1p in Candida parapsilosis, Candida orthopsilosis, and Candida metapsilosis accounts for reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 52:2305–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Garcia-Effron G, Lee S, Park S, Cleary JD, Perlin DS. 22 June 2009. Effect of Candida glabrata FKS1 and FKS2 mutations on echinocandin sensitivity and kinetics of 1,3-β-D-glucan synthase: implication for the existing susceptibility breakpoint. Antimicrob. Agents Chemother. doi:10.1128/AAC.00443-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kartsonis N, et al. 2005. Caspofungin susceptibility testing of isolates from patients with esophageal candidiasis or invasive candidiasis: relationship of MIC to treatment outcome. Antimicrob. Agents Chemother. 49:3616–3623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Katiyar S, Pfaller M, Edlind T. 2006. Candida albicans and Candida glabrata clinical isolates exhibiting reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 50:2892–2894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lortholary O, et al. 2011. Recent exposure to caspofungin or fluconazole influences the epidemiology of candidemia: a prospective multicenter study involving 2,441 patients. Antimicrob. Agents Chemother. 55:532–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Messer SA, Jones RN, Moet GJ, Kirby JT, Castanheira M. 2010. Potency of anidulafungin compared to nine other antifungal agents tested against Candida spp., Cryptococcus spp., and Aspergillus spp.: results from the global SENTRY Antimicrobial Surveillance Program (2008). J. Clin. Microbiol. 48:2984–2987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nguyen MH, et al. 1996. The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. Am. J. Med. 100:617–623 [DOI] [PubMed] [Google Scholar]
- 21. Odabasi Z, Paetznick V, Rex JH, Ostrosky-Zeichner L. 2007. Effects of serum on in vitro susceptibility testing of echinocandins. Antimicrob. Agents Chemother. 51:4214–4216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Paderu P, et al. 2007. Serum differently alters the antifungal properties of echinocandin drugs. Antimicrob. Agents Chemother. 51:2253–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pappas PG, et al. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 48:503–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Perlin DS. 2009. Antifungal drug resistance: do molecular methods provide a way forward? Curr. Opin. Infect. Dis. 22:568–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perlin DS. 2007. Resistance to echinocandin-class antifungal drugs. Drug Resist Updat. 10:121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pfaller M, et al. 2011. Use of epidemiological cutoff values to examine 9-year trends in susceptibility of Candida species to anidulafungin, caspofungin, and micafungin. J. Clin. Microbiol. 49:624–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pfaller MA, et al. 2010. Wild-type MIC distributions and epidemiological cutoff values for the echinocandins and Candida spp. J. Clin. Microbiol. 48:52–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pfaller MA, et al. 2011. Clinical breakpoints for the echinocandins and Candida revisited: integration of molecular, clinical, and microbiological data to arrive at species-specific interpretive criteria. Drug Resist Updat. 14:164–176 [DOI] [PubMed] [Google Scholar]
- 29. Pfaller MA, et al. 2008. Correlation of MIC with outcome for Candida species tested against caspofungin, anidulafungin, and micafungin: analysis and proposal for interpretive MIC breakpoints. J. Clin. Microbiol. 46:2620–2629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pfeiffer CD, et al. 2010. Breakthrough invasive candidiasis in patients on micafungin. J. Clin. Microbiol. 48:2373–2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rex JH, Pfaller MA. 2002. Has antifungal susceptibility testing come of age? Clin. Infect. Dis. 35:982–989 [DOI] [PubMed] [Google Scholar]
- 32. Rex JH, et al. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Clin. Infect. Dis. 24:235–247 [DOI] [PubMed] [Google Scholar]
- 33. Segal BH, et al. 2008. Defining responses to therapy and study outcomes in clinical trials of invasive fungal disease: Mycoses Study Group and European Organization for Research and Treatment of Cancer consensus criteria. Clin. Infect. Dis. 47:674–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thompson GR, III, et al. 2008. Development of caspofungin resistance following prolonged therapy for invasive candidiasis secondary to Candida glabrata infection. Antimicrob. Agents Chemother. 52:3783–3785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Trajman A, Luiz RR. 2008. McNemar chi2 test revisited: comparing sensitivity and specificity of diagnostic examinations. Scand. J. Clin. Lab. Invest. 68:77–80 [DOI] [PubMed] [Google Scholar]
- 36. Zimbeck AJ, et al. 2010. FKS mutations and elevated echinocandin MIC values among Candida glabrata isolates from U.S. population-based surveillance. Antimicrob. Agents Chemother. 54:5042–5047 [DOI] [PMC free article] [PubMed] [Google Scholar]