Abstract
The increasing prevalence of multidrug-resistant Gram-negative infections has led to renewed interest in the use of systemic polymyxin B. However, the nephrotoxic properties of polymyxin B are still poorly understood. The objective of this study was to characterize nephrotoxicity associated with polymyxin B, with an emphasis on examining the impact of dosing frequencies on the onset of nephrotoxicity. Sprague-Dawley rats were divided into two groups and administered the same total daily dose of polymyxin B subcutaneously but with different dosing frequencies (either 20 mg/kg of body weight every 24 h [q24h] or 5 mg/kg q6h). Drug concentrations in renal tissue were compared between the two groups at 24 h. Kidney tissues were harvested at 48 h and compared histologically. Serum creatinine was measured daily for up to 10 days, and nephrotoxicity was defined as a significant elevation in serum creatinine (≥2× baseline). Kaplan-Meier analysis was used to compare the onset of nephrotoxicity. Polymyxin B-induced nephrotoxicity manifested as elevation in serum creatinine and acute tubular necrosis. Extensive injury of the proximal tubules was observed. The lesions were more severe and higher drug concentrations were achieved in the kidneys of the q6h dosing group. The q24h dosing group experienced a more gradual onset of nephrotoxicity, which could be attributed to the lower kidney tissue drug concentrations (48.5 ± 17.4 μg/g versus 92.1 ± 18.1 μg/g of polymyxin B1, P = 0.04). Preferential accumulation of polymyxin B in the kidneys suggests that uptake to renal cells is a nonpassive process and q24h dosing was less nephrotoxic than q6h dosing.
INTRODUCTION
Multidrug resistance in Gram-negative bacteria, causing infections that are extremely difficult to treat, has become a medical crisis worldwide (21). Moreover, no new antibiotics are expected to become available within the next 5 to 10 years to combat infections caused by these resistant strains. These situations have led to a renewed interest in the use of systemic polymyxins, as a last-resort treatment for infections caused by multidrug-resistant Gram-negative bacteria (13, 27).
Polymyxins (polymyxin B and polymyxin E) are polypeptide antibiotics that first became available for clinical use in the 1950s. Shortly afterwards, their use fell out of favor when concerns were raised about their nephrotoxicity potential. Despite the fact that polymyxins have been available for decades, their pharmacological properties are still poorly understood. Current dosages of polymyxins are mostly based on convention, rather than being supported by robust pharmacokinetic and pharmacodynamic information. Recently, low levels of resistance to polymyxins have been reported (10, 14). Attempts to overcome resistance by escalating the dose are hindered by concerns of toxicity. Since the acquired resistance to polymyxins is still relatively low, studies are urgently needed to delineate their nephrotoxicity potential to guide optimal clinical dosing.
Several reports published in the 1960s and 1970s showed that parenteral administration of polymyxin was associated with considerable nephrotoxicity (9, 11, 20, 24). However, since their recent revival, published experiences of systemic polymyxin use suggested that the incidence of nephrotoxicity was less common and less severe than those reported 40 years ago. In one recent study that involved 26 patients administered polymyxin B for treatment of multidrug-resistant Gram-negative infections, the nephrotoxicity rate reported was 0% (13). In other reports, the nephrotoxicity rate ranged from 8% to 24% following the use of systemic polymyxin B or colistimethate sodium (3, 4, 18, 23). Conversely, a recent study that involved 126 patients treated with intravenous colistin showed that nearly half of the patients suffered from some degree of renal insufficiency in a dose-dependent manner, as defined by the RIFLE (risk, injury, failure, loss, and end-stage kidney disease) criteria (19). The discrepancy in the rates of nephrotoxicity among different reports makes it difficult to draw a definite conclusion on the risk of nephrotoxicity associated with administration of systemic polymyxins. Many of these studies involved critically ill patients who were coadministered other nephrotoxic drugs. Besides, the number of patients enrolled in these studies was usually small. Additionally, the definitions of nephrotoxicity, dosing, and routes of administration varied considerably among the different studies. The lack of control also makes extrapolation of the results to the general population challenging. Collectively, these factors could have contributed (at least partially) to the existing conflict in the incidence rate of nephrotoxicity associated with the polymyxins.
In order to minimize the effect of confounding factors that are commonly associated with noncomparative clinical case series, we examined the nephrotoxicity potential of polymyxin B using an animal model. Specifically, we examined the impact of different polymyxin B dosing frequencies on the onset of nephrotoxicity. Since the polymyxins are currently the last line of defense against multidrug-resistant bacteria, a better understanding of their nephrotoxic properties is essential to minimize the incidence of nephrotoxicity and to improve therapeutic outcomes.
(This study was presented in part at the 51st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, 17 to 20 September, 2011 [abstract A2-1182].)
MATERIALS AND METHODS
Antimicrobial agents.
For the in vitro cytotoxicity studies, polymyxin B sulfate (USP) and gentamicin sulfate (USP) powders were purchased from Sigma-Aldrich (St. Louis, MO). For the animal studies, polymyxin B for injection (USP; 1 mg of polymyxin B is equivalent to approximately 10,000 U) was purchased from APP Pharmaceuticals, LLC (lot number 6100814) (Schaumburg, IL). Prior to each experiment, the drug was reconstituted and diluted to the desired concentrations with growth medium or 5% dextrose injection (USP). The reconstituted drug solutions were stored at −70°C and used within 10 days. A stability study confirmed that the reconstituted drug was stable under these storage conditions for up to 10 days (data not shown).
Cell lines.
Three renal cell lines from different mammalian species were used to assess the in vitro cytotoxicity of polymyxin B: NRK-52E (rat renal proximal tubular epithelial cells), MDCK (canine renal distal tubular epithelial cells), and HEK 293 (human embryonic renal cells). All cells were purchased from ATCC (Manassas, VA).
Animals.
Female Sprague-Dawley rats (225 to 249 g, 12 to 16 weeks old) (Harlan, Indianapolis, IN) were used. The rats received food and water ad libitum. The rats were given at least 72 h to acclimate before the start of the experiments. All animals were cared for in accordance with the highest humane and ethical standards, as approved by the Institutional Animal Care and Use Committee of the University of Houston.
In vitro cytotoxicity.
The toxicity of polymyxin B was assessed using the three kidney cell lines described above. Cells were grown in Dulbecco's modified Eagle medium (DMEM) and exposed to increasing concentrations of polymyxin B (up to 4 mg/ml). After incubation for 48 h, cell viability was assessed by absorbance at 595 nm in triplicate, using the Cell Proliferation Kit I (MTT) purchased from Roche (Indianapolis, IN). A placebo (negative) control was used for each experiment; the percent cell viability was reported after normalizing to the absorbance of placebo control on the same day (fixed at 100%). Gentamicin sulfate (up to 60 mg/ml) was used as a positive control. A sigmoid inhibitory maximum effect (Emax) model was used to fit the mean data, and the relative toxicity was compared using the drug concentration resulting in 50% of the maximal reduction in cell viability (IC50).
Dose fractionation design.
The impacts of the dosing frequency on the accumulation of polymyxin B in renal tissue, the severity of the renal lesions, and the onset of nephrotoxicity were studied. For each of these purposes, two groups of rats were used (the number of rats per group is provided below). The animals were administered the same total daily dose of polymyxin B subcutaneously, but with different dosing frequencies (either 20 mg/kg of body weight every 24 h [q24h] or 5 mg/kg q6h). Selection of the dose was based on prior pharmacokinetic studies to achieve total daily drug exposure comparable to that achieved in humans after standard recommended doses (1.5 to 2.5 mg/kg/day) (12) and prior dose escalation studies to observe nephrotoxicity within 10 days. The two groups were compared using two-tailed tests, and P values of <0.05 were considered significant.
Drug concentration in kidney tissue and urine.
The animals were housed in metabolic cages. Two groups of three rats each were administered polymyxin B for 24 h. The rats were sacrificed, and the kidneys were collected, wrapped in Parafilm, and stored at −70°C until analysis. Cumulative urine was collected over 24 h. On the day of the analysis, the kidneys were thawed at room temperature and homogenized in deionized water. The kidney homogenates and urine were then assayed for the major components of polymyxin B (polymyxin B1, isoleucine-polymyxin B1, polymyxin B2, and polymyxin B3) using a validated ultraperformance liquid chromatography-tandem mass spectrometry (UPLC-MS-MS) method (12). The lower limit of quantification was determined based on the signal-to noise ratio of 10:1 and was found to be 50 ng/ml for all components. The precision of the method was well within the 15% acceptance range. The drug concentrations observed were compared using Student's t test.
Renal histological examination.
Microscopic examination of kidney sections was performed to assess the renal cellular injury associated with polymyxin B exposure. Two groups of two rats each were administered polymyxin B for 2 days. At 48 h, whole-body perfusion-fixation of the rats with 10% formalin was performed to preserve the renal tissues. Briefly, under sodium pentobarbital anesthesia (50 mg/kg given intraperitoneally), the rats were perfused intracardially (using a hydraulic pump) with Dulbecco's phosphate-buffered saline, 1× (Cellgro, Mediatech, Inc., Manassas, VA), at a rate of 20 ml/min at room temperature. After the blood was removed, the body was perfused with 10% formalin solution at 20 ml/min for 20 min to fix the renal tissues. Kidneys were then excised and postfixed in the same fixative at 4°C (8). The fixed kidneys were cut into 1-mm-thick sections. The sections were then stained with hematoxylin and eosin (H&E) and examined using a light microscope. For this purpose, the assessor (L.D.T.) was blinded to the treatment assignment. In order to compare the severities of the lesions between the two dosing groups semiquantitatively, the percentage of areas of renal cortex, outer medulla, and inner medulla showing lesions per microscopic field (for 13 randomly selected fields per anatomical region) under ×200 magnifications were compared. The results were compared using the Mann-Whitney test.
Onset of nephrotoxicity.
Two groups of 8 rats each were administered polymyxin B for up to 10 days. A low-dose control group (n = 3) administered polymyxin B at 5 mg/kg once daily was used as a negative control. Serum creatinine was monitored at baseline and daily. Blood samples (∼200 μl) were collected from the rats following tail tip amputation. The blood was allowed to clot and serum was separated by centrifugation at 4,000 × g for 10 min. Serum samples (100 μl) were assayed for creatinine concentration using a clinical chemistry analyzer (Piccolo Xpress, Abaxis, Inc., Union City, CA). Nephrotoxicity was defined as significant elevation in serum creatinine (≥2× the baseline level). The onset of nephrotoxicity was compared between the two dosing groups using Kaplan-Meier survival analysis and the Gehan-Breslow-Wilcoxon test.
RESULTS
In vitro cytotoxicity.
A significant decline in cell viability was observed for all cell lines treated with polymyxin B. Polymyxin B was found to be more toxic to the 3 types of renal cells from different mammalian species than was gentamicin (Table 1).
Table 1.
Cytotoxicity of polymyxin B and gentamicin in various cell lines after 48 h of drug exposure
| Cell line | IC50 (mg/ml) |
|
|---|---|---|
| Polymyxin B | Gentamicin | |
| NRK-52E | 0.4 | 12.4 |
| MDCK | 1.4 | 8.1 |
| HEK 293 | 0.5 | 6.1 |
Kidney tissue concentration and urinary drug excretion.
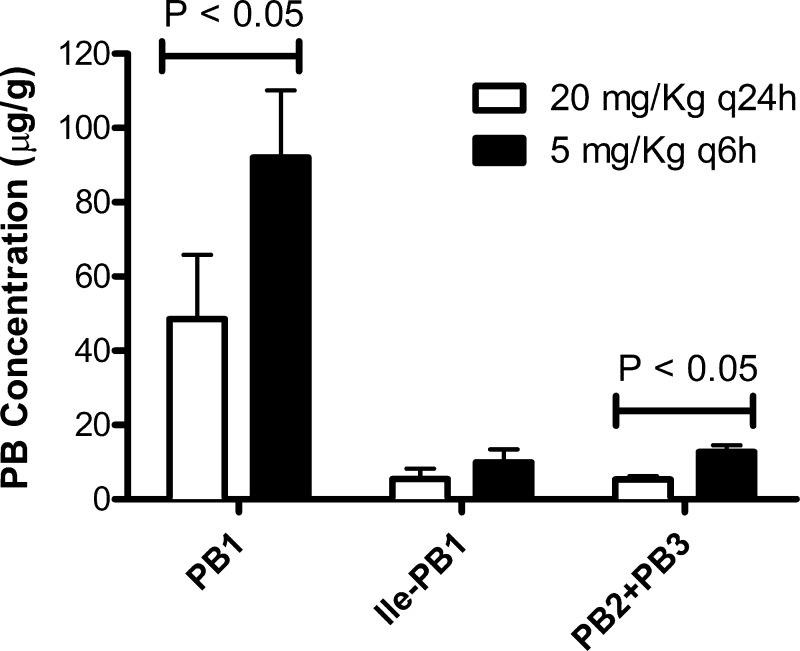
Significantly higher drug concentrations in kidney tissue were observed in the q6h dosing group than in the q24h dosing group (Fig. 1). These data implied that the uptake of polymyxin B into renal cells could be a nonpassive process. For both groups, less than 5% of the dose was recovered unchanged in urine (data not shown), implying that a mechanism(s) other than renal excretion could be involved in the systemic elimination of polymyxin B.
Fig 1.
Concentrations of the major components of polymyxin B (PB) in rat kidneys. Data are shown as means ± standard deviations. PB1, polymyxin B1; ile-PB1, isoleucine polymyxin B1; PB2, polymyxin B2; PB3, polymyxin B3.
Renal histological examination.
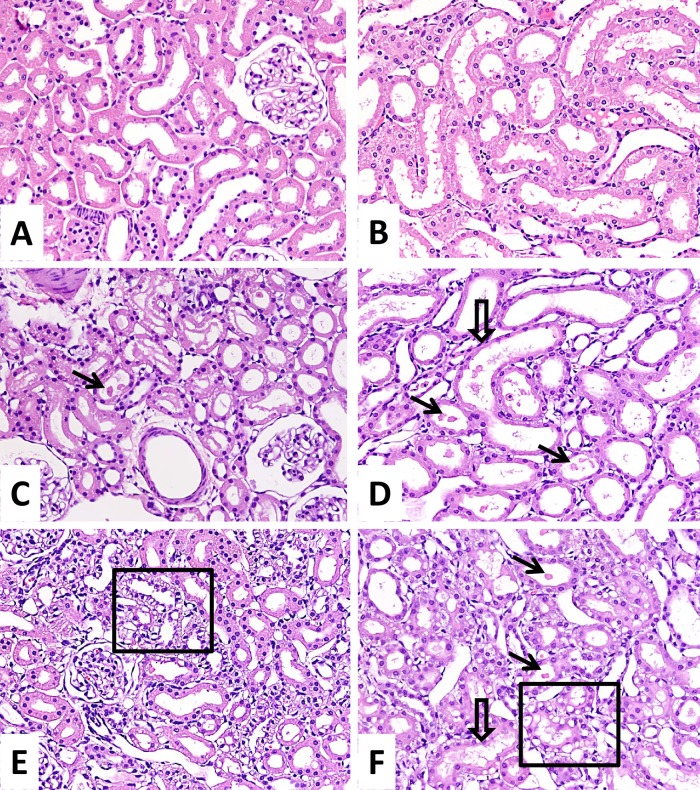
Histological examination of renal tissue of both dosing groups revealed cytoplasmic vacuolization of the proximal tubular epithelial cells, as well as focal tubular cell necrosis and tubular dilatation. Some of the tubules showed denuded basement membrane. The lesions were confined to the outer stripe of the outer medulla (S3 segment of the proximal tubules) and, to a lesser extent, the renal cortex. Interstitial edema was also observed. In contrast, the distal tubules and the glomeruli were mostly intact (Fig. 2). The percentages of areas showing lesions in renal cortex and outer medulla per microscopic field were significantly higher in the q6h dosing group (P < 0.001) (Table 2). The extent of renal injury was more severe with more frequent dosing, which could be attributed (at least in part) to the higher concentration of drug in renal tissue achieved with this regimen.
Fig 2.
Histological comparison of renal lesions. Shown are the renal cortical tissue and the outer stripe of the medulla of normal kidney (A and B), with q24h dosing (C and D), and with q6h dosing (E and F). Hematoxylin and eosin stain and ×200 magnification were used for all panels. For panels C and D, the q24h dosing was associated with focal tubular cell necrosis (arrows) in both locations, together with focal tubular cell cytoplasmic attenuation (open arrow). For panels E and F, the q6h dosing was associated with more severe changes in both locations, including tubular cell necrosis (arrows), extensive cytoplasmic attenuation (open arrow), and vacuolization (box).
Table 2.
Semiquantitative comparison of the kidney lesions
| Anatomic region | % Area showing lesion at indicated dosing schedulea |
P | |
|---|---|---|---|
| 20 mg/kg q24h | 5 mg/kg q6h | ||
| Cortex | 0.50 (0.00–3.25) | 11.50 (6.75–16.25) | <0.001 |
| Medulla (outer zone) | 3.50 (0.75–8.00) | 89.50 (83.75–95.50) | <0.001 |
| Medulla (inner zone) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | NSb |
Values represent medians of percentages of areas showing lesions per microscopic field, with 25th and 75th percentiles in parentheses.
NS, not significant.
Onset of nephrotoxicity.
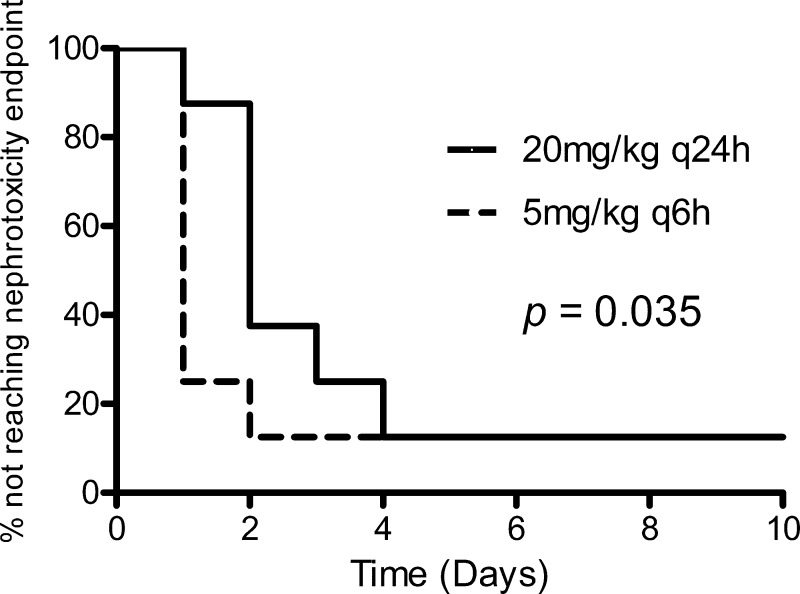
The q24h dosing group experienced a more gradual onset of nephrotoxicity than did the q6h dosing group (P = 0.035) (Fig. 3). The majority of the animals (6 out of 8) that received polymyxin B 5 mg/kg q6h experienced doubling of the baseline serum creatinine concentration 24 h after treatment. On the other hand, only 1 (out of 8) of the animals that received polymyxin B at 20 mg/kg q24h experienced doubling of baseline serum creatinine concentration within the first 24 h of treatment. However, the percentages of rats that did not reach the predefined endpoint of nephrotoxicity at the end of the experiment were comparable between the two groups (12.5%). No significant fluctuation in serum creatinine was observed for any of the rats that received polymyxin B at 5 mg/kg daily for up to 10 days. More frequent dosing was associated with an earlier onset of nephrotoxicity. These findings are consistent with those in the previous sections, which could also be attributed to the higher concentration of drug observed in renal tissue.
Fig 3.
Comparison of the onset of nephrotoxicity between the dosing groups.
DISCUSSION
Until new drugs for the treatment of Gram-negative bacterial infections become available commercially, the use of polymyxin B is expected to continue to increase worldwide. Polymyxin B exhibits potent in vitro bactericidal activity (25). However, current clinical dosing is based on convention rather than robust pharmacokinetic and pharmacodynamic data. Tam et al. have previously shown that the use of the conventional clinical dose of polymyxin B was associated with regrowth and resistance emergence in an in vitro infection model of Pseudomonas aeruginosa (25). These results suggested that higher doses of polymyxin B might be needed to suppress resistance development and to improve clinical outcomes. However, nephrotoxicity remains a major hindrance to considerable polymyxin B dose escalation in clinical settings.
Given the discrepancy in the reported nephrotoxicity rates of polymyxin B, we examined its nephrotoxicity potential using three cell lines from three different mammalian species representing various anatomical regions of the kidney. Polymyxin B was invariably found to be more toxic than gentamicin in all the kidney cell lines investigated. However, renal injury observed in animals was primarily confined to the epithelium of the proximal tubules, consistent with the findings in previous clinical reports (9, 22). Given that the proximal tubules are the most common sites of nephrotoxin-induced renal damage, the absence of diffuse injury all over different segments of the nephrons is not unexpected. This could be attributed to the numerous transporters located in the proximal tubules, resulting in the selective uptake and accumulation of drugs as well as other nephrotoxins in the proximal tubules, rendering the proximal tubules more vulnerable to injury.
We further examined the impact of dosing frequencies of polymyxin B on various aspects of nephrotoxicity. Although elevation in serum creatinine is the most clinically relevant to demonstrate functional deficit associated with renal injury, such investigations are labor-intensive and nonspecific, and the results may not be evident until there has been considerable reduction in renal function. Consequently, pilot studies were performed using early surrogate endpoints; the results of these served to strengthen our postulation incrementally and provide support for progressively more in-depth investigations. Despite using the same daily dose, the concentration of polymyxin B in the kidney tissues at 24 h was found to be lower when the drug was administered as a single daily dose. A possible explanation is that the accumulation of polymyxin B in the renal cells might be mediated by a transporter and that this uptake mechanism was saturable. When the drug was administered as a single dose, the concentration of the drug in the lumen of the renal tubules was high enough to saturate the uptake. As a result, less accumulation of the drug was observed in the kidney tissues. On the other hand, more fractionated administration resulted in repeated exposure of the renal cells to relatively lower drug concentrations and consequently more drug uptake into the cells. Only female animals were used in this study; investigations in a limited number of male animals also showed a similar trend (data not shown).
The difference in renal tissue drug concentration between the dosing groups could have implications for the severity and onset of nephrotoxicity associated with polymyxin B. Histological examination of the renal tissues at 48 h affirmed that more frequent administration resulted in more severe kidney damage, which functionally translated subsequently to an earlier onset of nephrotoxicity. These results suggest that different dosing frequencies of polymyxin B vary in their nephrotoxicity potential as well as the onset of nephrotoxicity. Tam et al. have previously illustrated that the bactericidal activity of polymyxin B against P. aeruginosa was most closely linked to the area under the concentration-time curve (AUC)/MIC ratio (25). This means that altering the dosing frequency would not likely impact bacterial killing or resistance suppression. Collectively, these results show that once-daily dosing appears promising in delaying the onset of nephrotoxicity without adversely affecting the bactericidal activity of polymyxin B.
Studying the relationship between antibiotic exposure and the likelihood of toxicity is a topic of increasing interest (1, 2, 6, 7, 15, 16). Pharmacokinetic and toxicodynamic studies of aminoglycosides showed that once-daily dosing was associated with reduced renal drug uptake, lower incidence of nephrotoxicity, and better clinical outcome compared to multiple-daily dosing (2). However, to date little is known about the relationship between polymyxin exposure and the likelihood of occurrence of nephrotoxicity. Given the similar pattern observed, it is possible that aminoglycosides and polymyxin B share a pathway for intracellular uptake and accumulation within renal cells (17).
In a recent study, Wallace et al. examined the effect of dosing frequency on the nephrotoxicity potential of intravenous colistin methanesulfonate (26). They found that once daily-dosing was associated with more severe kidney lesions than was twice-daily dosing of a clinically relevant dose for humans. However, colistin methanesulfonate is an inactive prodrug of colistin that is cleared predominantly through renal excretion (5). On the other hand, similar to the active moiety colistin (5), our results indicate that elimination of polymyxin B is unlikely due to renal excretion only. Given the different clearance mechanism, results from colistin methanesulfonate may not be directly applicable to polymyxin B.
In summary, our data suggested that administration of polymyxin B every 6 h was associated with an earlier onset of nephrotoxicity than found with once-daily administration of the same total daily dose. Furthermore, accumulation of polymyxin B in the kidneys suggested that drug uptake by renal cells involved a nonpassive mechanism. Further investigations on the mechanisms of uptake and accumulation of polymyxin B in renal cells are ongoing and could provide further insights for minimizing the nephrotoxicity of polymyxin B. Validation of these findings in clinical studies is warranted.
ACKNOWLEDGMENTS
This study was supported by the National Institutes of Health (R15AI089671-01).
We thank Jie He from the University of Houston for refining the polymyxin B assay and Laurie Minze and Alan Collins from the Methodist Hospital Research Institute for their technical assistance on perfusion fixation.
Footnotes
Published ahead of print 11 June 2012
REFERENCES
- 1. Bhavnani SM, Rubino CM, Ambrose PG, Drusano GL. 2010. Daptomycin exposure and the probability of elevations in the creatine phosphokinase level: data from a randomized trial of patients with bacteremia and endocarditis. Clin. Infect. Dis. 50:1568–1574 [DOI] [PubMed] [Google Scholar]
- 2. Drusano GL, et al. 2007. Back to the future: using aminoglycosides again and how to dose them optimally. Clin. Infect. Dis. 45:753–760 [DOI] [PubMed] [Google Scholar]
- 3. Falagas ME, Rafailidis PI, Kasiakou SK, Hatzopoulou P, Michalopoulos A. 2006. Effectiveness and nephrotoxicity of colistin monotherapy vs. colistin-meropenem combination therapy for multidrug-resistant Gram-negative bacterial infections. Clin. Microbiol. Infect. 12:1227–1230 [DOI] [PubMed] [Google Scholar]
- 4. Garnacho-Montero J, et al. 2003. Treatment of multidrug-resistant Acinetobacter baumannii ventilator-associated pneumonia (VAP) with intravenous colistin: a comparison with imipenem-susceptible VAP. Clin. Infect. Dis. 36:1111–1118 [DOI] [PubMed] [Google Scholar]
- 5. Garonzik SM, et al. 2011. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob. Agents Chemother. 55:3284–3294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ingram PR, Lye DC, Fisher DA, Goh WP, Tam VH. 2009. Nephrotoxicity of continuous versus intermittent infusion of vancomycin in outpatient parenteral antimicrobial therapy. Int. J. Antimicrob. Agents 34:570–574 [DOI] [PubMed] [Google Scholar]
- 7. Ingram PR, et al. 2008. Risk factors for nephrotoxicity associated with continuous vancomycin infusion in outpatient parenteral antibiotic therapy. J. Antimicrob. Chemother. 62:168–171 [DOI] [PubMed] [Google Scholar]
- 8. Johnson WD, Lang CM, Johnson MT. 1978. Fixatives and methods of fixation in selected tissues of the laboratory rat. Clin. Toxicol. 12:583–600 [DOI] [PubMed] [Google Scholar]
- 9. Katz R. 1963. Renal and possibly hepatic toxicity from Coly-Mycin. Report of a case. Med. Ann. Dist. Columbia 32:408–413 [PubMed] [Google Scholar]
- 10. Ko KS, et al. 2007. High rates of resistance to colistin and polymyxin B in subgroups of Acinetobacter baumannii isolates from Korea. J. Antimicrob. Chemother. 60:1163–1167 [DOI] [PubMed] [Google Scholar]
- 11. Koch-Weser J, et al. 1970. Adverse effects of sodium colistimethate. Manifestations and specific reaction rates during 317 courses of therapy. Ann. Intern. Med. 72:857–868 [DOI] [PubMed] [Google Scholar]
- 12. Kwa AL, et al. 2008. Pharmacokinetics of polymyxin B1 in patients with multidrug-resistant Gram-negative bacterial infections. Diagn. Microbiol. Infect. Dis. 60:163–167 [DOI] [PubMed] [Google Scholar]
- 13. Kwa AL, Tam VH, Falagas ME. 2008. Polymyxins: a review of the current status including recent developments. Ann. Acad. Med. Singapore 37:870–883 [PubMed] [Google Scholar]
- 14. Landman D, Bratu S, Alam M, Quale J. 2005. Citywide emergence of Pseudomonas aeruginosa strains with reduced susceptibility to polymyxin B. J. Antimicrob. Chemother. 55:954–957 [DOI] [PubMed] [Google Scholar]
- 15. Lodise TP, Lomaestro B, Graves J, Drusano GL. 2008. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob. Agents Chemother. 52:1330–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL. 2009. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin. Infect. Dis. 49:507–514 [DOI] [PubMed] [Google Scholar]
- 17. Moestrup SK, et al. 1995. Evidence that epithelial glycoprotein 330/megalin mediates uptake of polybasic drugs. J. Clin. Invest. 96:1404–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ouderkirk JP, Nord JA, Turett GS, Kislak JW. 2003. Polymyxin B nephrotoxicity and efficacy against nosocomial infections caused by multiresistant gram-negative bacteria. Antimicrob. Agents Chemother. 47:2659–2662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pogue JM, et al. 2011. Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin. Infect. Dis. 53:879–884 [DOI] [PubMed] [Google Scholar]
- 20. Price DJ, Graham DI. 1970. Effects of large doses of colistin sulphomethate sodium on renal function. Br. Med. J. 4:525–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rice LB. 2007. Emerging issues in the management of infections caused by multidrug-resistant gram-negative bacteria. Cleve. Clin. J. Med. 74(Suppl 4):S12–S20 [DOI] [PubMed] [Google Scholar]
- 22. Ryan KJ, Schainuck LI, Hickman RO, Striker GE. 1969. Colistimethate toxicity. Report of a fatal case in a previously healthy child. JAMA 207:2099–2101 [DOI] [PubMed] [Google Scholar]
- 23. Sobieszczyk ME, et al. 2004. Combination therapy with polymyxin B for the treatment of multidrug-resistant Gram-negative respiratory tract infections. J. Antimicrob. Chemother. 54:566–569 [DOI] [PubMed] [Google Scholar]
- 24. Tallgren LG, Liewendahl K, Kuhlbaeck B. 1965. The therapeutic success and nephrotoxicity of colistin in acute and chronic nephropathies with impaired renal function. Acta Med. Scand. 177:717–728 [DOI] [PubMed] [Google Scholar]
- 25. Tam VH, et al. 2005. Pharmacodynamics of polymyxin B against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 49:3624–3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wallace SJ, et al. 2008. Subacute toxicity of colistin methanesulfonate in rats: comparison of various intravenous dosage regimens. Antimicrob. Agents Chemother. 52:1159–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yuan Z, Tam VH. 2008. Polymyxin B: a new strategy for multidrug-resistant Gram-negative organisms. Expert Opin. Invest. Drugs 17:661–668 [DOI] [PubMed] [Google Scholar]