Abstract
Phenotypic tolerances to antibiotics of mature and young Pseudomonas aeruginosa PAO1 biofilms and released planktonic bacteria were compared for four antibiotics. Resistance levels were similar for gentamicin and ciprofloxacin but differed for ceftazidime and meropenem. β-Lactamase mapping showed that, after 5 h of ceftazidime exposure, mature biofilms produced more β-lactamase than young biofilms, facilitating the growth of released planktonic bacteria. This shows the importance of early treatment and choice of antibiotics for P. aeruginosa biofilm infections.
TEXT
Most chronic lung infections are caused by biofilm growth of colonizing bacteria. In biofilm infections, a cycling of symptoms occurs between periods of antibiotic treatments until a point is reached where antibiotic treatment is no longer capable of alleviating disease symptoms (8). Initially, antibiotic treatment appears to eliminate infection; however, only the planktonic bacteria released from biofilms are killed, temporarily reducing symptoms, but the biofilm remains intact and able to persist (13). In patients with Pseudomonas aeruginosa lung infection, there is increasing evidence that repeated antibiotic treatment, such as administration of the β-lactam ceftazidime, becomes less effective at eliminating symptoms (7). Since most symptoms of P. aeruginosa lung infection are caused by planktonic bacteria, it has been assumed that the loss of effective ceftazidime treatment is due to the selection of highly resistant planktonic strains (14). The purpose of this paper is to examine the differences in antibiotic susceptibility between young recently formed biofilm and mature chronic biofilm in order to help guide antibiotic therapy in patients with chronic lung infections. The results indicate that increased expression of β-lactamases by stable mature biofilms contributes to ceftazidime resistance of planktonic bacteria.
A novel device for concurrent minimum biofilm eradication concentration (MBEC) and MIC determination has been previously described (4). Ceri et al. used a young, 4-h biofilm to determine the MBEC and to also serve as the inoculum for an MIC determination. In this study, we used this device to examine antibiotic susceptibilities of both young (4-h) biofilms and mature (24-h) biofilms of P. aeruginosa PAO1. After the biofilm was established, it was exposed to increasing concentrations of gentamicin, ciprofloxacin, ceftazidime, and meropenem (obtained as analytical grade powders from Sigma-Aldrich Canada, Oakville, Ontario, Canada) in cation-adjusted Mueller-Hinton broth and the MIC and MBEC values were determined in quadruplicate as previously described (4). The inocula of the young and mature biofilms were standardized by using planktonic inocula of similar concentrations at each stage and comparing the fold increases in MIC values. The minimum bactericidal concentration (MBC) was determined by transferring 10 μl from each well of the challenge plate to a plate containing fresh media to facilitate the growth of all bacteria not eradicated by the antibiotic.
β-Lactamase expression of young and mature biofilms was measured using a nitrocefin colorimetric assay as previously described (6). Briefly, after 5 h and 24 h of antibiotic exposure, biofilm pegs exposed to 1 μg/ml, 16 μg/ml, and 512 μg/ml of ceftazidime or meropenem were broken off and immersed in sodium phosphate buffer. The supernatant from the corresponding well was spun down to isolate the released planktonic bacteria. The planktonic bacteria and the biofilm were lysed by freeze-thaw, and lysate was recovered after centrifugation. The nitrocefin assay to determine the β-lactamase concentration was performed by mixing 10 pM nitrocefin–50 mM sodium phosphate buffer (pH 7.0) and 20 μl of lysate or supernatant to give a total volume of 100 μl. The increase in absorbance was linear over a 30-min incubation at 37°C, and final absorbance was read at 482 nm, determining the amount of β-lactamase present. It should be noted that only the nitrocefin assay was used to quantitate β-lactamase expression and that it was not quantitated by high-performance liquid chromatography (HPLC). The protein concentration of the biofilm and planktonic bacteria was normalized using a bicinchoninic acid (BCA) assay as previously described (15).
The MIC results of gentamicin and ciprofloxacin treatment of P. aeruginosa PAO1 from a young biofilm were 4 μg/ml and 0.25 μg/ml, respectively, and both ceftazidime and meropenem had an MIC value of 2 μg/ml. These results were similar to MBECs and MICs determined by Ceri et al. (4), indicating that the methods used were reproducible (Fig. 1a). When the experiments were repeated using a mature biofilm, we observed results for gentamicin and ciprofloxacin similar to those determined for the young biofilm (Fig. 1b). The planktonic bacteria released from the mature biofilm were only slightly more phenotypically tolerant (less susceptible) to gentamicin and ciprofloxacin than the bacteria released from an early biofilm. We believe that these differences were likely due to small inoculum effects rather than resistance mechanisms, since the fold increases were similar to those seen with slightly higher inocula of planktonic bacteria (data not shown). These increases were smaller than we would expect if antibiotic sequestering or transportation were affected (5, 9). However, during ceftazidime exposure, the planktonic bacteria released from a mature biofilm were significantly more phenotypically tolerant than those from young biofilms, with MIC and MBC values of >2,048 μg/ml compared to MIC and MBC values of 2 μg/ml and 64 μg/ml, respectively, for young biofilm. The planktonic bacteria released from the mature biofilm were confirmed to be the same strain of P. aeruginosa as the initial inoculum through pulse-field gel electrophoresis (SpeI digests). The phenotypic tolerance was further assessed and determined to not be a stable change, since using these tolerant bacteria to form a new biofilm inoculum resulted in the same susceptibilities of bacteria released from the new young biofilm. These results agree with the observation that chronic P. aeruginosa biofilm infections become less susceptible to ceftazidime treatment over time (7). Since new, acute infections most likely involve strictly planktonic bacteria or a young biofilm, these results suggest that ceftazidime treatment would alleviate symptoms caused by these bacterial forms; however, chronic infections, especially P. aeruginosa infections, are primarily caused by mature biofilms. It has been shown that biofilms cycle through phases of releasing planktonic bacteria under ideal conditions in the absence of antibiotic treatments, resulting in recurring disease symptoms (10).
Fig 1.
Antibiotic susceptibilities of a young (4-h) biofilm (a) and a mature (24-h) biofilm (b), showing the log-transformed MBEC of the biofilm and the MIC and MBC values of the bacteria released from the biofilm following 24 h exposure to antibiotics.
We then compared the MIC and MBC of meropenem, a carbapenem with increased resistance to β-lactamases, for P. aeruginosa PAO1 (11). While being more resistant to β-lactamases, meropenem has also been shown to induce β-lactamase expression to a greater degree than ceftazidime (12). As shown in Fig. 1, the MBC and MIC of the bacteria released from the mature biofilm (Fig. 1b) were slightly greater than the MBC and MIC of bacteria released from the young biofilm (Fig. 1a); however, this was significantly less than the difference observed with ceftazidime, where the MIC was over 1,000-fold higher for the mature biofilm. These results suggest that there may be differences between the young and mature biofilms in the expression of factors involved in β-lactam resistance.
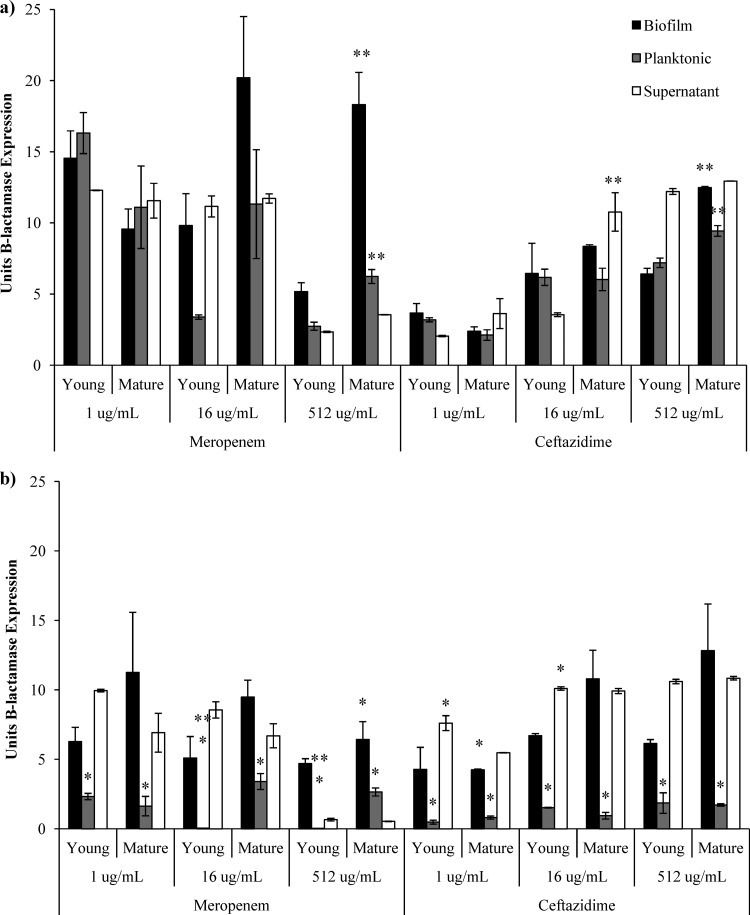
The lower phenotypic tolerance to meropenem shown by mature biofilms further suggests that β-lactamase production is crucial for tolerance of ceftazidime in P. aeruginosa biofilms. We found, as previously reported (6), that meropenem exposure stimulated significantly more β-lactamase production than ceftazidime exposure. After 5 h of exposure to high concentrations of both meropenem and ceftazidime, the young biofilms produced less β-lactamase than the mature biofilms (P = 0.03 and P = 0.005, respectively) (Fig. 2a); however, after 24 h of meropenem exposure, the mature biofilm had decreased its production of β-lactamase (P = 0.04) (Fig. 2b). There was decreased β-lactamase production by all planktonic bacteria exposed to ceftazidime or meropenem after 24 h (P < 0.05). It has been shown that ceftazidime stimulates β-lactamase production in the outer layers of P. aeruginosa biofilms and that this production peaks after 4 to 5 h of induction and is nonexistent after 10 h (1, 2). This was not the case with mature biofilms, which maintained β-lactamase production after 24 h of exposure to both high and low concentrations of ceftazidime. β-Lactamase production by released planktonic bacteria appears to drop dramatically after 24 h; however, the amount of β-lactamase activity in each sample maintained high levels and appeared lower after protein normalization due only to the high growth of bacteria.
Fig 2.
β-Lactamase activity from young (4-h) and mature (24-h) biofilms and levels of their released planktonic bacteria and the supernatant per indicated protein concentration after 5 h (a) and 24 h (b) of antibiotic exposure. All values represent the averages of the results of three replicate experiments, with error bars indicating standard errors. * indicates a significant difference between the results of 5-h and 24-h antibiotic exposure, and ** indicates a significant difference between the results determined for young and mature biofilms (P < 0.05).
From these results, it appears that mature biofilms are the major producers of β-lactamase after 5 h of high ceftazidime exposure. The concentration of antibiotics may be lowered by enzymatic hydrolysis, allowing the growth of planktonic bacteria. At the 24-h time point, the biofilm maintains β-lactamase production when exposed to ceftazidime; however, it is unable to maintain high levels of production against meropenem and no released planktonic bacteria are able to survive. This implies that mature biofilms are able to initially weather the storm of antibiotic exposure and then create an environment that allows their own survival and the survival of any planktonic bacteria released as well as of any other opportunistic bacteria, since the β-lactam breakdown benefits all microorganisms (3). This could give insight into the observation that repeated treatment with ceftazidime in cystic fibrosis patients decreases its ability to eliminate symptoms while not resulting in selection of hyperresistant P. aeruginosa mutants (15). In conclusion, these results suggest that ciprofloxacin or gentamicin should be beneficial to treat P. aeruginosa infection whenever such treatment is possible, since they are effective against all forms of P. aeruginosa growth. Ceftazidime use against these infections should be avoided and certainly limited to early acute infections.
ACKNOWLEDGMENTS
This work was supported by an Industrial Post-Graduate Scholarship from The National Sciences and Engineering Council as well as further financial support from Cangene Corporation.
Footnotes
Published ahead of print 9 July 2012
REFERENCES
- 1. Bagge N, et al. 2004. Dynamics and spatial distribution of β-lactamase expression in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 48:1168–1174 doi:10.1128/AAC.48.4.1168-1174.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bagge N, et al. 2004. Pseudomonas aeruginosa biofilms exposed to imipenem exhibit changes in global gene expression and β-lactamase and alginate production. Antimicrob. Agents Chemother. 48:1175–1187 doi:10.1128/AAC.48.4.1175-1187.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baquero F, Vicente MF, Perez-Diaz JC. 1985. β-Lactam coselection of sensitive and TEM-1 β-lactamase-producing subpopulations in heterogeneous Escherichia coli colonies. J. Antimicrob. Chemother. 15:151–157 [DOI] [PubMed] [Google Scholar]
- 4. Ceri H, Olson ME, Stremick C, Read RR, Morck D. 1999. The Calgary Biofilm Device: new technology for rapid determination of antibiotic susceptibilities. J. Clin. Microbiol. 37:1771–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. De Kievit TR, et al. 2001. Multidrug efflux pumps: expression patterns and contribution to antibiotic resistance in Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 45:1761–1770 doi:10.1128/AAC.45.6.1761-1770.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Galarneau A, Primeau M, Trudeau LE, Michnick SW. 2002. β-Lactamase protein fragment complementation assays as in vivo and in vitro sensors of protein-protein interactions. Nat. Biotech. 20:619–622 doi:10.1038/nbt0602-619 [DOI] [PubMed] [Google Scholar]
- 7. Gold R. 1987. Randomized trial of ceftazidime versus placebo in the management of acute respiratory exacerbations in patients with cystic fibrosis. J. Pediatr. 111:907–913 doi:10.1016/S0022-3476(87)80217-2 [DOI] [PubMed] [Google Scholar]
- 8. Griffith DP, Klein AS. 1983. Infection-induced urinary stones, p 210–227 In Roth RA, Finlayson B. (ed), Stones: clinical management of urolithiasis. Lippincott, Williams & Wilkins, New York, NY [Google Scholar]
- 9. Mah TF, et al. 2003. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 426:306–310 doi:10.1038/nature02122 [DOI] [PubMed] [Google Scholar]
- 10. Marrie TJ, Nelligan J, Costerton JW. 1982. A scanning and transmission electron microscopic study of an infected endocardial pacemaker lead. Circulation 66:1339–1341 doi:10.1161/01.CIR.66.6.1339 [DOI] [PubMed] [Google Scholar]
- 11. Masuda N, Ohya S. 1992. Cross-resistance to meropenem, cephems, and quinolones in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 36:1847–1851 doi:10.1128/AAC.36.9.1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nouda H, Harabe ET, Sumita Y, Okuda T, Fukasawa M. 1992. β-Lactamase stability and inhibitory activity of meropenem combined with a potent antibacterial activity. Chemotherapy 38:218–224 doi:10.1159/000239004 [DOI] [PubMed] [Google Scholar]
- 13. Stewart PS, Costerton JW. 2001. Antibiotic resistance of bacteria in biofilms. Lancet 358:135–138 doi:10.1016/S0140-6736(01)05321-1 [DOI] [PubMed] [Google Scholar]
- 14. VanDevanter DR, Van Dalfsen JM. 2005. How much do Pseudomonas biofilms contribute to symptoms of pulmonary exacerbation in cystic fibrosis? Pediatr. Pulmonol. 39:504–506 doi:10.1002/ppul.20220 [DOI] [PubMed] [Google Scholar]
- 15. Wu PJ, Shannon K, Phillips I. 1994. Effect of hyperproduction of TEM-1 beta-lactamase on in vitro susceptibility of Escherichia coli to beta-lactam antibiotics. Antimicrob. Agents Chemother. 38:494–498 doi:10.1128/AAC.38.3.494 [DOI] [PMC free article] [PubMed] [Google Scholar]