Abstract
An EZ::TN<R6Kγori/KAN-2>Tnp transposon insertion in an open reading frame of unknown function (ncr) in Acinetobacter baumannii resulted in an 8-fold increase in ciprofloxacin resistance (Cipr). Transposon insertions in an ncr mutant that reduced Cipr back to wild type mapped to three genes encoding subunits of the RecCBD exonuclease. The ncr mutation increased transcription of the recCBD genes, and overexpression of the recCBD genes in a wild-type background resulted in a 4-fold increase in Cipr.
TEXT
Acinetobacter baumannii is a Gram-negative bacterium that is capable of causing a wide variety of human infections (2, 3, 7, 10, 16). The ability to treat these infections has been complicated by the rapid increase in antibiotic resistance in this bacterium (8, 12, 13, 15, 17, 18). To better define the mechanisms that contribute to antibiotic resistance, a transposon insertion library, created in A. baumannii strain M2 with the EZ::TN<R6Kγori/KAN-2>Tnp transposon (Epicentre, Madison WI), was used to identify mutants with increased resistance to ciprofloxacin. Our original goal was to identify insertions that increased the expression of efflux systems resulting in multiple antibiotic resistance. One mutant, designated AB-4B, was obtained that exhibited an 8-fold increase in ciprofloxacin resistance (Cipr): 2 μg/ml versus 0.25 μg/ml for the wild-type M2 parent (Table 1). The mutation in AB-4B also resulted in a 4-fold increase in the levels of resistance to ofloxacin and gatifloxacin (data not shown). However, the mutation in mutant AB-4B did not significantly alter the levels of resistance to chloramphenicol, gentamicin, tigecycline, rifampin, or ampicillin (data not shown). This suggested that the fluoroquinolone resistance was not due to increased expression of a multidrug efflux system. The EZ::TN<R6Kγori/KAN-2>Tnp insertion and flanking A. baumanii DNA were isolated by rescue cloning after digestion of chromosomal DNA with XbaI, followed by religation and transformation into Escherichia coli CC118 to identify plasmids containing chromosomal DNA along with the R6K plasmid origin present in the transposon. The site of insertion was mapped to an open reading frame (ORF) designated A1S_0815, based on the genome sequence of ATCC 17978 (19), and this gene is present in all A. baumannii isolates sequenced to date. In ATCC 17978, this ORF encodes a protein of 132 amino acids. However, in other A. baumannii isolates, this protein was 186 amino acids. Further analysis of the sequence surrounding the A1S_0815 gene indicated a frameshift error that likely accounted for the smaller size of the protein. In the AB-4B mutant, the EZ::TN<R6Kγori/KAN-2>Tnp transposon inserted at a position corresponding to amino acid 50 of the 186-amino-acid protein. The protein encoded by A1S_0815 had a conserved domain (DUF177 superfamily) that suggested a possible metal binding function. The protein encoded by A1S_0815 was similar to those present in other bacteria, including Psychrobacter arcticus (37% identity, 59% similarity; YP 263815.1), Azotobacter vinelandii (37% identity, 54% similarity; YP002798678.1), Pseudomonas aeruginosa (31% identity, 53% similarity), and E. coli YcdE (27% identity, 44% similarity). Given the role for this gene in Cipr, it was renamed ncr (novel ciprofloxacin resistance). In A. baumannii and the bacteria listed above, the A1S_0815-like gene was encoded immediately upstream of a putative rpmF (A1S_0816) gene encoding the 50S ribosomal protein L32. Reverse transcription-PCR (RT-PCR) analysis indicated that both genes formed an operon (data not shown).
Table 1.
Ciprofloxacin resistance levels of A. baumannii strains in this study
| Strain | Ciprofloxacin MIC (μg/ml) |
|---|---|
| M2 | 0.25 |
| AB-4B ncr::EZ::TN<R6Kγori/KAN-2>Tnp | 2 |
| AB-0815 ncr::Smr | 2 |
| AB-4B/pWH1266 | 2 |
| AB-4B/pWH1266-ncr | 1 |
| AB-4B::pKNG101-ncr | 0.25 |
To verify that the ncr::EZ::TN<R6Kγori/KAN-2>Tnp insertion was responsible for the increased Cipr in strain AB-4B, a null allele in the ncr gene of the wild-type M2 strain was recreated by the insertion of a suicide plasmid containing an internal region of the ncr gene generated by PCR using the primers 5′-GTGATCTAGATGCTCGTATTGCTCGTGAAG-3′and 5′-ACATGTCGACTGATGTTTATGTTCACAAGC-3′. These primers contained restriction sites for XbaI and SalI that were used to clone the fragment into pKNG101 (11). The plasmid was moved into A. baumannii by conjugation with E. coli SM10 λpir. Exconjugants were verified to contain the ncr::pKNG101 (Smr) disruption by Southern blot analysis, and one strain, designated AB-0815, was used for further analysis. AB-0815 ncr::Smr exhibited a similar phenotype to strain AB-4B, with an 8-fold increase in Cipr (Table 1). Both the ncr::Smr mutant and the original ncr::EZ::TN<R6Kγori/KAN-2>Tnp mutant exhibited a slow-growth phenotype and formed smaller colonies on agar plates. To further verify the role of the ncr mutation in resistance, we amplified the wild-type ncr gene by PCR using the primers 5′-TGCACCACGTCAGGTAAAAGAG-3′ and 5′-GCGCGGATCCAAATGGATCAGAACAAATTC-3′ and cloned this insert as a BamHI fragment into the A. baumannii/E. coli shuttle plasmid pWH1266 (9). In this plasmid (pWH1266-ncr), transcription of the ncr gene is driven by its native promoter. When pWH1266-ncr was introduced into the AB-4B ncr::EZ::TN<R6Kγori/KAN-2>Tnp mutant, the levels of Cipr were decreased 2-fold to 1 μg/ml (Table 1), a level intermediate between those of the wild type and the ncr mutant. The basis for the partial complementation is unclear. However, when the same ncr-containing fragment was cloned into the suicide vector pKNG101 and integrated in single copy in AB-4B, the levels of Cipr were reduced to 0.25 μg/ml.
As mentioned above, the genomic organizations of the A. baumannii ncr and rpmF genes were identical to that seen in E. coli, where the ncr homolog is designated ycdE. Null alleles of the E. coli ycdE and rpmF genes were obtained from the Keio collection (1) to determine if mutations in either of these genes resulted in a ciprofloxacin-resistant phenotype. Neither mutation had a significant effect on Cipr, with MICs for the parent strain BW25113 of 0.5 μg/ml (data not shown).
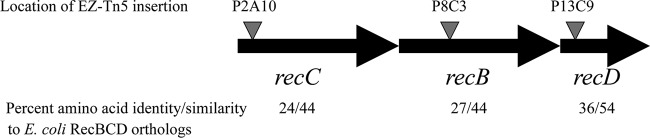
To understand the molecular basis for the increased ciprofloxacin resistance in the ncr::Smr mutant, we utilized EZ::TN<KAN-2>Tnp transposon mutagenesis to generate mutations in AB-0815 that reversed the high-level Cipr. Three insertions with this phenotype were mapped to a contiguous set of open reading frames that encoded products highly similar to the recCBD genes encoding the gamma, beta, and alpha subunits of the exonuclease V complex (Fig. 1) (4). The A. baumannii proteins exhibited the following amino acid identities and similarities, respectively, compared to the E. coli proteins: RecC, 27% and 44%; RecB, 24% and 44%; and RecD, 36% and 54%. The insertion in strain P13C9 disrupted recD, encoding the alpha subunit. The insertion in mutant P8C3 disrupted recB, encoding the beta subunit, and the insertion in mutant P2A10 disrupted recC, encoding the gamma subunit (Fig. 1). Each of these insertions resulted in a reduction in Cipr in the ncr::Smr background from 2 μg/ml down to 0.25 to 0.125 μg/ml (Table 2). To verify that the above insertions in the recCBD locus were responsible for the decreased Cipr in each of the mutants, the wild-type recCBD genes were amplified by PCR using the primers 5′-CGTCGGATCCGTCAACGCATCCATTACAGG-3′ and 5′-TAGCGGATCCTATCTCGAATCCATGTAAGC-3′ and cloned into pWH1266. When the ncr::Smr mutant containing each of the EZ-Tn5 insertions in the recCBD locus was transformed with pWH1266 plus recCBD, the levels of Cipr were increased back to that seen in the original mutant (2 μg/ml), indicating that loss of RecCBD function was responsible for the ciprofloxacin sensitivity (Table 2).
Fig 1.
Transposon insertions that reverse ciprofloxacin resistance in AB-4B. The location of EZ::TN transposon insertions that reverse the high-level ciprofloxacin resistance is shown. The organization of the recC, recB, and recD genes is shown, and the percentages of amino acid identity and similarity to the corresponding E. coli gene products are shown below each gene.
Table 2.
Effect of recCBD mutations on ciprofloxacin resistance
| Strain | Genotype | Ciprofloxacin MIC (μg/ml) |
|---|---|---|
| AB-0815/pWH1266 | ncr::Smr | 2 |
| P2A10/pWH1266 | ncr::Smr recB::EZ::TN<KAN-2>Tnp | 0.125 |
| P8C3/pWH1266 | ncr::Smr recC::EZ::TN<KAN-2>Tnp | 0.125 |
| P13C9/pWH1266 | ncr::Smr recD::EZ::TN<KAN-2>Tnp | 0.25 |
| AB-0815/pWH-recCBD | ncr::Smr | 2 |
| P2A10/pWH-recCBD | ncr::Smr recB::EZ::TN<KAN-2>Tnp | 2 |
| P8C3/pWH-recCBD | ncr::Smr recC::EZ::TN<KAN-2>Tnp | 2 |
| P13C9/pWH-recCBD | ncr::Smr recD::EZ::TN<KAN-2>Tnp | 2 |
The above data indicated that the ncr mutation required a functional recCBD locus to mediate increased Cipr. This predicted that the Ncr gene product might be involved in negative regulation of the recCBD genes and that, in turn, their overexpression was responsible for ciprofloxacin resistance. To investigate this possibility, transcription of the recCBD genes was monitored by cloning a 510-bp fragment extending 458 bp upstream and 52 bp downstream of the ATG start codon for RecC into the transcriptional promoter probe plasmid pQF50 (5) to create a recC-lacZ transcriptional fusion. This plasmid is unable to replicate in A. baumannii, but it can integrate into the chromosome by homologous recombination at the recCBD region, creating a lacZ fusion in single copy. The expression of recC-lacZ was measured at 10.1 ± 0.5 Miller units in the wild-type M2 background and 25.0 ± 0.4 Miller units in the AB-4B background, indicating that transcription of the recCBD operon was increased 2.5-fold by the ncr mutation (Fig. 2A). To independently confirm the increase in recCDB transcription in the ncr mutant, total RNA was prepared from both the wild type and the ncr mutant and semiquantitative RT-PCR was used to examine recCDB transcript levels in both strains. Using this analysis, a similar 2-fold increase in recCDB transcript levels was observed in the ncr mutant (Fig. 2B).
Fig 2.
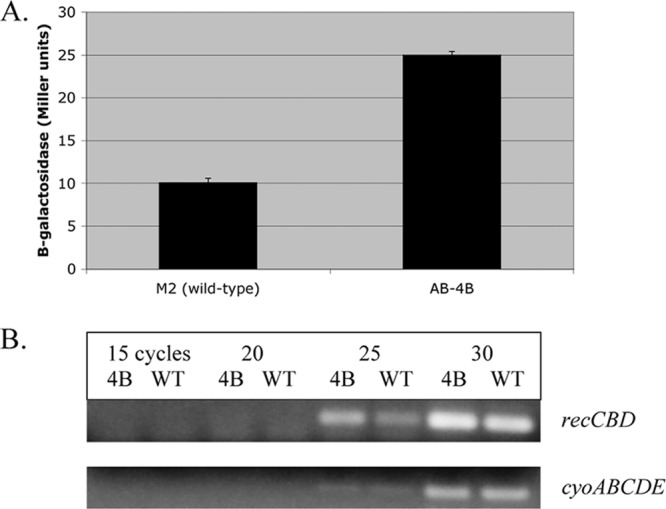
Effect of the ncr mutation on recCBD transcription. In panel A, the expression of a recC-lacZ fusion in both the M2 (wild-type) and AB-4B (ncr::EZ::TN<R6Kγori/KAN-2>Tnp) backgrounds is shown. In panel B, the expression of recCBD in wild-type M2 and AB-4B was monitored by semiquantitative RT-PCR. As an internal control, expression of the cyoABCDE operon was also examined from the same RNA samples. Samples were analyzed at 15, 20, 25, and 30 cycles. The absence of contaminating DNA from both samples was confirmed by the inability to generate PCR products in the absence of cDNA synthesis. The primers used for cDNA synthesis and subsequent RT-PCR for recCBD were 5′-ATTAAATGTAGCGTGTTCAG-3′, 5′-GAGCATCCTGAGCGCCAGAAG-3′, and 5′-CAATGTATTGCCCTAAACGGC-3′. For the cyo operon, the primers were 5′-ATGCGGATCCCAAGAGAAGATTTTCACACC-3′, 5′-TGGTGATTCCTTCATTCATCATG-3′ and 5′-ACTAAATGCTCGATTTGGTGC-3′.
To determine if overexpression of the recCBD genes was sufficient to confer increased Cipr, the plasmid pWH-recCBD was introduced into the wild-type M2 strain. M2 cells containing only the pWH1266 vector exhibited a MIC of 0.25 μg/ml. However, the presence of pWH-recCBD increased ciprofloxacin resistance 4-fold, with a MIC of 1 μg/ml.
In this study, two separate null alleles in the ncr gene conferred the same phenotype—an 8-fold increase in Cipr. This phenotype was exclusively due to loss of Ncr function, as the cloned ncr gene on a plasmid could complement these phenotypes, demonstrating that loss of ncr function and not polar effects on the downstream rpmF gene was responsible for Cipr. Analysis of the Ncr protein did not reveal an obvious role in Cipr, and although a number of Ncr orthologs are present in other bacteria, their function is unknown. The Ncr protein is annotated as having a metal binding domain (CO1399), and the lack of obvious signal sequences or transmembrane regions suggests it is localized to the cytoplasm. It was hypothesized that the loss of Ncr function altered the activity or expression of one or more gene products that then directly mediated Cipr. A genetic analysis was then conducted to identify mutations that reversed the high-level Cipr in the ncr mutant background. This revealed a key role for the recCBD genes in mediating the Cipr. Moreover, the transcription of the recCBD genes was shown to be increased 2.5-fold in the ncr mutant. Taken together, this strongly suggested that the overexpression of the recCBD genes in the ncr background is responsible for the increased Cipr. Consistent with this, overexpression of the recCBD genes on a multicopy plasmid was sufficient to increase the levels of Cipr 4-fold. Although the Ncr protein acts as a negative regulator of the recCBD genes, the lack of clear homology to DNA binding proteins suggests this regulation may be indirect.
A relationship between the RecCBD system and Cipr has been previously described by Gomez and Neyfakh, where loss of recD decreased Cipr in Acinetobacter baylyi (6). Given that RecCBD functions in both DNA recombination and repair, the increased sensitivity to ciprofloxacin is likely due to the reduced ability to repair DNA damage mediated by the inhibition of DNA gyrase and topoisomerase. However, the ability of the recCBD genes to increase Cipr when overexpressed appears to be a novel mechanism. Studies by Lopez et al. have demonstrated that ciprofloxacin stimulates recombination in a RecCBD-dependent manner in E. coli (14). Therefore, it is possible that increased expression of RecCBD in A. baumannii allows for a greater capacity to repair the DNA damage that results from the inhibition of DNA gyrase and topoisomerase.
ACKNOWLEDGMENTS
This work was supported by grant R01AI072219-01A1 from the National Institutes of Health. P.N.R. is supported by a Research Career Scientist award and a Merit Review from the Department of Veterans Affairs. R.A.B. is supported by VISN 10 GRECC, a Merit Review Award by the VHA, and NIH RO1-AI072219-05.
Footnotes
Published ahead of print 25 June 2012
REFERENCES
- 1. Baba T, et al. 2006. Construction of Escherichia coli K-12 in-frame single gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2:2006.0008 doi:10.1038/msb4100050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bergogne-Bérézin E, Towner KJ. 1996. Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin. Microbiol. Rev. 9:148–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dijkshoorn L, Nemec A, Seifert H. 2007. An increasing threat in hospitals: multidrug resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 5:939–951 [DOI] [PubMed] [Google Scholar]
- 4. Dillingham MS, Kowalczykowski SC. 2008. RecBCD enzyme and the repair of double stranded DNA breaks. Microbiol. Mol. Biol. Rev. 72:642–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Farinha MA, Kropinski AM. 1990. Construction of broad host range plasmid vectors for easy visible selection and analysis of promoters. J. Bacteriol. 172:3496–3499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gomez MJ, Neyfakh AA. 2006. Genes involved in intrinsic antibiotic resistance in Acinetobacter baylyi. Antimicrob. Agents Chemother. 50:3562–3567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gootz TD, Marra A. 2008. Acinetobacter baumannii: an emerging multidrug resistant threat. Exp. Rev. Anti Infect. Ther. 6:309–325 [DOI] [PubMed] [Google Scholar]
- 8. Hujer KM, et al. 2006. Analysis of antibiotic resistance genes in multidrug resistant Acinetobacter sp. isolates from military and civilian patients treated at the Walter Reed Army Medical Center. Antimicrob. Agents Chemother. 50:4114–4123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hunger M, Schmucker R, Kishan V, Hillen W. 1990. Analysis and nucleotide sequence of an origin of DNA replication in Acinetobacter calcoaceticus and its use for Escherichia coli shuttle plasmids. Gene 87:45–51 [DOI] [PubMed] [Google Scholar]
- 10. Joly-Guillou ML. 2005. Clinical impact and pathogenicity of Acinetobacter. Clin. Microbiol. Infect. 11:868–873 [DOI] [PubMed] [Google Scholar]
- 11. Kaniga K, Delor I, Cornelis GR. 1991. A wide host range suicide vector for improving reverse genetics in gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene 109:137–141 [DOI] [PubMed] [Google Scholar]
- 12. Keen EF, et al. 2010. Changes in the incidence of multidrug resistant and extensively drug resistant organisms isolated in a military care center. Infect. Control Hosp. Epidemiol. 31:728–732 [DOI] [PubMed] [Google Scholar]
- 13. Livermore DM. 2003. The threat from the pink corner. Ann. Med. 35:226–234 [DOI] [PubMed] [Google Scholar]
- 14. Lopez E, Elez M, Matic I, Blazquez J. 2007. Antibiotic mediated recombination: ciprofloxacin stimulates SOS independent recombination of divergent sequences in Escherichia coli. Mol. Microbiol. 64:83–93 [DOI] [PubMed] [Google Scholar]
- 15. Murray CK, Hospenthal DR. 2005. Treatment of multidrug resistant Acinetobacter. Curr. Opin. Infect. Dis. 18:502–506 [DOI] [PubMed] [Google Scholar]
- 16. Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen Clin. Microbiol. Rev. 21:538–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Perez F, et al. 2007. Global challenge of multidrug resistant Acinetobacter baumannii. Antimicrob. Agents Chemother. 51:3471–3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rice LB. 2006. Challenges in identifying new antimicrobial agents effective for treating infections with Acinetobacter baumannii and Pseudomonas aeruginosa. Clin. Infect. Dis. 43(Suppl 2):S100–S105 [DOI] [PubMed] [Google Scholar]
- 19. Smith MG, et al. 2007. New insights into Acinetobacter baumannii pathogenesis revealed by high-density pyrosequencing and transposon mutagenesis. Genes Dev. 21:601–614 [DOI] [PMC free article] [PubMed] [Google Scholar]