Abstract
Prolonged infusion of meropenem has been suggested in studies with population pharmacokinetic modeling but has not been tested in neonates. We compared the steady-state pharmacokinetics (PK) of meropenem given as a short (30-min) or prolonged (4-h) infusion to very-low-birth-weight (gestational age, <32 weeks; birth weight, <1,200 g) neonates to define the appropriate dosing regimen for a phase 3 efficacy study. Short (n = 9) or prolonged (n = 10) infusions of meropenem were given at a dose of 20 mg/kg every 12 h. Immediately before and 0.5, 1.5, 4, 8, and 12 h after the 4th to 7th doses of meropenem, blood samples were collected. Meropenem concentrations were measured by ultrahigh-performance liquid chromatography. PK analysis was performed with WinNonlin software, and modeling was performed with NONMEM software. A short infusion resulted in a higher mean drug concentration in serum (Cmax) than a prolonged infusion (89 versus 54 mg/liter). In all but two patients in the prolonged-infusion group, the free serum drug concentration was above the MIC (2 mg/liter) 100% of the time. Meropenem clearance (CL) was not influenced by postnatal or postmenstrual age. In population PK analysis, a one-compartment model provided the best fit and the steady-state distribution volume (Vss) was scaled with body weight and CL with a published renal maturation function. The covariates serum creatinine and postnatal and gestational ages did not improve the model fit. The final parameter estimates were a Vss of 0.301 liter/kg and a CL of 0.061 liter/h/kg. Meropenem infusions of 30 min are acceptable as they balance a reasonably high Cmax with convenience of dosing. In very-low-birth-weight neonates, no dosing adjustment is needed over the first month of life.
INTRODUCTION
Patients in the neonatal intensive care unit (NICU), especially premature newborns with immature organ systems, frequently suffer nosocomial infections caused by microorganisms resistant to narrow-spectrum antibiotics like ampicillin and gentamicin and thus require the introduction of agents with a wider spectrum of activity (7, 14, 29). Meropenem is active against a wide variety of Gram-negative and Gram-positive microorganisms and offers good penetration of body fluids and tissues (1, 9). It has been shown to be well tolerated by children and neonates (6), including preterm babies (10, 18), with the advantage of allowing monotherapy instead of combined therapy (37).
The pharmacokinetic (PK) characteristics of meropenem for children <3 months old have been described in four studies (6, 28, 35, 36) indicating that for babies with a gestational age (GA) of <32 weeks, 20 mg/kg every 12 h during the first 14 days of life and every 8 h thereafter ensures an adequate serum drug concentration profile.
Meropenem is given mostly via a 30-min infusion, as some data indicate rapid degradation after reconstitution (4, 24). Dose recommendations from two pediatric studies using Monte Carlo simulation have emphasized that a 4-h infusion may be needed for microorganisms with increased MICs, more specifically, for Pseudomonas aeruginosa (6, 35). A prolonged-infusion strategy, however, has not been tested in neonates, although some data suggest that, apart from patient-associated variability, extremely small infusion volumes may significantly affect the drug amount actually delivered (27).
We aimed to compare the steady-state PK and safety of meropenem given via short or prolonged infusion to neonates with a GA of <32 weeks to define the most appropriate dosing regimen for a phase 3 efficacy study of neonatal late-onset sepsis (LOS) (20).
MATERIALS AND METHODS
Study design.
A prospective open-label study was carried out from 7 April 2010 to 1 February 2011 in the NICUs of Tartu University Hospital, Tartu, Estonia, and Tallinn Children's Hospital, Tallinn, Estonia. Neonates requiring meropenem treatment for sepsis, pneumonia, or necrotizing enterocolitis due to a pathogen with proven or highly suspected resistance or for clinical deterioration on empirical antibiotics were eligible for this study if they had (i) a GA of ≤32 weeks and a birth weight (BW) of <1,500 g, (ii) a postnatal age (PNA) of ≤56 days, (iii) written consent signed by a parent or guardian, and (iv) an arterial or central venous cannula settled on clinical indications. Infants with major uncorrected congenital malformations or expected to die within 24 h were excluded.
Study drug administration.
Meropenem (AstraZeneca Limited, Macclesfield, United Kingdom) was reconstituted in normal saline to a final concentration of 10 mg/ml immediately prior to administration. Each dose of 20 mg/kg was given intravenously every 12 h to the first 9 neonates over 30 min (short infusion, group 1) and to the next 10 neonates as a 4-h infusion (prolonged infusion, group 2). In the latter group, the first dose was given over 30 min and after informed consent (IC) was obtained, at least two prolonged infusions were administered prior to the study dose to ensure a steady state. After PK sampling, meropenem administration was changed back to a 30-min infusion.
Sampling and sample handling.
Immediately before and 0.5, 1.5, 4, 8, and 12 h after the 4th to 7th doses of meropenem (study dose), 200 to 300 μl of blood was drawn from an arterial cannula into dry vials. Blood was centrifuged immediately, and serum was stored at −20°C for a maximum of 24 h and then transferred to −70°C until analyzed within 7 months. Five infusion lines collected at the end of the 4-h infusion were stored for meropenem concentration measurement as described for other samples.
Urine samples were collected at 4-h intervals within 12 h after administration of the meropenem study dose. The quantity of urine collected was measured, and possible losses were estimated by weighing the diapers. The samples were stored as described above for serum samples.
Meropenem assay.
Samples were melted at room temperature, and 50-μl serum were transferred into 250-μl PCR tubes. For serum sample extraction, 50 μl of methanol (containing ertapenem at a concentration of 10 μg/ml as an internal standard [IS]) was added. After vigorous shaking with a Vortex mixer for 1 min, the sample was centrifuged at 8,000 rpm (3,500 × g) for 10 min and the supernatant (approximately 75 μl) was separated, filtered through 0.22-μm Millex-GV polyvinylidene difluoride filters, and transferred into a high-performance liquid chromatography autosampler vial.
Urine samples were melted at room temperature and diluted with ultrapure water (1/9 or more). A 3-μl volume of the prepared sample was injected into the Agilent 1290 Infinity UHPLC system. Gradient elution with methanol and 0.1% formic acid (pH 2.6) at a flow rate of 0.3 ml/min was used for chromatographic separation. Samples were chromatographed using a Waters Acquity UPLC ethylene bridged hybrid (BEH) C18 column (2.1 by 100 mm, 1.7 μm) equipped with a Waters VanGuard Acquity UPLC BEH C18 guard column (2.1 by 5 mm, 1.7 μm). Samples were analyzed with a diode array detector at 306 nm, and an electrospray interface Varian 320-MS triple-quadrupole liquid chromatography-mass spectrometry apparatus was used to analyze samples in the single-reaction monitoring mode. Transitions of the parent ion with m/z 384 [M + 1] to daughter ions with m/z 254, 298, and 340 were used for meropenem quantification and qualification.
The calibration curves were linear from 0.1 to 200 μg/ml in serum and from 1 to 250 μg/ml in urine. The limit of detection (LOD) and limit of quantification (LOQ as 10 times the standard deviation) were estimated from five replicate analyses of spiked blank serum samples. The LOQ for serum samples was 0.1 μg/ml, and the LOD was 0.01 μg/ml. The LOQ for urine samples, as the lowest concentration of calibration samples, was 1 μg/ml with accuracy and precision of 100% ± 3% and a coefficient of variation (CV) of <2%.
Method within-day accuracy ranged from 100% ± 2% to 100% ± 8% for the serum calibration curve and from 100% ± 4% to 100% ± 6% (as relative standard deviation) for the urine calibration curve. The between-day precision was <5% for serum samples and <4% for urine samples. Sample preparation recovery was 84% ± 7% (CV, 8%) over the calibration curve in duplicate, which is the same as previously described (17).
Patient monitoring.
All infants were monitored for adverse events. Laboratory and vital parameters and positive microbiological cultures were monitored for at least 7 days after the end of meropenem treatment. Serum creatinine was measured by a Jaffe kinetic method. Electroencephalography (EEG) of all but four patients was performed at least once during meropenem therapy. Doppler echocardiography was performed when clinically indicated. Concomitant medications were recorded from 7 days before to 12 h after study dose infusion.
Statistical and PK analyses.
Statistical analysis was performed with the R version 2.12.0 software. The means of the groups were compared by Mann-Whitney test. Spearman rank correlation was used to test the relationships between variables. In calculations of the fraction of time in the dose interval when the plasma drug level exceeds the MIC (fT>MIC), the EUCAST susceptibility breakpoint (≤2 mg/liter) was used (13).
PK analysis was performed with WinNonlin software (version 6.1; Pharsight Corporation) by applying a noncompartmental model that assumed the use of a 30-min or 4-h intravenous infusion, as appropriate. Creatinine clearance (CLCR) was calculated directly from the urine and serum creatinine concentrations at 12 h postdose.
A population PK model was developed by using the serum drug concentration data. One- and two-compartment models were considered, and volume parameters were scaled with linear body weight. An investigation to derive a maturation and weight function from the data as recommended by Tod et al. (34) was performed, compared with scaling of clearance with the size and maturation function proposed by Rhodin et al. (25). The influence of serum creatinine on clearance scaled by expected serum creatinine as described by Ceriotti et al. (8) was also investigated. Nonlinear mixed-effect modeling was performed in NONMEM version 7.1 (3).
The protocol was approved by the Ethics Committee of the University of Tartu. This study was registered at the EU Clinical Trials Register under number 2009-017823-24.
RESULTS
Study population and clinical observations.
Altogether, 21 families were approached and IC was obtained from the parents of 20 patients. One infant was withdrawn for lack of an arterial or central venous cannula, leaving 19 patients to constitute the study population. As presented in Table 1, the baseline demographics and concomitant use of potentially nephrotoxic drugs were similar in both groups, except for a lower median first-minute Apgar score in group 2 than in group 1. Meropenem was given to 16 patients for sepsis and to 3 patients for pneumonia with no differences in the distribution of diagnoses between groups. Eleven patients (58%) had altogether 13 positive blood cultures (coagulase-negative staphylococci, n = 5; Enterobacteriaceae, n = 4; Enterococcus faecalis, n = 2; Staphylococcus aureus, n = 1; Pseudomonas aeruginosa, n = 1). Most of the patients were severely ill, with 95% requiring respiratory support and 42% requiring vasoactive treatment (Table 1). One neonate in each group died, each more than 7 days after the completion of meropenem therapy, for reasons not related to the study drug. No side effects, EEG changes, or drug-related laboratory abnormalities were registered. The mean meropenem concentration in the infusion lines at the end of the 4-h infusion was 11.68 ± 0.5 mg/ml.
Table 1.
Demographic data of the study patients in the short- and prolonged-infusion groupsa
| Parameter | Group 1 (n = 9) | Group 2 (n = 10) | P value |
|---|---|---|---|
| BW (g) | 895.6 (239.4) | 842.4(102.2) | 0.624 |
| Current body wt (g) | 984.6 (291.6) | 969.5 (102.9) | 0.967 |
| GA (wk) | 26.9 (1.4) | 25.8 (25.8) | 0.112 |
| PNA at PK sampling (days) | 15.6 (8.6) | 20.5 (6.6) | 0.191 |
| Male sex (no. of subjects) | 6 | 6 | |
| First-min Apgar score | 2.9 (2.2) | 5.2 (1.9) | 0.041 |
| AVLb/CPAPc (no. of subjects) | 5/3 | 8/2 | |
| Vasoactive support (no. of subjects) | 5 | 3 | |
| Serum creatinine at enrollment (μmol/liter) | 51.4 (21.2) | 44.8 (24.0) | 0.278 |
| Urine creatinine during PK sampling (μmol/liter) | 816.7 (338.8) | 670.8 (272.5) | 0.258 |
| Positive blood culture (no. of subjects) | 4 | 7 | |
| Duration of meropenem therapy (days) | 11 (1.9) | 9.2 (3.5) | 0.067 |
| Concomitant vancomycin, ibuprofen (no. of subjects) | 4 | 3 |
Groups: 1, short infusion; 2, long infusion. Data are presented as means (SD) if not stated otherwise.
AVL, artificial ventilation of lungs.
CPAP, continuous positive airway pressure.
Noncompartmental PK analysis.
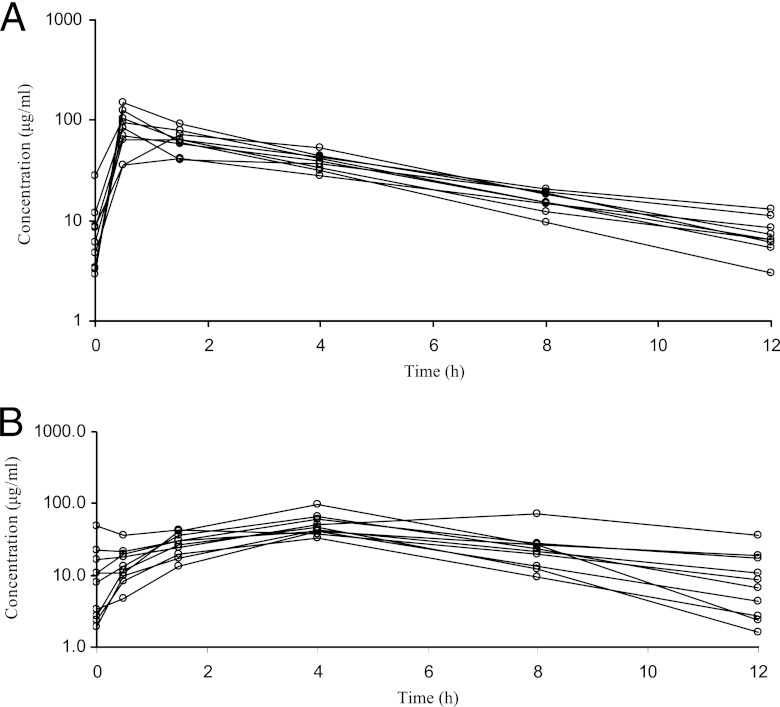
Except for a higher Cmax in the short-infusion group and a higher time to drug Cmax in serum (Tmax) in the prolonged-infusion group, all of the PK parameters of the two groups were similar (Table 2). Large interpatient variability was seen, especially in Cmax. The mean concentration-time curves by study group are presented in Fig. 1. All of the PK parameters of subjects with a PNA of <15 days (n = 6) and a PNA of ≥15 days (n = 13) were similar in both groups (data not shown). All of the patients in the short-infusion group and 8/10 in the long-infusion group achieved an fT>MIC of 100% for an MIC of 2 mg/liter. The fT >6.2× MIC (value required to prevent resistance development in P. aeruginosa [32]) was 80.2% (95% CI, 70.8 to 89.6) in the short-infusion group and 81.9% (95% CI, 69.6 to 94.4) in the prolonged-infusion group. No significant correlation between meropenem clearance (CLssM) and postmenstrual age or PNA was observed.
Table 2.
Results of noncompartmental analysis of PK parameters in the short- and prolonged-infusion groupsa
| Parameterb | Group 1 | Group 2 | P value |
|---|---|---|---|
| Actual meropenem dose (mg/kg) | 18.9 (5) | 18.3 (2.4) | 0,650 |
| t1/2 (h) | 3.4 (0.9) | 3.3 (1.7) | 0.436 |
| Cmax (mg/liter) | 89.3 (32.7) | 54.5 (19.0) | 0.005 |
| Tmax (h) | 0.7 (0.4) | 4.0 (1.9) | 0.002 |
| Cmin (mg/liter) | 6.5 (3.7) | 7.2 (6.1) | 0.780 |
| CLssM (ml/h/kg) | 52.4 (15.5) | 62.7 (31.6) | 0.842 |
| Vss (ml/kg) | 270.6 (83.3) | 342.9 (174.3) | 0.447 |
| AUClastc (h · μg/ml) | 369.2 (66.1) | 338.6 (121.6) | 0.497 |
| fT>MIC (%)d (95% CI) | 100 (100–100) | 99.9 (99.6–100) | 0.193 |
Groups: 1, short infusion; 2, prolonged infusion. Data are presented as means (SD) if not stated otherwise.
t½, half-life.
AUClast, area under the concentration-time curve from time zero to the last point of the curve.
Calculated for the EUCAST MIC susceptibility breakpoint of 2 mg/liter for P. aeruginosa and Enterobacteriaceae.
Fig 1.
Meropenem concentration-time curves of groups 1 (A) and 2 (B). Each line represents an individual patient. Dots indicate PK sampling times.
The mean (standard deviation [SD]) CLCR values in the 30-min and 4-h infusion groups were 18.7 (7.8) and 21.4 (9.2) ml/min/1.73 m2, respectively. The mean (SD) renal recovery of the meropenem dose was 86.4 (32.8)% for the four patients in the short-infusion group and 57.8% (19.3) for the five patients in the prolonged-infusion group, 100% of whose excreted urine was collected.
Population modeling.
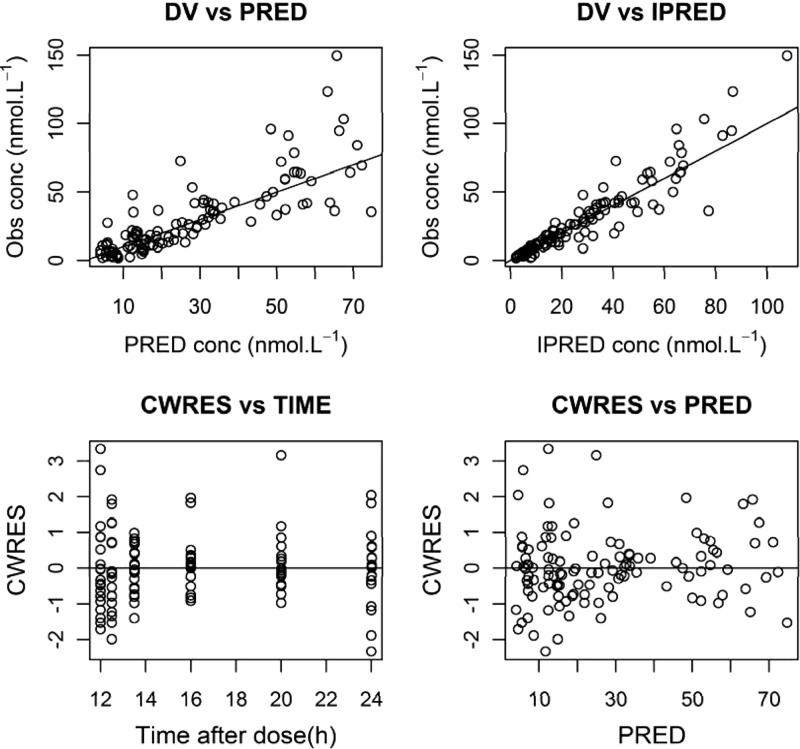
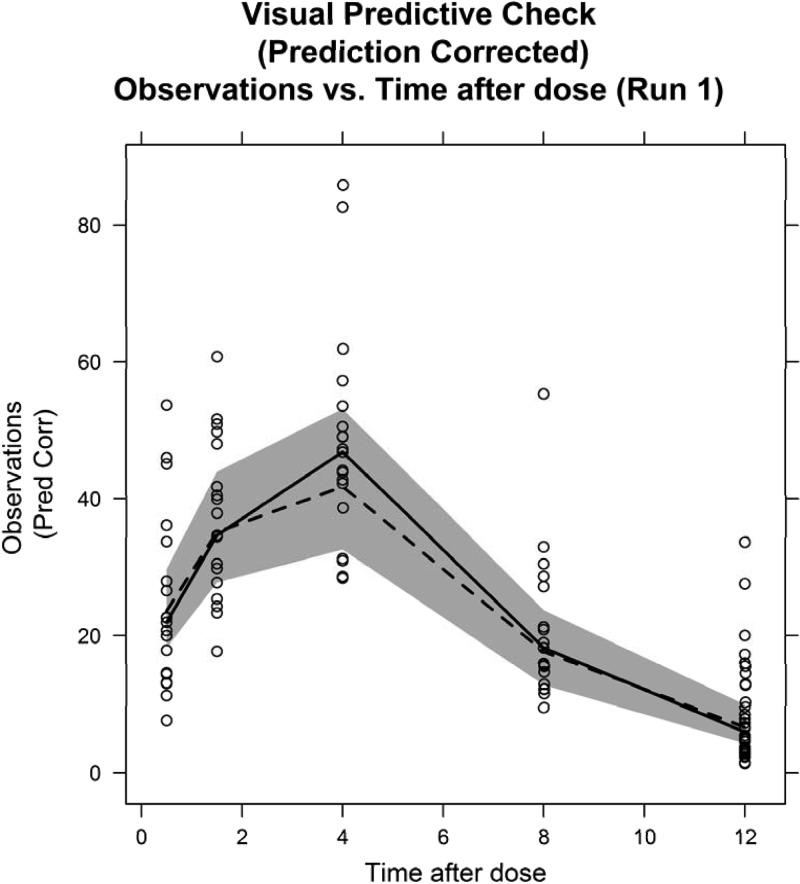
There was no significant difference in the NONMEM objective function value between one- and two-compartment models, so a one-compartment model was used. Maturation function parameters could not be estimated, and thus, the fixed Rhodin model (25) was used, and serum creatinine corrected for PNA did not significantly improve the fit. Goodness-of-fit plots and a visual predictive check of the final model are shown in Fig. 2 and 3.
Fig 2.
Basic goodness-of-fit plots from the final population model. VD, dependent variable; PRED, population prediction; IPRED, individual prediction; CWRES, conditional weighted residuals.
Fig 3.
Prediction-corrected visual predictive check of the final population model. The solid line shows the median prediction-corrected (Pred Corr) data, and the gray-shaded area represents the 95% simulated prediction interval from 1,000 simulations. The broken line represents the median of the original data.
DISCUSSION
To the best of our knowledge, this is the first study comparing the steady-state PK of meropenem given via short or prolonged infusion to seriously ill premature neonates. Despite some differences, in general, the PK parameters were similar; no difference in the fT>MIC or in the minimum drug concentration in serum (Cmin) was found with different infusion durations. Furthermore, the PK parameters of meropenem among those studied with a PNA of less than or more than 2 weeks were similar and no correlation between PNA and meropenem CLssM over the first month of life was seen. Thus, in contrast to the findings of Bradley et al. (6), our findings do not support the routine change of dosing frequency at the age of 2 weeks in severely ill neonates with a GA of ≤32 weeks. Still, given the small number of patients and the narrow range of GAs in our study, this approach should not be extended to more mature infants.
Our finding that prolongation of meropenem infusion does not necessarily result in an advantageous PK/pharmacodynamic (PD) profile when antibiotic-sensitive strains are encountered, is consistent with the results reported previously for neonates (6, 35). The lower clearance of meropenem by preterm neonates than by adults and even term neonates results in a 2 to 3 times longer half-life, and accordingly, even after a short infusion of 20 mg/kg, a greater fT>MIC and Cmin will be achieved (5, 11, 15, 28, 33). Furthermore, in all age groups, the short and long infusions of meropenem have not been different in terms of effect against Escherichia coli and Klebsiella spp., organisms that cause a significant proportion of the Gram-negative infections in neonates (16). For intermediate or resistant microorganisms (with meropenem MICs of >2 mg/liter) like Acinetobacter spp. and Pseudomonas aeruginosa, previous PK/PD simulation studies involving neonates (35) and pediatric patients (23) have suggested better PK/PD target attainment with 4-h infusions. Of note, both of the above-mentioned microorganisms are still very rare in the neonatal setting (22). However, treatment options for infections with these organisms are limited and carbapenems might be the only option. Based on our results at a MIC cutoff of 8 mg/liter with a short infusion, no neonate is expected to have an fT>MIC of <40%, with typical values of >95%. Modeling of various infusion times from 30 min to 6 h revealed only a minor decrease in the number of the few outliers not achieving an fT>MIC of at least 80%, with the vast majority having an fT>MIC between 95 and 100% (30).
Recently the Cmin/MIC ratio has been suggested to play a role in resistance suppression (32). However, by how much the drug concentration should exceed the MIC (Cmin/MIC) has not been studied in neonates. Tam et al. have demonstrated in vitro that to avoid non-plasmid-mediated resistance in P. aeruginosa, a Cmin/MIC ratio of at least 6.2 is needed (32). Unfortunately, even for in vitro conditions, these relationships are not straightforward, with inoculum size (i.e., the likelihood of the presence of resistant strains in a population) and resistance mechanisms but also the duration of the experiment likely affecting conclusions (12, 32) and making the uniform achievement of these values under clinical circumstances questionable.
The concentration at which a maximal bactericidal effect is achieved is an important determinant of efficacy (21, 31). Prolonged infusion results in a much lower Cmax and longer Tmax than administration over 30 min, potentially compromising the attainment of the 4× MIC necessary for the optimum killing properties of beta-lactams in the critically ill (21, 31). Potential drawbacks are associated with the degradation of meropenem after reconstitution (2), probably dependent on the formulation used. A recent study indicated that at a concentration of 4% at room temperature (≤25°C), the degradation will be <10% over 12 h (4). The same was observed by us in a limited number of infusion lines at the end of infusion.
Some limitations of this study should be noted. First, full covariate analysis was not possible because of the relatively small number of patients with a narrow GA range, allowing conclusions to be drawn for this specific patient group only (26). Second, meropenem PK may further be affected by unpredictable covariates like the severity of disease and therapeutic interventions (vasoactive treatment, volume replacement, etc.) (19). Still, we believe that these limitations have not prevented us from coming to appropriate conclusions.
Conclusions.
In VLBW neonates, meropenem infusions of 30 min are optimal, as they balance a reasonably high Cmax and fT>MIC for susceptible organisms with convenience of dosing with no dosing adjustment over the first month of life. On the basis of the results reported here, we have recommended that a dose of 20 mg/kg given as a 30-min infusion be used in a larger study of efficacy in patients with LOS (20).
ACKNOWLEDGMENTS
We thank all of the patients and their parents for their kind cooperation; our study nurses, Irina Bljudz, Marianna Mihhailova, Birgit Kiilaspää, and Pille Org, for their diligent work; and Merck & Co., Inc., for providing ertapenem for use as an analytical method IS.
This study was funded by the European Community's Seventh Framework Programme (FP7/2007-2013) under grant agreement 242146. J. Standing is supported by Methodology Fellowship G1002305 from the UK Medical Research Council. I. Lutsar and T. Metsvaht are partly supported by grants from the Estonian Science Foundation (8799) and Estonian Target Financing (SF0180004s12) and from the European Union through the European Regional Development Fund and the Archimedes Foundation.
Footnotes
Published ahead of print 25 June 2012
REFERENCES
- 1. Abramowicz M, Klein JO, Ingall D, Finland M. 1966. Levels of penicillin in serum of newborn infants. Am. J. Dis. Child. 111:267–271 [DOI] [PubMed] [Google Scholar]
- 2. AstraZeneca 2011. Meropenem: summary of product characteristics. State Agency of Medicines, Tallinn, Estonia [Google Scholar]
- 3. Beal S, Sheiner L, Boeckmann A, Bauer R. 2009. NONMEM user's guides (1989-2009). Icon Development Solutions, Ellicott City, MD [Google Scholar]
- 4. Berthoin K, Le Duff CS, Marchand-Brynaert J, Carryn S, Tulkens PM. 2010. Stability of meropenem and doripenem solutions for administration by continuous infusion. J. Antimicrob. Chemother. 65:1073–1075 [DOI] [PubMed] [Google Scholar]
- 5. Blumer JL. 1996. Pharmacokinetic determinants of carbapenem therapy in neonates and children. Pediatr. Infect. Dis. J. 15:733–737 [DOI] [PubMed] [Google Scholar]
- 6. Bradley JS, et al. 2008. Meropenem pharmacokinetics, pharmacodynamics, and Monte Carlo simulation in the neonate. Pediatr. Infect. Dis. J. 27:794–799 [DOI] [PubMed] [Google Scholar]
- 7. Bromiker R, Arad I, Peleg O, Preminger A, Engelhard D. 2001. Neonatal bacteremia: patterns of antibiotic resistance. Infect. Control Hosp. Epidemiol. 22:767–770 [DOI] [PubMed] [Google Scholar]
- 8. Ceriotti F, et al. 2008. Reference intervals for serum creatinine concentrations: assessment of available data for global application. Clin. Chem. 54:559–566 [DOI] [PubMed] [Google Scholar]
- 9. Condon RE, et al. 1997. Penetration of meropenem in plasma and abdominal tissues from patients undergoing intraabdominal surgery. Clin. Infect. Dis. 24(Suppl. 2):S181–S183 [DOI] [PubMed] [Google Scholar]
- 10. De Cunto A, et al. 2007. Use of meropenem in preterm newborns. Survey of the literature and case series. Minerva Pediatr. 59:755–760 [PubMed] [Google Scholar]
- 11. Drusano GL, Hutchison M. 1995. The pharmacokinetics of meropenem. Scand. J. Infect. Dis. Suppl. 96:11–16 [PubMed] [Google Scholar]
- 12. Drusano GL, Liu W, Fregeau C, Kulawy R, Louie A. 2009. Differing effects of combination chemotherapy with meropenem and tobramycin on cell kill and suppression of resistance of wild-type Pseudomonas aeruginosa PAO1 and its isogenic MexAB efflux pump-overexpressed mutant. Antimicrob. Agents Chemother. 53:2266–2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. EUCAST 2010. Antimicrobial wild type distributions of microorganisms. European Committee on Antimicrobial Susceptibility Testing, Växjö, Sweden [Google Scholar]
- 14. Gray JW, Patel M. 2011. Management of antibiotic-resistant infection in the newborn. Arch. Dis. Child. Educ. Pract. Ed. 96:122–127 [DOI] [PubMed] [Google Scholar]
- 15. Hurst M, Lamb HM. 2000. Meropenem: a review of its use in patients in intensive care. Drugs 59:653–680 [DOI] [PubMed] [Google Scholar]
- 16. Jaruratanasirikul S, et al. 2011. Pharmacodynamics of meropenem in critically ill patients with febrile neutropenia and bacteraemia. Int. J. Antimicrob. Agents 38:231–236 [DOI] [PubMed] [Google Scholar]
- 17. Kipper K, et al. 2009. Rapid determination of meropenem in biological fluids by LC: comparison of various methods for sample preparation and investigation of meropenem stability. Chromatographia 70:1423–1427 [Google Scholar]
- 18. Köksal N, Hacimustafaoglu M, Bagci S, Celebi S. 2001. Meropenem in neonatal severe infections due to multiresistant gram-negative bacteria. Indian J. Pediatr. 68:15–19 [DOI] [PubMed] [Google Scholar]
- 19. Lingvall M, Reith D, Broadbent R. 2005. The effect of sepsis upon gentamicin pharmacokinetics in neonates. Br. J. Clin. Pharmacol. 59:54–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lutsar I, et al. 2011. Meropenem vs standard of care for treatment of late onset sepsis in children of less than 90 days of age: study protocol for a randomised controlled trial. Trials 12:215 doi:10.1186/1745-6215-12-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Manduru M, et al. 1997. In vitro pharmacodynamics of ceftazidime against Pseudomonas aeruginosa isolates from cystic fibrosis patients. Antimicrob. Agents Chemother. 41:2053–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Muller-Pebody B, et al. 2011. Empirical treatment of neonatal sepsis: are the current guidelines adequate? Arch. Dis. Child. Fetal Neonatal Ed. 96:F4–F8 [DOI] [PubMed] [Google Scholar]
- 23. Ohata Y, et al. 2011. Optimal dosage regimen of meropenem for pediatric patients based on pharmacokinetic/pharmacodynamic considerations. Drug Metab. Pharmacokinet. 26:523–531 [DOI] [PubMed] [Google Scholar]
- 24. Patel PR, Cook SE. 1997. Stability of meropenem in intravenous solutions. Am. J. Health Syst. Pharm. 54:412–421 [DOI] [PubMed] [Google Scholar]
- 25. Rhodin MM, et al. 2009. Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr. Nephrol. 24:67–76 [DOI] [PubMed] [Google Scholar]
- 26. Ribbing J, Jonsson EN. 2004. Power, selection bias and predictive performance of the population pharmacokinetic covariate model. J. Pharmacokinet. Pharmacodyn. 31:109–134 [DOI] [PubMed] [Google Scholar]
- 27. Sherwin CM, McCaffrey F, Broadbent RS, Reith DM, Medlicott NJ. 2009. Discrepancies between predicted and observed rates of intravenous gentamicin delivery for neonates. J. Pharm. Pharmacol. 61:465–471 [DOI] [PubMed] [Google Scholar]
- 28. Smith PB, et al. 2011. Population pharmacokinetics of meropenem in plasma and cerebrospinal fluid of infants with suspected or complicated intra-abdominal infections. Pediatr. Infect. Dis. J. 30:844–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Smith PB, et al. 2009. Pharmacokinetics of an elevated dosage of micafungin in premature neonates. Pediatr. Infect. Dis. J. 28:412–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Standing J, Morris J, Germovsek E, Lutsar I, Cortina-Borja M. 2011. Methods for optimising neonatal antimicrobial use: time- and concentration-dependent agents, poster IV-12. 20th Annual Meeting of the Population Approach Group in Europe, Athens, Greece [Google Scholar]
- 31. Tam VH, McKinnon PS, Akins RL, Rybak MJ, Drusano GL. 2002. Pharmacodynamics of cefepime in patients with Gram-negative infections. J. Antimicrob. Chemother. 50:425–428 [DOI] [PubMed] [Google Scholar]
- 32. Tam VH, et al. 2005. Optimization of meropenem minimum concentration/MIC ratio to suppress in vitro resistance of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 49:4920–4927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Thalhammer F, et al. 1999. Continuous infusion versus intermittent administration of meropenem in critically ill patients. J. Antimicrob. Chemother. 43:523–527 [DOI] [PubMed] [Google Scholar]
- 34. Tod M, Jullien V, Pons G. 2008. Facilitation of drug evaluation in children by population methods and modelling. Clin. Pharmacokinet. 47:231–243 [DOI] [PubMed] [Google Scholar]
- 35. van den Anker JN, et al. 2009. Meropenem pharmacokinetics in the newborn. Antimicrob. Agents Chemother. 53:3871–3879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van Enk JG, Touw DJ, Lafeber HN. 2001. Pharmacokinetics of meropenem in preterm neonates. Ther. Drug Monit. 23:198–201 [DOI] [PubMed] [Google Scholar]
- 37. Yatsyk GV. 1998. Use of meropenem in the treatment of severe infections in newborns. Antibiot. Khimioter. 43:32–33 (In Russian.) [PubMed] [Google Scholar]