Abstract
ST-246 is being evaluated as a treatment for pathogenic orthopoxvirus infections in humans. To this end, a phase 2, double-blind, randomized, placebo-controlled, multicenter trial was conducted to assess the safety, tolerability, and pharmacokinetics (PK) of ST-246 when administered as a single daily oral dose (400 mg or 600 mg) for 14 days in fed adult volunteers. ST-246 was safe and well tolerated, with no deaths or serious adverse events reported during the study. There was a low incidence of treatment-emergent adverse events (TEAEs), the most common of which were mild nausea and headache. There were no clinically significant results from laboratory assessments, vital sign measurements, physical examinations, or electrocardiograms. The PK and dose proportionality of ST-246 were determined. The PK analysis showed that steady state was achieved by day 5 for the ST-246 400-mg treatment group and by day 6 for the 600-mg group. The dose proportionality analysis showed that the 400- and 600-mg ratio of dose-normalized peak drug concentration in plasma (Cmax) and relative exposure for each dosing interval (AUCτ) ranged from 80% to 85%. However, the 90% confidence intervals did not include 1.0, so dose proportionality could not be concluded. Overall, ST-246 was shown to be safe, and the PK was predictable. These results support further testing of ST-246 in a multicenter pivotal clinical safety study for licensure application.
INTRODUCTION
In recent history, variola virus, the etiologic agent of smallpox, was a major human pathogen, responsible for 300 million to 500 million deaths in the 20th century alone. Smallpox was highly communicable and carried exceptionally high morbidity. The rate of transmission to unvaccinated members of households was 30% to 80% (5). Mortality rates ranged from 1% for variola minor to 30% for variola major (12). Smallpox was declared to be eradicated from the natural environment in 1980 after an aggressive global surveillance, vaccination, and containment campaign conducted by the World Health Organization (WHO) from 1966 to 1977. Routine smallpox vaccination of the general public in the United States was discontinued in 1971.
Despite its extinction in nature, smallpox as a catastrophic epidemic disease remains a concern owing to the potential for a deliberate release of variola virus as an act of war or bioterrorism (9, 15). A single case of smallpox anywhere in the world would be considered a global health emergency. The deliberate or accidental release of the smallpox virus into today's largely unvaccinated and highly mobile population would wreak far-reaching medical, social, and economic havoc.
Although early vaccination can alter the outcome of disease or potentially prevent disease in a population that has been exposed, smallpox vaccination is not always effective, carrying a risk for immunosuppressed and even certain healthy recipients (8, 12). Also, it is no longer universally available to all who potentially might require it. Therefore, other modalities, such as safe and effective small molecules, would be useful for smallpox therapeutic intervention and for treatment of zoonotic poxvirus diseases, such as monkeypox, cowpox, and vaccinosis. There are currently no U.S. Food and Drug Administration (FDA)-approved therapies on the market for these diseases, but development of therapeutics is being pursued (7) and includes recent development and testing of antiviral drugs for treatment of orthopoxvirus diseases and vaccination complications (2, 3, 4, 13).
ST-246 (tecovirimat monohydrate), a low-molecular-weight synthetic compound, benzamide, N-[(3aR,4R,4aR,5aS,6S,6aS)-3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-2(1H)-yl]-4-(trifluoromethyl), rel-(monohydrate), was discovered through a deliberate effort to develop orally available antiviral drugs for use in biodefense (1). This compound inhibits egress of orthopoxvirus from mammalian cells. It is chemically unrelated to any substance currently in use for human or veterinary therapy and shows broad-spectrum antiviral activity against multiple members of the orthopoxvirus family, including vaccinia virus, cowpox virus, ectromelia virus, camelpox virus, monkeypox virus, and variola virus.
Given the potential utility of antiviral therapy against smallpox and the limitations of currently available drugs, there is clearly a need for a safe medication that can be taken orally and that is highly active against variola virus. In previous phase 1 clinical studies, ST-246 has been shown to be well tolerated when given daily for up to 21 days to nonfasted healthy volunteers at dose levels up to 800 mg/day (11). In a 2009 nonhuman primate (NHP) study (10), 3 mg/kg-of-body-weight ST-246 resulted in 100% survival. The blood exposure level at this dose is lower than that for 400- or 600-mg doses in humans (comparable to an NHP dose of 8 to 10 and 12 to 14 mg/kg, respectively; unpublished data). This phase 2 study was conducted to assess the safety, tolerability, and pharmacokinetics (PK) of ST-246 as a single daily oral dose of 400 or 600 mg for 14 days in 18- to 74-year-old fed volunteers. In addition, the inclusion/exclusion criteria selected for this study were less restrictive than those utilized in previous phase 1 studies to allow a safety evaluation that was more representative of the general population likely to be affected in a bioterrorism event.
MATERIALS AND METHODS
ST-246 product.
ST-246 was provided in orange and black, hard gelatin, size 0 capsules containing 200 mg of the ST-246 active ingredient. The placebo capsules were identical in appearance but did not contain the active ingredient. All inactive ingredients/excipients are generally recognized as safe and are U.S. Pharmacopeia (USP)/National Formulary (NF) grade. ST-246 was administered at an oral dose of 400 mg or 600 mg (2 × 200-mg or 3 × 200-mg capsules, respectively).
Study design and population.
This was a double-blind, randomized, placebo-controlled, multicenter trial to assess the safety, tolerability, and PK of the anti-orthopoxvirus compound ST-246 (400 mg and 600 mg) when administered as a single daily oral dose for 14 days in fed adult volunteers.
The study protocol and informed consent forms were reviewed and approved by an institutional review board. At the screening visit, subjects or their legally acceptable representatives provided written consent to participate in the study after having been informed about the nature and purpose of the study, participation and termination conditions, and risks and benefits of treatment. Following a 14-day screening period at 3 clinical sites in the United States, 107 subjects (males and nonpregnant females, 18 to 74 years old inclusive) were randomly assigned to receive either active drug (400 mg, n = 45; 600 mg, n = 46) or placebo (n = 16) over a 14-day treatment period. The inclusion/exclusion criteria were designed to also include those who were moderately obese, i.e., having a body mass index (BMI) (kg/m2) of 30 to 35, those with well-controlled medical conditions (excluding asthma treated with systemic steroids, serious angioedema episodes, poorly controlled hypertension with repeat readings of >140 systolic and/or >90 diastolic, a history of head trauma or seizures, cardiac disease resulting in any limitation of activity, a history of bleeding disorder, malignancy, abnormal electrocardiogram [ECG], clinically significant viral infection, including hepatitis B or C virus and HIV infection or AIDS, bacterial, fungal, or mycobacterial infection, and chronic bacterial, mycobacterial, fungal, parasitic, or protozoal infection) and those taking concomitant medications (excluding insulin, immunosuppressant/immunomodulatory medication, and anticonvulsive or anticoagulation therapy). Therefore, subjects whose screening laboratory results met grade 1 criteria on the Division of AIDS (DAIDS) table for grading the severity of adult adverse events (AEs) were enrolled.
Subjects self-administered a once-daily oral dose of ST-246 or placebo at home during the treatment period, with the exception of in-clinic visits on days 1, 2, 5, 6, 8, 12, 13, and 14, when ST-246 was administered in the presence of study staff. All subjects received their dose of ST-246 or placebo within 30 min after a light breakfast consisting of 400 to 450 cal and approximately 25% fat. Subjects were instructed to eat their standard light meal and take their study drug at the same time each day and to record the times on their diary card.
Safety assessments.
Evaluated safety parameters included general safety (AEs graded for severity in accordance with the DAIDS table), vital sign measurements, physical examination findings, laboratory test results (hematology, blood chemistry, including liver enzyme function tests, and urinalysis) and 12-lead ECG heart rate, morphological waveform analysis, and PR, QRS, QT, QTcB (Bazett's formula) and QTcF (Fridericia's formula) interval assessments on day 1 pretreatment and day 14, 3 h posttreatment. Subjects were asked to record all concomitant medications and all potential AEs on the diary card, to be reviewed at all study visits.
Venous blood collection.
To determine the PK of ST-246, venous blood samples were collected at specific time points, including 0 (baseline) and 2, 4, 6, and 12 h after administration of study medication on day 1 and before dosing on days 2, 5, 6, 8, 12, and 13. Day 14 PK assessments were done before dosing and at 2, 4, 6, 12, 24, 48, 72, 96, and 120 h after dosing. Follow-up clinic visits for PK sampling occurred at 24, 48, 72, 96, and 120 h (days 15 to 19) after the final dose of the study drug. Patients also were asked to return for a final follow-up visit 4 weeks (28 + 2 days) after the treatment period.
Plasma samples were collected and stored at −70°C until analyzed. The following PK parameters were evaluated: (i) following the initial dose, maximum drug concentration (Cmax), time to maximum drug concentration (Tmax), area under the concentration-time curve from time zero to time of the last measurable concentration (AUClast), and area under the plasma concentration-time curve for each dosing interval (AUCτ); following multiple dosing, Cmax, minimum drug concentration (Cmin), Tmax, AUClast, AUCτ, terminal rate constant (λz), elimination half-life (t1/2), apparent total clearance of drug from plasma after oral administration (Cl/F), apparent volume of distribution during the terminal phase after oral administration (Vz/F), accumulation ratio (Rac), average plasma drug concentration during multiple-dose administration (Cavg), and percent fluctuation.
Bioanalysis.
Plasma concentrations of ST-246 were determined for both doses by a validated, sensitive, and specific liquid chromatographic/tandem mass spectrometric (LC/MS/MS) assay method using a stable isotope analog of ST-246 as an internal standard. Human plasma (50 μl) was extracted using a liquid-liquid extraction method followed by chromatographic separation (6).
Statistical analysis.
Treatment groups included placebo, ST-246 400 mg, and ST-246 600 mg. The intent-to-treat (ITT) population consisted of all subjects who were randomly assigned to treatment, the safety population consisted of all subjects who received study medication, and the PK population consisted of all subjects who received at least 1 dose of ST-246 and had sufficient ST-246 concentration in plasma data for PK analysis. All statistical analyses were performed using the software program SAS, version 9.2 (SAS, Cary, NC).
The safety population was used for all safety and tolerability data summaries. Adverse events were coded by using the Medical Dictionary for Regulatory Activities. The frequency of treatment-emergent adverse events (TEAEs) was calculated for each system organ class (SOC) by preferred term and by treatment for the number of subjects reporting the event. The overall AE rate was compared between treatment groups by using the appropriate statistical test, accounting for age (18 to 45 and 46 to 75 years). The number and percentage of participants experiencing each specific adverse event was tabulated by severity and relationship to treatment.
The PK population was used for preparation of PK summaries and analysis. The PK parameter values were analyzed by noncompartmental methods using the software program WinNonlin, version 5.0.1 (Pharsight Corporation, Mountain View, CA), and were determined directly from the plasma concentration-time data. Plasma concentrations were summarized by using descriptive statistics for each scheduled time point. A statistical assessment of time of steady-state concentrations was performed using a repeat-measures analysis of variance (ANOVA) and the Helmert transformation.
RESULTS
Demographics.
A total of 107 subjects were enrolled and randomized to one of the following treatments: 16 subjects in the placebo group, 45 subjects in the ST-246 400-mg treatment group, and 46 subjects in the ST-246 600-mg treatment group. The ages of subjects in the study ranged from 18 to 74 years, with a mean (± standard deviation [SD]) age of 42.5 (±15.64) years. Just over half of the subjects were female (54.2%), and the majority of subjects were white (67.3%) and not Hispanic or Latino (80.4%). Body weights for all subjects ranged from 42.7 to 133.0 kg, and the mean (± SD) body weight was 78.01 (±15.262) kg.
Enrolled subjects were in good general health with no clinically significant medical history, had not been hospitalized for a chronic medical condition in the previous 2 years, and had no clinically significant findings from physical examination and laboratory results, other than high blood pressure in some subjects in all treatment groups, within the 14 days prior to receipt of the study drug. Of the 107 total subjects, 53 were overweight (BMI, 25 to 29.9 kg/m2) and 26 were moderately obese (BMI, 30 to 35 kg/m2). Mean and median values for vital sign measurements at screening and day 1 before dosing were similar for all treatment groups. Concomitant medications included acetaminophen, nonsteroidal anti-inflammatory drugs (e.g., ibuprofen, aspirin, or naproxen sodium), and oral contraceptives.
Safety.
Of the 107 subjects in the safety population, 3 subjects in each of the ST-246 400-mg (6.7%) and 600-mg (6.5%) treatment groups did not complete the study. Withdrawal from the study in the ST-246 400-mg treatment group was due to withdrawal of consent by one subject and AEs in two subjects (an upper respiratory tract infection and a postprocedural hematoma; neither was considered related to the study medication), and that for subjects in the ST-246 600-mg treatment group was due to subject request, loss to follow-up, and protocol violation. No deviations met IRB reporting criteria.
A total of 48 subjects (44.9%) reported at least 1 TEAE (149 TEAEs in total): 6 subjects (37.5%) in the placebo group reported a total of 15 TEAEs, 23 subjects (51.1%) in the ST-246 400-mg group reported a total of 54 TEAEs, and 19 subjects (41.3%) in the ST-246 600-mg group reported a total of 80 TEAEs. There were no statistically significant differences noted among the treatment groups for the factors of age or age and site.
The most commonly reported TEAEs were nervous system disorders (17.8%), gastrointestinal disorders (10.3%), and musculoskeletal and connective tissue disorders (10.3%). Within these SOCs, subjects in both the ST-246 400-mg and ST-246 600-mg groups most often reported headache (11.1% and 17.4%, respectively; 75% mild) and nausea (6.7% and 4.3%, respectively; all mild). Only 1 subject in the placebo group reported TEAEs.
Overall, 20 subjects (18.7%) reported 67 TEAEs that were considered by the investigator to be drug related (TEAEs with a definite, probable, or possible relationship in time and pattern of response to the study drug): 1 subject (6.3%) in the placebo group reported 5 related TEAEs, 11 subjects (24.4%) in the ST-246 400-mg group reported 21 related TEAEs, and 8 subjects (17.4%) in the ST-246 600-mg group reported 41 related TEAEs. Headache was the most commonly reported TEAE that was considered to be related to study medication, reported by 1 subject (6.3%), 2 subjects (4.4%), and 5 subjects (10.9%) in the placebo, ST-246 400-mg, and ST-246 600-mg groups, respectively. Most TEAEs were mild (127 events) or moderate (21 events). Only 1 TEAE was reported to be severe: 1 subject in the ST-246 400-mg group reported a severe TEAE of streptococcal pharyngitis that was considered to be unrelated to study medication.
There were no deaths or serious adverse events (SAEs) reported during this study. Two subjects in the ST-246 400-mg group withdrew from the study because of TEAEs: 1 subject withdrew because of an upper respiratory tract infection TEAE and the other because of a postprocedural hematoma TEAE. Both of these TEAEs were considered by the investigator to be unrelated to study medication. There were no remarkable or clinically significant results from laboratory assessments, vital sign measurements, physical examinations, or ECGs, and none were reported as AEs (data not shown).
Pharmacokinetics.
The pharmacokinetics (Table 1) and dose proportionality of ST-246 were determined after once-daily oral administration of ST-246 for 14 days to healthy fed adult volunteers. Trough concentrations of ST-246 in plasma were assessed before dosing on days 2, 5, 6, 8, 12, 13, and 14.
Table 1.
Day 1 and 14 ST–246 plasma PK variable estimates
| Day and ST–246 dose (mg) | Parameter | Cmax (ng/ml) | Cmin (ng/ml) | Tmax (h) | AUClast (ng · h/ml) | AUCτ (ng · h/ml) | λz (1iters/h) | t1/2 (h) | Cl/F (liters/h) | Vz/F (liters) | Rac | Cavg (ng/ml) | Fluctuation (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | |||||||||||||
| 400 | Mean (SD) | 1,170 (429) | 4 (1) | 9,926 (4,044) | 11,329 (4,945) | ||||||||
| Median | 1,100 | 4 | 8,527 | 11,589 | |||||||||
| Range | 449–2,560 | 2–6 | 3,889–20,692 | 3,889–20,606 | |||||||||
| No. of subjects | 43 | 43 | 43 | 22 | |||||||||
| 600 | Mean (SD) | 1,467 (626) | 4 (1) | 12,469 (5,280) | 13,895 (5,702) | ||||||||
| Median | 1,350 | 4 | 12,212 | 12,762 | |||||||||
| Range | 410–3,670 | 2–6 | 30,90a–29,790 | 5,602–29,784 | |||||||||
| No. of subjects | 44 | 44 | 44 | 23 | |||||||||
| Day 14 | |||||||||||||
| 400 | Mean (SD) | 1,286 (449) | 174 (80) | 4 (1) | 17,183 (7,708) | 12,026 (4,255) | 0.03 (0.02) | 26 (11) | 38 (14) | 1,248 (588) | 1.3 (0.5) | 501 (177) | 226 (58) |
| Median | 1,210 | 177 | 4 | 15,923 | 10,769 | 0.03 | 24 | 37 | 1,176 | 1.3 | 449 | 220 | |
| Range | 534–2,420 | 0–437 | 2–6 | 4,787–37,908 | 4,829–21,263 | 0.02–0.08 | 8–46 | 19–83 | 464–2,537 | 0.6–3.1 | 201–886 | 85–378 | |
| No. of subjects | 41 | 41 | 41 | 41 | 40 | 24 | 24 | 40 | 24 | 41 | 40 | 40 | |
| 600 | Mean (SD) | 1,523 (607) | 201 (112) | 3 (1) | 19,448 (9,800) | 1,4,791 (5,712) | 0.05 (0.04) | 24 (15) | 48 (25) | 1,356 (790) | 1.3 (0.6) | 616 (238) | 225 (51) |
| Median | 1,635 | 177 | 4 | 19,592 | 14,333 | 0.04 | 20 | 42 | 1,120 | 1.3 | 597 | 229 | |
| Range | 0–3,120 | 0–461 | 2–6 | 0b–41,664 | 3,793–34,966 | 0.01–0.23 | 3–58 | 17–158 | 286–3,341 | 0.6–4.1 | 158–1,457 | 54–330 | |
| No. of subjects | 42 | 41 | 41 | 42 | 40 | 26 | 26 | 40 | 26 | 41 | 40 | 40 | |
| Day 14 (body wt normalized) | |||||||||||||
| 400c | Mean (SD) | 1,206 (365) | 168 (84) | 4 (1) | 16,154 (6,938) | 11,378 (3,573) | 0.03 (0.02) | 26 (13) | 38 (19) | 1,260 (666) | 1.3 (0.6) | 474 (149) | 221 (73) |
| Median | 1,137 | 159 | 4 | 15,240 | 11,351 | 0.03 | 26 | 36 | 1,194 | 1.2 | 473 | 202 | |
| Range | 647–2,294 | 0–442 | 1–8 | 5,591–38,342 | 5,640–20,040 | 0.02–0.09 | 9–51 | 15–97 | 369–2,831 | 0.4–4.0 | 235–835 | 101–386 | |
| No. of subjects | 41 | 41 | 41 | 41 | 40 | 24 | 24 | 40 | 24 | 41 | 40 | 40 | |
| 600c | Mean (SD) | 1,501 (561) | 199 (110) | 3 (2) | 19,186 (9,537) | 14,739 (5,376) | 0.05 (0.04) | 24 (14) | 51 (33) | 1,349 (839) | 1.3 (0.6) | 614 (224) | 230 (63) |
| Median | 1,525 | 183 | 3 | 18,179 | 14,530 | 0.04 | 22 | 42 | 1,157 | 1.2 | 605 | 224 | |
| Range | 0–3,033 | 0–493 | 2–7 | 0b–42,681 | 3,991–33,988 | 1.01–0.19 | 3–57 | 17–166 | 238–3,673 | 0.5–3.4 | 166–1,416 | 44–359 | |
| No. of subjects | 42 | 41 | 41 | 42 | 40 | 26 | 26 | 40 | 26 | 41 | 40 | 40 |
The lowest value is associated with subject 002033. This subject did not have blood samples for PK analysis collected at the 12- and 24-h time points on day 1. The lack of these data could cause the AUC for this subject to be underestimated by as much as 50%. However, this low estimate is expected to have little effect on the mean AUClast value for the 600-mg ST-246 treatment group for day 1.
The value of zero is associated with subject 002046, who had measurable concentrations of ST-246 only on day 1 and before dosing on day 6 (section 11.3).
Body weight-normalized variables were derived by multiplying each variable by (observed body weight/median body weight). Median body weight was 78.6 kg.
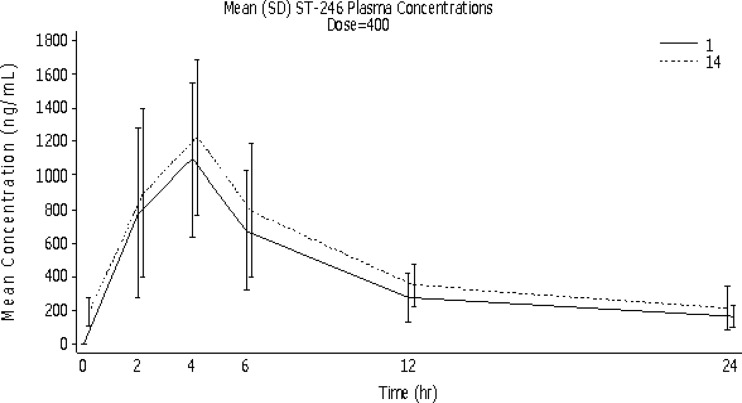
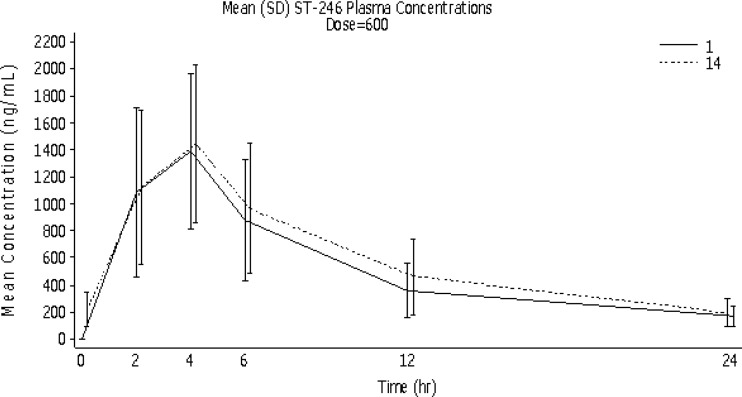
On day 1, mean (± SD) Cmaxs increased by 25% in response to a 50% increase in dose from 400 mg to 600 mg (from 1,170 ± 429 to 1,467 ± 626 ng/ml, respectively), compared to an 18% increase on day 14 (from 1,286 ± 449 to 1,523 ± 607 ng/ml, respectively). However, mean day 14 Cmaxs were 10% and 4% higher than the day 1 values for the ST-246 400-mg and 600-mg groups, respectively. Median time to reach Tmax was 4 h (range, 2 to 6 h) for both treatment groups on both days 1 and 14. Mean (± SD) exposure of ST-246 (Fig. 1 and 2), as measured by AUClast, increased by 26% in response to a 50% increase in dose (from 9,926 ± 4,044 to 12,469 ± 5,280 h · ng/ml) on day 1 and by 22% (from 17,183 ± 7,708 to 19,448 ± 9,800 h · ng/ml) on day 14, illustrating minimal accumulation of ST-246 after 14 days of dosing. Mean (± SD) exposure for each dosing interval (AUCτ) increased by 23% in response to a 50% increase in dose on both days 1 and 14 (from 11,329 ± 4,945 to 13,895 ± 5,702 h · ng/ml and from 12,026 ± 4,255 to 14,791 ± 5,712 h · ng/ml, respectively).
Fig 1.
Mean (SD) ST-246 concentrations in plasma over time by dosing day for the ST-246 400-mg group (PK population).
Fig 2.
Mean (SD) ST-246 concentrations in plasma over time by dosing day for the ST-246 600-mg group (PK population).
On day 14, the mean (± SD) Cmins were 174 ± 80 ng/ml and 201 ± 112 ng/ml for the ST-246 400-mg and 600-mg groups, respectively, representing a 16% increase in response to a 50% increase in dose. The mean (± SD) Cavg values increased by 23% in response to a 50% increase in dose (from 501 ± 177 to 616 ± 238 ng/ml for the ST-246 400-mg and 600-mg groups, respectively). The terminal ST-246 half-life was comparable between the 2 treatment groups; mean (± SD) half-lives were 25.8 ± 11.3 h and 24.3 ± 15.2 h for the ST-246 400-mg and 600-mg groups, respectively. The mean (± SD) percent fluctuation at steady-state value was also comparable: 226% ± 58% and 225% ± 51% for the ST-246 400-mg and 600-mg groups, respectively. Respective accumulation factors (Rac) were 1.31 ± 0.46 and 1.33 ± 0.60 for the two treatment groups.
Statistical analysis using repeated-measures ANOVA and Helmert transformation determined that steady state was achieved by day 5 for the ST-246 400-mg treatment group and by day 6 for the ST-246 600-mg group. Therefore, the steady-state assumptions used for the PK analyses of day 14 data were valid.
The statistical analyses of ST-246 PK variables in plasma revealed significant differences (P < 0.05) in Cmax and AUCτ between the two treatment groups on days 1 and 14. On day 1, there were no differences between male and female subjects or among the age groups for Cmax or AUCτ. On day 14, there were statistically significant differences (P < 0.05) between male and female subjects for AUCτ, half-life, and clearance but not for Cmax (P > 0.05). Because male subjects were generally heavier than female subjects, body weight was included as a covariate when gender differences were significant. The analyses showed that weight was a significant covariate for both AUCτ and clearance. Because of the significant differences caused by gender in AUCτ, summary statistics of the body weight-normalized PK variables were also assessed (Table 1). After normalizing AUCτ to the population median body weight (78.6 kg), AUCτ values for the ST-246 400-mg and 600-mg groups were 11,378 ± 3,573 h · ng/ml and 14,739 ± 5,376 h · ng/ml, respectively. Therefore, body weight-normalized AUCτ increased by 30% in response to a 50% increase in dose.
The dose proportionality analysis (Table 2) showed that the ratios of both dose-normalized Cmax and AUCτ values ranged from 80% to 85%, but the 90% confidence intervals did not include 1.0. Therefore, dose proportionality could not be concluded.
Table 2.
Dose proportionality analysis of ST-246 plasma PK parameter estimates (PK population)
| Variable at day 14 | Geometric meana |
90% CIb |
|||
|---|---|---|---|---|---|
| Test (600 mg) | Ref (400 mg) | Ratio (%)c (test/ref) | Lower | Upper | |
| ln (Cmax/dose) | 2.4 | 3.0 | 79.8 | 69.4 | 91.7 |
| ln (AUCτ/dose) | 22.8 | 28.3 | 80.7 | 69.9 | 93.1 |
| ln (Cmax/dose)d | 2.4 | 2.9 | 83.6 | 74.0 | 94.5 |
| ln (AUCτ/dose)d | 23.0 | 27.1 | 84.9 | 74.7 | 96.6 |
Geometric mean for test formulation (test; 600 mg) and reference formulation (ref; 400 mg) based on least-squares mean of log-transformed parameter values. For the test formulation, n = 44; for ref, n = 43.
Ninety percent confidence interval: two products are considered bioequivalent if the 90% CI of the relative mean Cmax and AUCτ of the test to reference are within 80% to 125%.
Ratio (%) = geometric mean value (test)/geometric mean value (ref).
Body weight-normalized parameter derived by multiplying the parameter by (observed body weight/median body weight [78.6 kg]).
DISCUSSION
This fourth clinical study with ST-246 was conducted as a phase 2, double-blind, randomized, placebo-controlled, multicenter trial. ST-246 was found to be safe, well tolerated, and predictable when administered as a single daily oral dose of 400 or 600 mg for 14 days to 18- to 74-year-old fed volunteers.
The two doses of 400 mg and 600 mg of ST-246 were chosen based on safety data at higher doses (800 mg/day for 21 days) in phase I human clinical trials and efficacy data in monkey studies. Both 400- and 600-mg doses provide plasma exposure well above the concentration demonstrated for monkeypox virus efficacy in NHPs (10). Using survival as a primary endpoint, an oral dose of approximately 3 mg/kg/day (36 mg/m2; lower limit of protection), for a period of 14 days starting at 3 days postinfection in fed monkeys, conferred 100% protection from death and significantly reduced viremia and lesion counts (10). The human therapeutic doses of 400 and 600 mg/day in the fed state are anticipated to provide exposure levels comparable to those of doses of 8 to 10 and 12 to 14 mg/kg/day, respectively, in monkeys (unpublished data). This study showed that these doses achieved the anticipated exposure levels.
The treatment duration of 14 days was selected to ensure adequate suppression of viral replication to allow enough time for the host immune system to clear the infection. This time period was based upon observations with mice that showed that a 5-day course of treatment provided the minimum duration required to protect mice from lethal orthopoxvirus infection (16). Moreover, it has been demonstrated in monkeys that humoral immunity can be measured by day 10 postinfection and function to clear remaining virus after dosing is complete (14).
In this study, the 400- and 600-mg dose proportionality analysis showed that the ratios of dose-normalized Cmax and AUCτ values ranged from 80% to 85%, but the 90% confidence intervals did not include 1.0. Therefore, dose proportionality could not be concluded. The low ratios of the dose-normalized Cmax and AUCτ values indicated that absorption of ST-246 was moving toward saturation as the dose increased within the 400- to 600-mg range.
The most commonly reported TEAEs were mild nausea and headache. There were no remarkable or clinically significant results from laboratory assessments, vital sign measurements, physical examinations, or ECGs. No deaths or SAES occurred during this study. These results support further testing of ST-246 in a multicenter pivotal clinical safety study.
ACKNOWLEDGMENTS
We acknowledge our colleagues at the National Institutes of Health, National Institute of Allergy and Infectious Diseases, and the Office of Biodefense Research Affairs for their continued support of this program.
This project has been funded in whole or in part with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under contract no.HHSN266200600014C.
Robert Jordan, Tove' C. Bolken, Kevin F. Jones, Shanthakumar R. Tyavanagimatt, and Dennis E. Hruby are shareholders of Siga Technologies, Inc.
Footnotes
Published ahead of print 9 July 2012
REFERENCES
- 1. Bailey TR, et al. 2007. N-(3,3a,4,4a,5,5a,6,6a-octahydro-1,3-dioxo-4,6-ethenocycloprop[f]isoindol-2-(1H)-yl) carboxamides: identification of novel orthopoxvirus egress inhibitors. J. Med. Chem. 50:1442–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baker RO, Bray M, Huggins JW. 2003. Potential antiviral therapeutics for smallpox, monkeypox and other orthopoxvirus infections. Antiviral Res. 57:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bray M. 2003. Pathogenesis and potential antiviral therapy of complications of smallpox vaccination. Antiviral Res. 58:101–114 [DOI] [PubMed] [Google Scholar]
- 4. Bray M, Roy CJ. 2004. Antiviral prophylaxis of smallpox. J. Antimicrob. Chemother. 54:1–5 [DOI] [PubMed] [Google Scholar]
- 5. Buller RM. 1999. Poxviruses. In Armstrong D, Cohen J. (ed), Infectious diseases, p 8.7.4–8.7.6 Harcourt, London, United Kingdom [Google Scholar]
- 6. Chen Y, et al. 2011. Comparison of the safety and pharmacokinetics of ST-246 after IV infusion or oral administration in mice, rabbits and monkeys. PLoS One 6(8):e23237 doi:10.1371/journal.pone.0023237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fenner F, Henderson DA, Arita I, Jazek Z, Ladnyi ID. 1988. Smallpox and its eradication. World Health Organization, Geneva, Switzerland [Google Scholar]
- 8. Fulginiti VA, Papier A, Lane JM, Neff JM, Henderson DA. 2003. Smallpox vaccination: a review, part II. Adverse events. Clin. Infect. Dis. 37:251–271 [DOI] [PubMed] [Google Scholar]
- 9. Henderson DA, et al. 1999. Smallpox as a biological weapon: medical and public health management. JAMA 281:2127–2137 [DOI] [PubMed] [Google Scholar]
- 10. Jordan R, et al. 2009. ST-246 antiviral efficacy in a nonhuman primate monkeypox model: determination of the minimal effective dose and human dose justification. Antimicrob. Agents Chemother. 53:1817–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jordan R, et al. 2008. Single-dose safety and pharmacokinetics of ST-246, a novel orthopoxvirus egress inhibitor. Antimicrob. Agents Chemother. 52:1721–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kemper AR, Davis MM, Freed GL. 2002. Expected adverse events in a mass smallpox vaccination campaign. Eff. Clin. Pract. 5:84–90 [PubMed] [Google Scholar]
- 13. LeDuc JW, Damon I, Relman DA, Huggins J, Jahrling PB. 2002. Smallpox research activities: U.S. interagency collaboration, 2001. Emerg. Infect. Dis. 8:743–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Panchanathan V, Chaudhri G, Karupiah G. 2006. Protective immunity against secondary poxvirus infection is dependent on antibody but not on CD4 or CD8 T-cell function. J. Virol. 80:6333–6338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smith GL, McFadden G. 2002. Smallpox: anything to declare? Nat. Rev. Immunol. 2:521–527 [DOI] [PubMed] [Google Scholar]
- 16. Yang G, et al. 2005. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus challenge. J. Virol. 79:13139–13149 [DOI] [PMC free article] [PubMed] [Google Scholar]