Abstract
The food system dominates anthropogenic disruption of the nitrogen cycle by generating excess fixed nitrogen. Excess fixed nitrogen, in various guises, augments the greenhouse effect, diminishes stratospheric ozone, promotes smog, contaminates drinking water, acidifies rain, eutrophies bays and estuaries, and stresses ecosystems. Yet, to date, regulatory efforts to limit these disruptions largely ignore the food system. There are many parallels between food and energy. Food is to nitrogen as energy is to carbon. Nitrogen fertilizer is analogous to fossil fuel. Organic agriculture and agricultural biotechnology play roles analogous to renewable energy and nuclear power in political discourse. Nutrition research resembles energy end-use analysis. Meat is the electricity of food. As the agriculture and food system evolves to contain its impacts on the nitrogen cycle, several lessons can be extracted from energy and carbon: (i) set the goal of ecosystem stabilization; (ii) search the entire production and consumption system (grain, livestock, food distribution, and diet) for opportunities to improve efficiency; (iii) implement cap-and-trade systems for fixed nitrogen; (iv) expand research at the intersection of agriculture and ecology, and (v) focus on the food choices of the prosperous. There are important nitrogen-carbon links. The global increase in fixed nitrogen may be fertilizing the Earth, transferring significant amounts of carbon from the atmosphere to the biosphere, and mitigating global warming. A modern biofuels industry someday may produce biofuels from crop residues or dedicated energy crops, reducing the rate of fossil fuel use, while losses of nitrogen and other nutrients are minimized.
The agriculture and food system disrupts the biogeochemical nitrogen cycle at various spatial scales. Limiting the impact of the agriculture and food system on the nitrogen cycle is increasingly important, as that system grows to feed a larger and more affluent world population.
Managing the food-nitrogen connection is likely to resemble managing the energy-carbon connection, a task that already has begun. The parallels between food and energy are myriad. In both the food and energy systems, alarms regarding a crisis of global supply were sounded in the 1970s, innovations and adaptations followed that permitted growth to continue, and the focus now is on addressing adverse impacts of further expansions of supply. Problems of scarcity share the stage with problems of abundance.
This paper reviews the nitrogen cycle, its disruptions by human activity, and some of the adverse environmental consequences of these disruptions. It then suggests principles, extracted from the energy-and-carbon arena, that might guide modification of the agriculture and food system to increase its responsiveness to nitrogen management objectives.
How the Nitrogen Cycle Works and How It Is Being Disrupted
The Nitrogen Cycle.
Given that extensive introductions to the biogeochemical nitrogen cycle are found elsewhere (1–5), a quick tour here may suffice. Nitrogen is found in three forms. It is bound to itself in a two-atom molecule, dinitrogen, or N2; this form is the most abundant, but it is almost unavailable to life because it is so stable that only a few specialized bacteria (and lightning) can break it apart. Nitrogen is bound to carbon, as organic nitrogen, in a magnificent variety of organic molecules, critical to life and present long after death, including proteins and their component amino acids. And it is bound neither to itself nor to carbon, in nitrogen nutrients. Nitrogen nutrients are relatively small molecules, both nitrogen ions and nitrogen gases. The principal nitrogen ions are ammonium (NH4+) and nitrate (NO3−). The nitrogen gases include ammonia (NH3); various oxides of nitrogen, including nitric oxide (NO), nitrogen dioxide (NO2), dinitrogen pentoxide (N2O5), and nitrous oxide (N2O); and nitric acid vapor (HNO3).
A specialized vocabulary describes the transformations from one form to another. Fixation is the process of making N2 into nitrogen nutrients (largely NH4+), and denitrification (in effect, unfixing) is the process of rebuilding N2 from nitrogen nutrients (largely NO3−). Nitrification oxidizes ammonium to nitrate. (A complication: Side reactions of both nitrification and denitrification produce N2O.) Assimilation and immobilization are the processes by which nutrients become organic nitrogen (plants assimilate, microorganisms immobilize), and mineralization is the process by which organic nitrogen is decomposed back into nitrogen nutrients. Assimilation, immobilization, and mineralization are capabilities found widely in nature, but fixation and denitrification can be accomplished only by specialized microorganisms.
Both air routes and water routes connect nutrient systems across large distances. Denitrification, mineralization, and nitrification all produce nitrogen gases. Once volatilized into the atmosphere, these gases undergo further chemical transformations before returning to the Earth’s surface by wet or dry deposition. Alternatively, nitrogen nutrients and organic nitrogen can be leached into groundwater or carried in runoff into surface water, then transported down waterways in solution or attached to solid particles.
The nitrogen cycle is captured quantitatively by the magnitudes of the stocks of nitrogen in the various biological and geophysical “reservoirs” [measured in millions of metric tons of nitrogen, Mt(N), for example] and the flows of nitrogen between pairs of reservoirs (in Mt(N)/yr). The stock of N2 in the atmosphere is so large, 3.9 × 1015 Mt(N), as to be effectively infinite. The stock of terrestrial organic nitrogen is about 100,000 Mt(N); very little terrestrial fixed nitrogen is in the form of nutrient, because uptake by plants is rapid. Only 4% of the 100,000 Mt(N) of terrestrial organic nitrogen is in living organisms, and the rest is in dead organic matter (5). Of the terrestrial dead organic matter, roughly 15% is labile, and 85% is recalcitrant, the distinction referring to the ease of mineralization. The stock of fixed nitrogen in the ocean, about half nutrients and about half dead organic matter, is roughly 10 times larger than the stock of terrestrial fixed nitrogen (5, 6).
Evidence for Human Impact on a Global Scale.
The concentration of nitrous oxide in the atmosphere gives indirect information about human impacts on the nitrogen cycle. Records from ice cores reveal that the concentration of nitrous oxide fluctuated only a few percent in the period from 2,000 years ago until about a century ago, when a statistically significant upward climb began. The current concentration, about 310 parts per billion by volume (ppbv), is about 10% higher than the average value before this century, and the current rate of increase is about 0.8 ppbv per year, or 0.3% per year, corresponding to a flow of 4 Mt(N)/yr (5). The stable concentration in earlier times and the rising concentration in the past century are presumed to be evidence that a stable dynamic equilibrium governed the flows of nitrogen among soils, waterways, oceans, and the atmosphere until human activity was boisterous enough to create a detectable signal. A similar story is told by the ice-core record of the atmospheric concentration of carbon dioxide (CO2): the concentration started its upward climb 200 years ago, is currently climbing 0.5% per year, and is now about 30% above its earlier average value. The earlier period of dynamic equilibrium and nearly constant atmospheric concentration is called the preindustrial period; its features are crude averages over several centuries of data, ending roughly in 1800.
Specifically, for the preindustrial global nitrogen cycle to have been in dynamic equilibrium requires a constant flow of fixed nitrogen (i) through the fixed-nitrogen subcycle, where nutrient is transformed into organic nitrogen and back (through a loop of assimilation, death, and mineralization); and (ii) through the fixing-unfixing subcycle, where N2 is transformed into nutrient and back. Quantitatively, and restricting attention to the terrestrial component of these subcycles, 1,200 Mt(N)/yr flowed through the fixed nitrogen cycle and 140 Mt(N)/yr flowed through the fixing-unfixing subcycle. Ocean and land nitrogen cycles are linked by river runoff, and coastal zones are active regions of nitrogen transformation. Ocean and land nitrogen cycles also are linked by atmospheric transport of nitrogen gases between land and sea. The flows that form the ocean components of the global nitrogen cycle are poorly known (5).
The rate at which nitrogen is being fixed on land today is approximately 300 Mt(N)/yr, roughly double its preindustrial value. Thus, the incremental fixation today from the global industrial and agricultural system of human beings is roughly equal to natural fixation in preindustrial times. This startling result captures the essence of the human impact on the nitrogen cycle. Its consequences depend strongly on the extent to which denitrification has kept pace with fixation. Unfortunately, a quantitative understanding of denitrification rates in various managed and unmanaged terrestrial and aquatic environments is largely missing, probably the biggest obstacle thwarting accurate modeling of the present-day nitrogen cycle (3).
Anthropogenic Additions of Fixed Nitrogen.
The additional flow of approximately 160 Mt(N)/yr from human nitrogen-fixation activity has three principal components. The two largest are directly related to agriculture: the synthesis of ammonia, largely for nitrogen fertilizer, and land use that enhances biological fixation. Ammonia synthesis is accurately known to be contributing about 95 Mt(N)/yr globally, of which 80 Mt(N)/yr is incorporated into synthetic nitrogen fertilizer, and the rest is “consumed by chemical industries and lost during processing and transportation” (7). The third contributor is high-temperature combustion, estimated, also very roughly, at 30 Mt(N)/yr (8, 9). I examine each of these three in turn.
Fertilizer.
Nitrogen fertilizer is made from ammonia, and ammonia is made, in effect, fixed, from nitrogen in the air. An ammonia factory requires very high pressures and moderately high temperatures to accomplish what bacteria accomplish at ordinary pressures and temperatures. The single human activity of nitrogen fertilizer production provides more than half of all anthropogenic fixed nitrogen. Fertilizer is the fossil fuel of food.
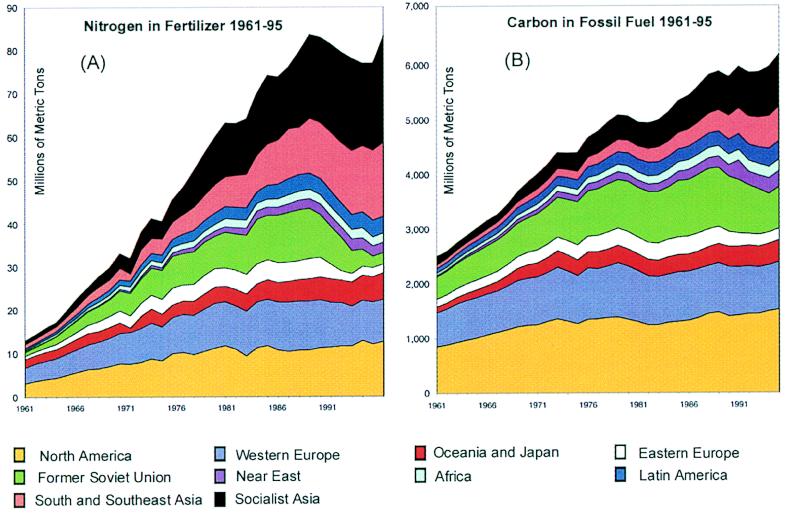
Fig. 1A displays 35 years of global nitrogen fertilizer use (1961–1995), disaggregated into 10 geographical regions (International Fertilizer Industry Association, http://www.fertilizer.org.). The rate of global nitrogen fertilizer use crossed 20 Mt(N) in 1965, 40 Mt(N) in 1973, and 60 Mt(N) in 1979. It remained within a band from 75 Mt(N) to 85 Mt(N) from 1986 to 1995, the consequence of continuous growth of consumption in Asia and a precipitous fall in consumption in the former Soviet Union and Eastern Europe.
Figure 1.
Comparable global data on release of nitrogen in fertilizer and release of carbon in fossil fuel, 1961–1995, with site of release disaggregated into 10 world regions. (A) Nitrogen in fertilizer; data from http://www.fertilizer.org. (B) Carbon in fossil fuel; data from http://cdiac.esd.ornl.gov/ndps/ndp030.html.
The plausibility of both saturation effects and upward pressures complicates attempts to predict future consumption of nitrogen fertilizer (10). The saturation in nitrogen fertilizer use in North America and Western Europe seen in Fig. 1A reflects fundamental physiological limits to yields and diminishing returns to single-factor inputs. A falling ratio of nitrogen fertilizer use to gross domestic product should be anticipated, inasmuch as nitrogen fertilizer contributes only to the production of a commodity and not to the downstream processing and services that account for an increasing fraction of wealth as incomes rise. By contrast, energy is a needed input at every stage in a complex economy, and hence has fewer built-in features leading to saturation. Saturation in fertilizer use will be reinforced as fertilizer subsidies are removed and external environmental costs are internalized in the fertilizer price.
Other pressures act to counter saturation. Upward pressure on nitrogen fertilizer use will be felt, as latent demand in many developing countries is expressed. Fig. 1A shows that the growth in fertilizer use across the developing world has been very uneven, and comparisons of fertilizer use on the same crop across countries confirms that fertilizer use and yield are correlated (ref. 11; http://www.fertilizer.org/CROPS/CROPS/harris.htm.). Upward pressure also will be felt if agricultural biotechnology continues to raise ceilings on yield potential with new crop variants dependent on nitrogen. Still other pressure may come from an expansion of fertilizer use on commercial forests and nonfood crops. As an alternative to fossil fuel, fast-growing energy crops may be established on dedicated, fertilized plantations.
Nitrogen’s share, by weight, of total fertilizer (nitrogen plus phosphorus plus potassium) has climbed steadily; today, nitrogen’s share is about 60%, in sharp contrast to 1960, when roughly equal amounts of nitrogen, phosphorus, and potassium were applied. This phenomenon reflects a comparative advantage of nitrogen fertilizer resulting from both plant physiology and economics. It also reflects the unavailability of phosphorus or potassium in some parts of the world, leading to inappropriate ratios of application (ref. 12 and http://www.fertilizer.org/PUBLISH/PUBENV.).
It is interesting to compare the history of the use of nitrogen fertilizer and fossil fuels. Fig. 1B mimics Fig. 1A: the carbon content of fossil fuel use is shown for the same 10 geographical subregions and the same time period (ref. 50; Carbon Dioxide Information and Analysis Center, http://cdiac.esd.ornl.gov/ndps/ndp030.html). Total global fossil-carbon use grew more slowly than total fertilizer use, crossing 3,000 million metric tons of carbon [Mt(C)] in 1965 and 4,000 Mt(C) in 1971, then remaining within a band between 4,800 and 5,100 Mt(C) from 1977 to 1984 and within a second band between 5,800 and 6,000 Mt(C) between 1988 and 1994. Growth of fossil-carbon use was slowed substantially by investments in energy efficiency.
Comparing Fig. 1 A and B reveals that developing-country Asia gained global share more rapidly for nitrogen fertilizer than for fossil carbon. From 1961 to 1995, its nitrogen share climbed from 14% to 50% whereas its carbon share climbed only from 9% to 26%. In 1995, the nitrogen shares for North America and Western Europe were 15% and 12%, respectively, whereas the corresponding carbon shares were larger, 25% and 14%. In the former Soviet Union and in Eastern Europe nitrogen fertilizer fell far more than fossil-carbon use during the last years shown: In the former Soviet Union the 1995 level of nitrogen fertilizer use was 19% of the 1987 level, and in Eastern Europe it was 47% of the 1987 level, whereas the 1995 levels of fossil-carbon use were 70% and 63% of 1987 levels, respectively. Careful study of the fall in fertilizer use in the former Soviet Union and in Eastern Europe seems warranted.
Land use.
Land devoted to legumes, such as soybean and alfalfa, is the site of anthropogenic fixed-nitrogen production. Legumes are extraordinary, relative to other crops, in hosting nitrogen-fixing microorganisms in their roots; legumes are botanical fertilizers. At harvest, there is far more nitrogen in legumes than in other crops: soybeans are about 5% nitrogen (dry mass), wheat is 2%, rice is 1%. Land devoted to wet rice cultivation promotes asymbiotic nitrogen fixation, and thus is another site of anthropogenic fixed-nitrogen production. Schlesinger (5), citing Burns and Hardy (13) and including only legumes, estimates total anthropogenic land-use-related nitrogen fixation to be 40 Mt(N). Galloway (8), including both legumes and rice, makes the same estimate. Successive improvements in such estimates can be imagined, which compare preindustrial and contemporary nitrogen fixation rates for more and more kinds of land-use change. Included, for example, would be land converted from forests to fields.
Land use also affects denitrification. Agricultural practices that reduce anaerobic environments, such as plowing (which aerates the soil) and draining wetlands, decrease denitrification, whereas irrigation increases denitrification. Denitrification also generally increases where fertilizer is applied, where legumes are planted, and where crops accelerate the mineralization of recalcitrant nitrogen in the soil. Greater burning of biological material also increases denitrification, because some of the nitrogen in biological material (wood, for example) recombines into N2 in flames, a process known as pyrodenitrification (2).
The management of nitrogen, like the management of carbon, is likely to evolve from a focus only on sources to a balanced focus on sources and sinks. A strategy of engineered denitrification to reduce fixed-nitrogen build-up might ensue, based on manipulating land use to enhance natural denitrification processes. Presuming the simultaneous goal of managing the global greenhouse, such a strategy would entail only options with favorable ratios of wanted N2 to unwanted N2O.
Combustion.
Where the temperature of combustion exceeds about 1,500°C, there is enough concentrated energy to break apart atmospheric N2 and to form nitrogen oxides (14). Nitrogen oxide production also results as the “fuel nitrogen” in fossil fuels is burned. Estimating the rate of nitrogen fixation caused by anthropogenic combustion requires taking into account both the amount of combustion and the amount of pollution control. Control of nitrogen oxide emissions is increasingly widespread and increasingly strict.
Why the Increase in Stocks of Fixed Nitrogen Is Troublesome
Schematically, one can identify seven distinct adverse impacts of anthropogenic disruption of the nitrogen cycle, two expressed at the global scale and five at the regional scale. The two global impacts are caused by nitrous oxide in the atmosphere both contributing to the greenhouse effect and reducing the concentration of stratospheric ozone. Two of the five regional effects have direct impacts on public health: air pollution and unhealthy nitrate concentrations in drinking water. The other three regional effects are mediated by ecological processes: acid deposition, eutrophication of bays and estuaries, and ecosystem disruption resulting from uneven responses to nitrogen fertilization across species.
These nitrogen effects can “cascade” (8). A nitrogen atom leaking away from an agricultural area can contribute to an air pollution problem over a city, then to a nitrate concentration problem in a municipal water supply, then to an acidification problem in a lake, then to a eutrophication problem in an estuary, and then to the destruction of ozone in the stratosphere. Such sequences, of course, can be interspersed with second passes through the agriculture and food system: through a cycle of fodder, cow, and manure, for example.
I review each of the seven impacts briefly below. Note that in six of the seven instances (all except ecosystem disruption) a regulatory regime either is already in place or is being designed.
Nitrous Oxide (N2O) Is a Greenhouse Gas.
Because N2O has a long (120-year) residence time in the atmosphere and absorbs IR radiation, it is the one nitrogen gas emitted into the atmosphere that contributes significantly to the greenhouse effect: It is the fourth largest contributor to the natural greenhouse effect, after water vapor, carbon dioxide, and methane. The increase in N2O concentration since preindustrial times contributes about one-fifteenth as much to the greenhouse effect as the increase in CO2 concentration in the same period; about one N2O molecule has been added for every 3,000 CO2 molecules, but each is about 200 times as effective. Nitrous oxide is included explicitly in international climate agreements.
Nitrous Oxide Depletes Stratospheric Ozone.
The long atmospheric residence time of nitrous oxide is a consequence of its lack of reactivity in the troposphere and its very low solubility in water. Nitrous oxide is destroyed only in the stratosphere, where energetic UV light breaks it apart. One product of its decomposition is nitric oxide (NO), which acts catalytically to lower the concentration of stratospheric ozone (15). The engines of subsonic and supersonic aircraft traveling at high altitudes also emit nitric oxide in regions affecting stratospheric ozone. Current international assessments of the impact of aircraft nitric-oxide emissions on stratospheric ozone are expected to influence the near-term future of supersonic aircraft, as well as the regulatory regime for engine emissions of subsonic aircraft. Thus, because agriculture and aviation share common stratospheric chemistry, agriculture is enmeshed for the indefinite future in a high-stakes aerospace debate.
Nitrogen Gases Generate Air Pollution.
Because of their high reactivity in the atmosphere, nitric oxide (NO) and nitrogen dioxide (NO2), collectively called NOx, control the production of tropospheric ozone. Nitrogen gases (both ammonia and nitrogen oxides) are also precursors of very small particulates that travel long distances in the atmosphere and that find their way deep into the lung when inhaled (51). The regulation of NOx and tropospheric ozone is at the core of air pollution control in the United States. Emerging attention to very small particulates may lead to further regulations on nitrogen emissions.
The Concentration of Nitrate Ions in Drinking Water Can Be a Threat to Infant Health.
Nitrite ions (NO2−) in blood can inactivate hemoglobin, with dangerous consequences. The inactivation occurs because nitrite ions change hemoglobin, whose iron is doubly charged (Fe++) and can carry oxygen, into methemoglobin, whose iron is triply charged (Fe+++) and cannot carry oxygen. Infants younger than about 3 months are particularly at risk, for reasons that are not fully understood. The American Academy of Pediatrics speculates that fetal hemoglobin (which remains in the infant for the first few months of life) “may be more susceptible to oxidation to methemoglobin by nitrite” (ref. 16; see http://www.aap.org/policy/356.html). Because the nitrite ions are formed in the gastrointestinal tract by the chemical reduction of nitrate ions (NO3−), the target of regulation is nitrate intake (17). The U.S. Environmental Protection Agency estimated in 1992 that 66,000 at-risk infants were drinking water whose nitrate concentration exceeded the U.S. health standard, 10 mg of nitrogen as nitrate (NO3−-N) per liter of water (18). Several water treatment options are available, all quite costly (18).
Nitrogen Oxides Emitted into the Atmosphere Contribute to Acid Deposition.
Acid deposition encompasses two related phenomena by which acidity is transferred from the atmosphere to the Earth’s surface: acid precipitation (including fog, rain, and snow) and dry deposition. The two principal contributors to acid deposition are nitrate and sulfate ions. Because precipitation is acidic even in the absence of air pollution (as a result of the effects of carbon dioxide and other gases on moisture in the atmosphere), acid precipitation is a term reserved for precipitation that is made still more acidic by pollution. Damage from acid deposition has been widely explored, and adverse consequences for lakes, forests, and buildings have been documented. To date, regulatory intervention to reduce acid deposition has focused far more on sulfate than nitrate, largely because a greater fraction of atmospheric sulfate arises from large emitters.
High Nitrate Concentrations in Aquatic Ecosystems Can Lead to Eutrophication.
Nitrogen is the limiting nutrient in many aquatic ecosystems, especially estuaries and bays, and thus the addition of nitrogen can lead to eutrophication, or excessive plant growth, followed by the depletion of dissolved oxygen and the development of aquatic “dead zones” where these plants decay (19). Because phosphorus, rather than nitrogen, is usually the limiting nutrient in fresh-water ecosystems, nitrate added to watersheds in their headwaters can be carried almost all the way to the sea before causing its first visible damage, thereby separating cause and effect both in space and time.
An example is the hypoxic zone in the Gulf of Mexico off Louisiana, presumed to be brought about by agriculture in the Mississippi River watershed. A region defined by a dissolved oxygen concentration of less than 2 mg/liter, unable to sustain most forms of life, this hypoxic zone grows along the bottom of the gulf each summer as the gulf stratifies, plankton in abundance die and sink, and the dissolved oxygen at lower depths is consumed. The area of the hypoxic zone has been approximately 13,000 square km in recent years. In drought years the area is smaller and in flood years the area is larger, compelling evidence that something is carried into the gulf that promotes the growth and subsequent decay of plankton. Excess nitrogen from fertilized fields and livestock in the Great Plains is implicated. An interagency Mississippi River/Gulf of Mexico Watershed Nutrient Task Force has been established to assist policy-making (ref. 20; see also the Gulf of Mexico Hypoxia Assessment Plan at http://www.cop.noaa.gov/HypoxiaPlan.html;†).
Nitrogen Addition to Ecosystems Reduces Biodiversity and Thereby Leads to Loss of Ecosystem Function.
Both by air and water routes, fixed nitrogen deliberately applied to crops finds its way to unmanaged ecosystems, leading to their inadvertent fertilization. Schlesinger (21) writes: “Vegetation on much of the Earth’s land surface exists in a state of nitrogen deficiency, due in part to the low natural rate of nitrogen fixation and persistent losses of available nitrogen to denitrification and nitrate leaching… We know from a large ecological literature that the fertilization of natural ecosystems, perhaps first noted in the eutrophication of lakes, is likely to result in a loss of species diversity… Any addition of a resource to [a natural community where that resource is scarce] will lead to the dominance of the species that can use that resource most efficiently.” Rather than having a net positive effect, inadvertent fertilization alters ecosystem composition and diminishes ecosystem function (22–25).
Lessons from Carbon and Energy for Nitrogen and Food
Efforts already underway to manage human impacts on the carbon cycle suggest five principles that could guide first steps to manage human impacts on the nitrogen cycle: (i) reach agreement on goals relevant to sustainability; (ii) improve efficiency of producers and consumers throughout the system; (iii) harness market forces; (iv) incorporate mechanisms to learn continuously from research; and (v) engage the consumer and the citizen. In a properly crafted management system, these five prescriptions can be mutually supportive.
Reach Agreement on Goals Relevant to Sustainability.
Management of human impact on the nitrogen cycle has not yet reached the stage where goals have been agreed on. The goal of carbon cycle management, however, has been widely endorsed: stabilization of the climate. In ratifying the United Nations Framework Convention on Climate Change, the nations of the world agreed to pursue, collectively, not a constant total emission of carbon dioxide into the atmosphere but a constant level of carbon dioxide in the atmosphere. Constant stocks, not constant flows, were judged to define sustainability.
Reasoning by analogy, the goal of nitrogen management would be ecological stabilization, reached through the achievement of constant stocks of fixed nitrogen in identified ecosystems. The stock in particular watersheds might be stabilized, as well as, perhaps, the total terrestrial stock. The goal of achieving a constant rate of production of fixed nitrogen would be rejected as inadequate.
The implications of a goal of constant stocks are formidable. The goal of achieving a constant carbon dioxide concentration in the atmosphere makes future use of fossil fuels hostage to the combined power of natural and engineered carbon sequestration (26–29). Similarly, the goal of achieving constant stocks of fixed nitrogen makes future use of nitrogen fertilizer hostage to the strength of natural plus engineered denitrification in the corresponding ecosystems. Globally, until nitrogen fixation is balanced by denitrification, the amount of excess fixed nitrogen in the world will grow relentlessly, with increasing consequences for ecosystems and public health.
In international negotiations, the choice of a specific target for the carbon dioxide concentration of the future atmosphere has been treated as separable from the decision that there should be such a target. The choice of a specific target is expected to require a depth of understanding of costs and benefits that may not be available for several decades. Meanwhile, there is broad-based participation in “what if” discussion of specific targets for the atmospheric carbon dioxide concentration. Similarly, choosing the specific ecosystems whose total fixed nitrogen is targeted, and the targets themselves, is premature, but “what if” discussion is already timely.
Improve Efficiency of Producers and Consumers Throughout the System.
The flow of nitrogen through the food system can be measured in various ways. By one measure, already discussed, the food system elicits an incremental fixation of 120 Mt(N)/yr in the form of chemical fertilizer and legumes. By another measure, 50 Mt(N)/yr is the flow of nitrogen that rides along with the annual global harvest of 2,600 Mt (dry weight) of crops (mid-1990s estimates); not included is the nitrogen in forage consumed by grazing animals (7). By a third measure, the nitrogen flow in the daily intake of food is about 23 Mt(N)/yr, the sum of 17 Mt(N) in plant food and 6 Mt(N)/yr in animal food (7); assuming that protein is 16% nitrogen and that there are 6 billion people, this works out, plausibly, to an average of 66 g of protein per person per day. Thus, less than half of the fixed nitrogen added by agriculture ends up in our harvested crops, and less than half of the fixed nitrogen in our harvested crops ends up in what we eat. Tracking material flows through a system containing both producers and consumers, documenting leakage at each stage, is an application of industrial ecology (30, 31).
To categorize ways of reducing nitrogen leakage, Galloway (32) suggests that all leakage be assigned to either crops, animals, or people. An ideal system would return all crop wastes, manure, and food wastes to the field, and no nitrogen would be lost through volatilization, runoff, or denitrification. As a result, maintaining constant food production would require no external inputs of nitrogen. Cohen (33) sets forth a similar utopia: “Required agricultural inputs of nutrients and energy [within half a century] will be derived from human, animal, and industrial wastes rather than from today’s fertilizers and fossil fuels. Unwanted effluents like eroded soil or agricultural runoff with pesticides and fertilizers will be eliminated or converted to productive inputs for industrial and urban use.”
Galloway’s or Cohen’s ideal system could have low throughput, in which case it would somewhat resemble preindustrial agriculture. But what is desired is high throughput, and no such system has ever been attempted. Any close approach to a high-throughput, zero-loss system would incur unacceptable costs for transporting wastes back to the field and probably would stress soil unacceptably. Nonetheless, the zero-loss system provides a useful point of reference from which to explore improved nitrogen-use efficiency in the crop system, the animal system, and the food consumption system.
Crops.
Of the many losses of fixed nitrogen in the crop system, probably the most important are the losses associated with suboptimal application of fertilizer. A report of the National Research Council (34), in the interest of pedagogy, set forth a representative relationship between fertilizer application rate and yield for a corn field. The yield is 4 metric tons per hectare (t/ha) in the absence of fertilizer, 7 t/ha when fertilizer is applied at 100 kg(N)/ha, and 8 t/ha when fertilizer is applied at 200 kg(N)/ha. “Thus, the first hundred kg of nitrogen per ha is three times as effective as the second hundred in adding to yield… The model can be taken further. Corn contains about 1.3% nitrogen. Thus the harvested grain retains 39 of the first 100 kg of nitrogen added as fertilizer, but only 13 of the second 100” (2). Efficiencies of 13% suggest a large potential for improvement through social and technical innovation. Diminishing returns to scale for a single factor of production suggest a sensitivity to price-related policy.
The opportunity for knowledge to substitute for nitrogen at the field is abundant. In precision agriculture, timing, quantity, and chemical form are controlled at the subfield level. Even within the constraints of traditional farming, there are measurable benefits from revising the timing of delivery (35) and fine-tuning the quantity applied to reflect soil variability (36). Smil (7) estimates that the “cumulative effect of adopting well-proven and low-cost measures aimed at increasing efficiency of nutrient uptake” would expand the effective global supply of nitrogen fertilizer by about 20 Mt(N)/yr. About half of the increment would come from improved fertilizer management and about half from reducing erosion, expanding the use of nitrogen-fixing crops, and increasing the recycling of organic wastes.
Animals.
Of the many losses of fixed nitrogen in the animal system, probably the most important are the losses associated with the nitrogen in manure. Geographic separation of feedlots and dairies from sites of crop production, a relatively recent phenomenon, has greatly raised the cost of recycling animal wastes. Even though consolidation of livestock management into large commercial units reduces collection costs, transportation costs make loop-closing uneconomic. With the Netherlands in the lead, public policy is forcing new management strategies for manure that are more responsive to environment and public health.
As per-capita income rises in most countries, this inefficiency grows in importance, because an increasing fraction of the flows of nitrogen from plants to people involves animals as intermediaries. In recent years, 40% of global grain production has gone to animal feed, but in the United States this fraction is 70%, and in Asia it has climbed in the last decade (1985–1987 versus 1995–1997) from 15% to 24% (37). There is a parallel in the energy system: as income rises, an increasing fraction of fossil fuel energy reaches the consumer through the intermediary of electricity. Meat is the electricity of food.
Food consumption.
There are losses of fixed nitrogen throughout the food system: in the ships and trucks transporting food, in the markets where it is sold, and in the kitchens and restaurants where it is prepared. Neither the most important losses nor the losses most easily reduced are easily identified.
Any analysis of nitrogen flows through the food system must take cultural factors into account. “Culture … defines which biological raw materials are seen as food and which are not” (33). For most of the world’s people, eating is a form of pleasure, enhanced by variety and free choice. In response, agriculture becomes more varied and more international. Overeating looms large.
How much nitrogen is required in food? The nutritionists’ recommended protein requirements (effectively, nitrogen requirements) have dropped over time, as knowledge has improved and as the ideal of a child growing as big as possible has been recognized to be a cultural construct. Sasson (38) writes: “It is not necessary to enjoy good health to have a diet where proteins represents 15% or more of the total caloric intake. A much lower proportion (5% in the case of good quality proteins such as those in eggs or milk, or 8% in the case of other types of proteins) is sufficient to cover the needs of an individual, child or adult, as long as he has an adequate calorie intake as well. Human milk contains only 5–6% of its energy in the form of proteins and yet it is an ideal food for the newborn child.”
The global grain yield (60% of all food production) has had almost the same nitrogen intensity (nitrogen percent by weight) over the past three decades, because the rates of growth of production of wheat, rice, and corn, each with its distinct nitrogen intensity, have been almost identical, about 2.5% per year (39). There appears to be no trend analogous to the “decarbonization” of the energy economy, the continuous decrease in the average carbon content of fuel throughout the 20th century that resulted from coal losing market share to petroleum and then petroleum losing market share to natural gas.
Harness Market Forces.
A general message from theory and experience is that market mechanisms are efficient. They stimulate the collective imagination, which is inevitably more powerful than the imagination of any small set of people who try to discern constructive behaviors on their own. Market mechanisms affect both producers and consumers. Market mechanisms reward those who do more than they need to do, relative to some yes-no measure of compliance. The “fertilizer sector” has not heeded this message. Instead, it “has been characterized by protection, subsidies, and price controls” (40).
Among the market mechanisms available for nitrogen management are cap-and-trade regimes, where a specified number of permits to fix nitrogen are issued and traded (41). Arguments in favor of setting caps on fixed-nitrogen inputs to a given region are beginning to be marshaled (42). The permit system could be organized at the watershed level, the national level, or the international level. A cap-and-trade precedent at the national level is the tradable permit system for atmospheric sulfur dioxide emissions from U.S. coal-fired power plants. The regime’s first years have been unexpectedly successful (43). This particular trading system had features that enabled it to survive a politically charged design process: it involved only a small number of traders (on the order of 100), the measurement of emissions was relatively straightforward, and the political consensus that made enforcement credible was in place. The U.S. has no cap-and-trade system for NOx emissions, even though the principal motivation (reducing acid precipitation) is the same. Beginning with trading in NOx emissions is a sensible way to gain the experience necessary to implement full-scale trading in fixed nitrogen.
Isn’t more expensive food the inevitable consequence of policy interventions to address previously ignored environmental impacts of agriculture? And isn’t more expensive food a scourge on the poor? Experience in the energy sector suggests two ways out of this trap. First, when priority is given to new problems, new ideas emerge; tradeoffs turn into joint gains. One learns to produce food more cheaply and with reduced environmental consequences. Second, general subsidies can be replaced by targeted subsidies for the poor. In the regulated rate schedules for electricity, the first few units of consumption (the first 100 kilowatt hours per month, for example) often are subsidized. Such “lifeline rates,” in both industrialized and developing countries, are a clever mediator between efficiency and equity, because the limited fraction of total use by poor families makes the impact of lifeline rates minimal (44).
Incorporate Mechanisms to Learn Continuously from Research.
Crop physiology and ecophysiology have been identified as areas of agricultural science that hold the key to substantial expansion of global food supply. Basic understanding of the soil-crop interactions that govern yield in today’s most productive regions should permit greater “ecological intensification,” higher “input end-use efficiency” and better protected “natural resource quality” (36).
Basic understanding of soil-crop interactions also should permit crops to be grown safely for the twin objectives of food (or feed) and energy, a strategy of interest in both industrialized and developing countries. The food component of a plant and the residue would be managed jointly, informed by a detailed understanding of how much residue should be returned to the land. Wherever, in high-yield agriculture, the soil’s need for carbon could be satisfied by a fraction of the total carbon in residues, the rest of the carbon in residues would be freed for commercial use. Today’s practice of burning residues on the field could be replaced by high-technology processing of residues for electricity and fuels (45). And if more of the nitrogen must be returned to the soil than would come along with the residues recycled for carbon, then the nitrogen and the carbon in the unrecycled residues might be unbundled and managed separately, the nitrogen meeting the needs at the field of origin, and, if some is left, serving as fixed-nitrogen input elsewhere. The requirements of the soil for other nutrients and micronutrients also would have to be met.
At the intersection of agricultural and environmental science, the priority is to understand the transport and fate of nitrogen nutrient, as well as the ecological consequences of increased nitrogen input (24). In nitrogen-cycle research there is a “missing fixed-nitrogen problem” analogous to the “missing carbon” problem in global climate research: little is known about the rate at which anthropogenic fixed nitrogen is denitrified in terrestrial and aquatic ecosystems (8). The recent result that terrestrial sinks for atmospheric carbon dioxide, per unit area at the continent level, are larger than ecologists expected (46) provides further reason to understand how nutrient-enriched ecosystems evolve over time. If, for example, 50 Mt(N)/yr of today’s anthropogenically fixed nitrogen (about one-third of the total) were forming recalcitrant organic compounds at a C/N mass ratio of 20:1 (a molar ratio of 23:1), carbon would be sequestered in organic matter at a rate of 1,000 Mt(C)/yr, one-sixth of the current rate of carbon emissions from fossil fuels.
Economists and agronomists are locked in debate about likely future yields. In the energy world, economists and geologists are locked in virtually the same debate, this time about likely additions to reserves of fossil fuels. The reason for lack of resolution is the same. The historical record shows a run of successes (higher yields, new reserves) for many decades. Because the method of the economists is to predict future outcomes from past performance, economists expect success to continue. And because for the scientists future success depends on discoveries they will have to make and do not now know how to make, the scientists are doubtful. At its core, this disagreement is about the pace of technical change. Agronomists are the geologists of food.
Engage the Consumer and the Citizen.
In the early 1970s the previously separate concerns for energy production and energy use merged into a single inquiry. The oil field, the gasoline station, the car engine, and alternatives to commuting to work now were linked. The system was further enlarged by thinking of the consumer not as someone who devours a certain amount of energy but as someone who has to be provided a service or amenity, such as transportation or lighting or a warm space. In the case of the agriculture and food system such a merger would integrate the perspectives of the agronomist and the nutritionist, the farmer and the eater.
The protein consumption of large numbers of ever better fed people dominates the impacts of food consumption on the nitrogen cycle. Consider what the Food and Agriculture Organization reports about the 110-g daily protein consumption of the average American. Of the 40 g of vegetable protein, 25 are from grains, and 15 are from other sources. Of the 70 g of animal protein, 40 are from meat, 20 from milk, five from eggs, and five from fish and seafood (Food and Agriculture Organization of the United Nations, http://apps.fao.org/lim500/nph-wrap.pl?FoodBalanceSheet&Domain=Food BalanceSheet). This diet is beckoning the rest of the world (47). Food preferences, beliefs about healthy eating, social norms, ethical constraints such as a concern for animal welfare: these are among the factors that determine how our eating disrupts the nitrogen cycle.
The individual is not only an eater but a citizen. Here energy again is a guide, this time to political discourse. Desire for autonomy drives interest in solar energy. Mistrust of expertise fuels arguments against nuclear power. Organic farming is the solar energy of food. Agricultural biotechnology is the nuclear power of food.
Agricultural biotechnology is at risk of repeating the course followed by nuclear power. Those in charge believe the way to deal with the public’s qualms is “to educate,” but listening would be more productive. The stakes are high. The development of nitrogen-fixing corn and wheat, for example, could transform nitrogen management, but with what other consequences?
Questions burgeon: How well understood is the underlying science, and how quickly is the science becoming better known? Where are the irreversibilities? If scientists are the guardians, who guards the guardians? And who protects all of us from guardians of the guardians who see their task as avoiding every potentially slippery slope, thereby annulling the spirit of experimentation so critical to our future?
Conclusions
Through numerous feedback loops the impacts of agriculture on the environment become impacts of agriculture on agriculture. Impacts on both the nitrogen and the carbon cycle result in changes in climate, changes in soil characteristics, changes in species mix, changes in pest populations, some of which will benefit agriculture, but many of which will not. Indeed, the existence of these closed loops is one of the principal reasons why impacts of agriculture on the environment merit the attention of the agriculture community.
The finding that the nitrogen cycle at several spatial scales is strongly impacted by food production should not surprise us. Consistently, when one investigates the effects of aggregate human activity on the natural world, one finds ecological systems that are stressed by this activity. Consistently, one finds that these stresses are only partially understood, that built-in self-correcting mechanisms to keep these stresses from becoming dangerous are largely absent; that deliberate mitigating actions are not hard to find once the problem receives sustained attention; and that at least some of the mitigating actions that emerge from such an exercise are ethically complex.
The societal response to the new knowledge that abundant fixed nitrogen produces negative environmental effects has been appropriately cautious. Nitrogen fertilizer, along with improved seed and irrigation, are the “technological trinity” responsible for the high-yield agriculture of the Green Revolution (40). High-yield agriculture, in turn, has been key to avoiding mass starvation in much of the world. Poorly devised strategies to reduce the use of fertilizer could lead, in the short term, not only to human distress but also to environmental adversity, were such efforts to result in production, on the margin, from lands relatively vulnerable to environmental damage (land on steep slopes, wetlands).
Nonetheless, it is inevitable that the agriculture and food system will evolve to contain its impacts on biogeochemical cycles. As rational policy regimes replace more opportunistic ones, the agriculture and food system will be subject to the same governance as other industrial systems (48, 49). At the level of the field, greater inputs of information and fewer inputs of chemicals (including nitrogen fertilizer) are a likely outcome.
Management of the nitrogen cycle can be informed by the greater experience to date in managing the carbon cycle. There are familiar objectives, uncertainties, environmental risks, and collisions of values. Among the promising approaches are: (i) setting a goal of ecosystem stabilization; (ii) searching the entire production and consumption system for opportunities to improve efficiency; (iii) implementing cap-and-trade systems for fixed-nitrogen; (iv) expanding research at the intersection of agriculture and ecology, and (v) focusing on the food choices of the prosperous.
Coordinated management of the nitrogen and carbon cycles is required to address key environmental issues. The global increase in fixed nitrogen may be fertilizing the Earth, transferring significant amounts of carbon from the atmosphere to the biosphere, and mitigating global warming. A modern biofuels industry someday may produce biofuels from crop residues or dedicated energy crops, reducing the rate of fossil fuel use, while losses of nitrogen and other nutrients are minimized. A basic research program that addresses the critical scientific questions today limiting agricultural productivity is likely to address nitrogen fertilization and biomass energy as well.
The agriculture and food community can be expected to resist external pressure. However, the long-run consequence of this pressure is likely to be beneficial. A new challenge stimulates fresh approaches that result in greater efficiency, here, in particular, in managing fixed nitrogen. Greater efficiency will reduce both the external intrusion and the impact on costs.
Acknowledgments
Among those who have tried to educate me about some of the themes of this paper are Braden Allenby, Allison Armour-Garb, Jesse Ausubel, Robert Ayres, Kenneth Cassman, James Galloway, Hiram Levy, William Keene, Ann Kinzig, Emily Matthews, Jeremiah Ostriker, Ted Parson, Jane Pitt, Vernon Ruttan, William Schlesinger, Vaclav Smil, Christopher Taylor, Valerie Thomas, David Tilman, Iddo Warnick, Bess Ward, and Robert Williams.
ABBREVIATIONS
- Mt(N)
metric tons of nitrogen
- Mt(C)
metric tons of carbon
- ha
hectare
Footnotes
Scavia, D., Workshop on Materials and Energy Flows, United States Geological Survey, November 3, 1998, Reston, VA.
References
- 1.Smil V. Carbon, Nitrogen, Sulfur: Human Interference in Grand Biospheric Cycles. New York: Plenum; 1985. [Google Scholar]
- 2.Ayres R, Schlesinger W, Socolow R. In: Industrial Ecology and Global Change. Socolow R, Andrews C, Berkhout F, Thomas V, editors. Cambridge, U.K.: Cambridge Univ. Press; 1994. pp. 121–155. [Google Scholar]
- 3.Galloway J N, Schlesinger W H, Levy H, II, Michaels A, Schnoor J L. Global Biogeochem Cycles. 1995;9:235–252. [Google Scholar]
- 4.Smil V. Cycles of Life: Civilization and the Biosphere. New York: Scientific American Library; 1996. [Google Scholar]
- 5.Schlesinger W H. Biogeochemistry: An Analysis of Global Change. 2nd Ed. New York: Academic; 1997. [Google Scholar]
- 6.Kinzig A, Socolow R. Physics Today. 1994;24:24–31. [Google Scholar]
- 7.Smil, V. (1999) Global Biogeochem. Cycles, in press.
- 8.Galloway J N. Environ Pollution. 1998;102:S1. , 15–24. [Google Scholar]
- 9.Olivier J G J, Bouwman A F, van der Hoek K W, Berdowski J J M. Environ Pollution. 1998;102:S1. , 135–148. [Google Scholar]
- 10.Frink C R, Waggoner P E, Ausubel J H. Proc Natl Acad Sci USA. 1999;96:1175–1180. doi: 10.1073/pnas.96.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris G. An Analysis of Fertilizer Application Rates for Major Crops. Paris: International Fertilizer Industry Association; 1998. [Google Scholar]
- 12.Isherwood K F. Fertilizer Use and the Environment. Paris: International Fertilizer Industry Association; 1998. [Google Scholar]
- 13.Burns R C, Hardy R W F. Nitrogen Fixation in Bacteria and Higher Plants. New York: Springer; 1975. [DOI] [PubMed] [Google Scholar]
- 14.Turns S. An Introduction to Combustion. New York: McGraw Hill; 1996. [Google Scholar]
- 15.Stevenson F J. Cycles of Soil: Carbon, Nitrogen, Phosphorus, Sulfur, Micronutrients. New York: Wiley; 1986. [Google Scholar]
- 16.American Academy of Pediatrics. Pediatrics. 1994;46:475–478. [Google Scholar]
- 17.National Research Council. Nitrate and Nitrite in Drinking Water: A Report of the Board on Environmental Studies and Toxicology. Washington DC: National Academy Press; 1995. [Google Scholar]
- 18.Lamarre L. Electric Power Res Inst J. 1998;23:18–23. [Google Scholar]
- 19.Carpenter S R, Caraco N F, Correll D L, Howarth R W, Sharpley A N, Smith V H. Ecol Appl. 1998;8:559–568. [Google Scholar]
- 20.Moffat A S. Science. 1998;279:988–989. [Google Scholar]
- 21.Schlesinger W H. In: Industrial Ecology and Global Change. Socolow R, Andrews C, Berkhout F, Thomas V, editors. Cambridge, U.K.: Cambridge Univ. Press; 1994. pp. 245–260. [Google Scholar]
- 22.Tilman D. Resource Competition and Community Structure. Princeton, NJ: Princeton Univ. Press; 1982. [PubMed] [Google Scholar]
- 23.Vitousek P M. Ecology. 1994;75:1861–1876. [Google Scholar]
- 24.Vitousek P M, Aber J D, Howarth R W, Likens G E, Matson P A, Schindler D W, Schlesinger W H, Tilman D. Ecol Appl. 1997;7:737–750. [Google Scholar]
- 25.Daily G, editor. Natureís Services: Societal Dependence on Natural Ecosystems. Washington, DC: Island; 1997. [Google Scholar]
- 26.Herzog, H. J., ed. (1997) Energy Conversion Management38, Suppl., 689 pp.
- 27.Hileman B. Chem Engin News. 1997;75:34–37. [Google Scholar]
- 28.Socolow R, editor. Fuels Decarbonization and Carbon Sequestration: Report of a Workshop. Princeton, NJ: Princeton Univ. Press; 1997. [Google Scholar]
- 29.Parson E A, Keith D W. Science. 1998;282:1053–1054. [Google Scholar]
- 30.Socolow R. In: Industrial Ecology and Global Change. Socolow R, Andrews C, Berkhout F, Thomas V, editors. Cambridge, U.K.: Cambridge Univ. Press; 1994. pp. 3–16. [Google Scholar]
- 31.Powers C W, Chertow M R. In: Thinking Ecologically: The Next Generation of Environmental Policy. Chertow M, Esty D, editors. New Haven, CT: Yale Univ. Press; 1997. pp. 19–36. [Google Scholar]
- 32.Galloway, J. N. (1999) J. Agroecosystems, in press.
- 33.Cohen J. Key Reporter. 1998;63:1–5. [Google Scholar]
- 34.National Research Council. Soil and Water Quality: An Agenda for Agriculture. Washington, DC: National Academy Press; 1992. [Google Scholar]
- 35.Matson P A, Naylor R, Ortiz-Monasterio I. Science. 1998;280:112–115. doi: 10.1126/science.280.5360.112. [DOI] [PubMed] [Google Scholar]
- 36.Cassman K G, Peng S, Olk D C, Ladha J K, Reichardt W, Dobermann A, Singh U. Field Crops Res. 1998;56:7–39. [Google Scholar]
- 37.World Resources Institute. World Resources: A Guide to the Global Environment 1998–99. Oxford, U.K.: Oxford Univ. Press; 1998. [Google Scholar]
- 38.Sasson A. Feeding Tomorrow’s World. Scientific and Cultural Organization, Paris: United Nations Educational; 1990. [Google Scholar]
- 39.Cassman K G. Proc Natl Acad Sci USA. 1999;96:5952–5959. doi: 10.1073/pnas.96.11.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bumb B, Baanante C. IFRPI 2020 Brief 40, October 1996. Washington, DC: International Food Policy Research Institute; 1996. [Google Scholar]
- 41.Runge C F. In: Thinking Ecologically: The Next Generation of Environmental Policy. Chertow M, Esty D, editors. New Haven, CT: Yale Univ. Press; 1997. pp. 200–216. [Google Scholar]
- 42.Cowling E B, Erisman J W, Smeulders S M, Holman S C, Nicholson B M. Environ Pollution. 1998;102:S1. , 599–608. [Google Scholar]
- 43.Kerr R. Science. 1998;282:1024–1027. [Google Scholar]
- 44.World Bank. Rural Energy and Development: Improving Energy Supplies for Two Billion People. Washington, DC: World Bank; 1996. [Google Scholar]
- 45.Williams R. In: Industrial Ecology and Global Change. Socolow R, Andrews C, Berkhout F, Thomas V, editors. Cambridge, U.K.: Cambridge Univ. Press; 1994. pp. 199–225. [Google Scholar]
- 46.Fan S, Gloor M, Mahlman J, Pacala S, Sarmiento J, Takahashi T, Tans P. Science. 1998;282:442–446. doi: 10.1126/science.282.5388.442. [DOI] [PubMed] [Google Scholar]
- 47.Stern P, Dietz T, Ruttan V, Socolow R, Sweeney J, editors. Environmentally Significant Consumption: Research Directions. Washington, DC: National Academy Press; 1997. [Google Scholar]
- 48.Armour-Garb A R. NY Univ Environ Law J. 1995;4:339–374. [Google Scholar]
- 49.Chertow M, Esty D, editors. Thinking Ecologically: The Next Generation of Environmental Policy. New Haven, CT: Yale Univ. Press; 1997. [Google Scholar]
- 50.Marland G, Boden T A, Andrews R J, Brenkert A L, Johnson C. Trends: A Compendium of Data on Global Change. Oak Ridge National Laboratory, Oak Ridge, TN: Carbon Dioxide Information Analysis Center; 1998. [Google Scholar]
- 51.Wilson R, Spengler J, editors. Particles in Our Air: Concentrations and Health Effects. Cambridge, MA: Harvard Univ. Press; 1996. [Google Scholar]