Abstract
The in vitro activity of tedizolid (previously known as torezolid, TR-700) against penicillin-resistant Streptococcus pneumoniae (PRSP) clinical isolates and the in vivo efficacy of tedizolid phosphate (torezolid phosphate, TR-701) in murine models of PRSP systemic infection and penicillin-susceptible S. pneumoniae (PSSP) pneumonia were examined using linezolid as a comparator. The MIC90 against 28 PRSP isolates was 0.25 μg/ml for tedizolid, whereas it was 1 μg/ml for linezolid. In mice infected systemically with a lethal inoculum of PRSP 1 h prior to a single administration of either antimicrobial, oral tedizolid phosphate was equipotent to linezolid (1 isolate) to 2-fold more potent than linezolid (3 isolates) for survival at day 7, with tedizolid phosphate 50% effective dose (ED50) values ranging from 3.19 to 11.53 mg/kg of body weight/day. In the PSSP pneumonia model, the ED50 for survival at day 15 was 2.80 mg/kg/day for oral tedizolid phosphate, whereas it was 8.09 mg/kg/day for oral linezolid following 48 h of treatment with either agent. At equivalent doses (10 mg/kg once daily tedizolid phosphate or 5 mg/kg twice daily linezolid), pneumococcal titers in the lungs at 52 h postinfection were approximately 3 orders of magnitude lower with tedizolid phosphate treatment than with linezolid treatment or no treatment. Lung histopathology showed less inflammatory cell invasion into alveolar spaces in mice treated with tedizolid phosphate than in untreated or linezolid-treated mice. These results demonstrate that tedizolid phosphate is effective in murine models of PRSP systemic infection and PSSP pneumonia.
INTRODUCTION
Streptococcus pneumoniae (pneumococcus) remains the most common cause of community-acquired pneumonia, meningitis, bacteremia, and otitis media (14). Clinical resistance of S. pneumoniae to penicillin, quinolones, macrolides, and other antimicrobial agents has been reported since the 1960s (3). The Centers for Disease Control and Prevention applied new Clinical and Laboratory Standards Institute (CLSI) penicillin breakpoints to S. pneumoniae isolates collected from patients with nonmeningitis-associated invasive pneumococcal disease in 10 states in the United States during 2006 and 2007 (4, 5). The percentages of these S. pneumoniae isolates that were categorized as susceptible (penicillin-susceptible S. pneumoniae [PSSP], MICs ≤ 0.06 μg/ml), intermediate, and resistant (penicillin-resistant S. pneumoniae [PSSP], MICs ≥ 2 μg/ml) to penicillin were found to be 93.2%, 5.6%, and 1.2%, respectively (4).
The oxazolidinones are being developed for treatment of infections caused by Gram-positive pathogens with resistance to penicillin and other antimicrobial agents, including those of the quinolone and macrolide classes. Oxazolidinones bind to the 50S subunit of the bacterial ribosome, thereby blocking bacterial protein synthesis (10, 12). Linezolid (Zyvox) is the only representative of the oxazolidinone class that regulatory agencies, including the U.S. Food and Drug Administration, have currently approved for use (16). Although resistance to linezolid remains infrequent, U.S. surveillance has identified the sporadic emergence of Gram-positive bacteria (mostly staphylococci) with resistance to linezolid, due to 23S rRNA or L3/L4 riboprotein mutations or the presence of the mobile multidrug resistance gene cfr (8).
Tedizolid phosphate (previously known as torezolid phosphate, TR-701) is a new oxazolidinone designed for improved antibacterial potency, especially against linezolid-resistant strains. It is being developed for the treatment of serious infections caused by Gram-positive bacteria, including PRSP. Tedizolid phosphate is an inactive prodrug that, after oral or intravenous (i.v.) administration, is readily converted by phosphatases to the active form, tedizolid (torezolid or TR-700). This study describes the in vitro activity of tedizolid against PRSP clinical isolates and the efficacy of tedizolid phosphate in murine models of PRSP systemic infection and PSSP pneumonia.
(This work was presented in part at the 44th Interscience Conference on Antimicrobial Agents and Chemotherapy, 2004, Washington, DC, and the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy, 2007, Chicago, IL.)
MATERIALS AND METHODS
Antimicrobial agents.
All antimicrobial agents used (tedizolid phosphate, tedizolid, and linezolid) were obtained in powder form from Dong-A Pharmaceutical Co., Yongin-Si, South Korea. Test solutions of these antimicrobial agents were freshly prepared prior to use.
Susceptibility testing.
PRSP (penicillin G MICs ≥ 2 μg/ml) clinical isolates were collected between 2002 and 2004 from patients at a South Korean tertiary-care hospital (19). The species were identified by conventional methods or by using either the ID 32 GN or the ATB 32A system (bioMérieux, Marcy l'Etoile, France). MIC values of tedizolid (active form) and linezolid against the 28 PRSP isolates were determined in agar dilution assays in accordance with NCCLS guidelines (15) using Mueller-Hinton agar supplemented with 5% sheep blood. Serial 2-fold dilutions of stock solutions of tedizolid or linezolid were made to yield 10× solutions that were mixed with 9 parts Mueller-Hinton agar, supplemented with 5% sheep blood, at 45 to 50°C. Final concentrations of antimicrobials were from 128 μg/ml to 0.0313 μg/ml. Agar was poured into 10-cm plastic petri dishes at a depth of 3 to 4 mm and allowed to solidify at room temperature. Inocula were prepared from single colonies of an overnight growth of PRSP isolates by suspending in broth, adjusting the turbidity to match that of the 0.5 McFarland standard, and applying 104 CFU, using a Steers replicator, onto prepared plates, starting with drug-free control plates and ending with the highest concentration of drug. Plates were incubated at 35°C for 16 to 20 h before inspection for growth. S. pneumoniae ATCC 49619 was used as a control. The MIC for each isolate was determined as the minimum concentration at which there was no growth.
Pneumococcal systemic lethal infection.
To induce a systemic S. pneumoniae infection, male ICR mice (weight, 18 to 20 g; Orient Bio Inc., Gyeonggi-do, South Korea) were inoculated intraperitoneally with 1 of 4 PRSP isolates (DR9, DR10, DR11, or DR14) suspended in 10% mucin (Becton Dickinson and Company, Sparks, MD). The suspension contained sufficient bacteria to kill 100% of untreated control mice. At 1 h postinfection, mice received a single dose of either tedizolid phosphate or linezolid, and survival was assessed daily for 7 days postinfection. Treatments were delivered both orally and intravenously at each of four doses (40 mg/kg of body weight, 13.33 mg/kg, 4.44 mg/kg, and 1.48 mg/kg), with 8 mice per group defined by dose, delivery method, and infecting strain. The 50% effective dose (ED50), i.e., the dose allowing survival of 50% of the infected mice, was calculated for each delivery route using probit analysis.
Pneumococcal lower respiratory infection.
A PSSP type III clinical isolate found to be highly virulent in mice when introduced into the lungs was used to compare the in vivo efficacy of oral tedizolid and oral linezolid in a subacute murine pneumonia model. Specific-pathogen-free 5-week-old female C57BL/6 mice (18 to 20 g; Orient Bio Inc., Gyeonggi-do, South Korea) were adapted to standardized environmental conditions for 3 days before inoculation. PSSP type III was grown to logarithmic phase in brain heart infusion broth with 10% horse serum, collected by centrifugation, washed twice, and resuspended in phosphate-buffered saline. Mice anesthetized with ketamine and xylazine were inoculated by intranasal instillation of a 25-μl suspension containing 2.5 × 107 CFU of PSSP. Animals were held in a vertical position for 5 min to facilitate distal migration of the bacteria to the alveoli by gravity. The size of the inoculum was confirmed by serial dilution and quantitative subculture. Treatments were initiated at 4 h postinfection (time zero) by oral gavage in groups of 20 mice each using the appropriate dose of tedizolid phosphate or linezolid in 0.2 ml distilled water.
To study effects on survival, infected mice (n = 10 per dose group) received either tedizolid phosphate at doses of 2.5, 5, 10, and 20 mg/kg once daily (QD) for 48 h or linezolid at doses of 2.5, 5, 10, and 20 mg/kg twice daily (BID) for 48 h. Untreated infected mice served as controls. Survival was recorded daily for 15 days, and cumulative survival rates were calculated. The mean survival day was defined as [f(d − 1)]/N, where f is the number of mice recorded to have expired on day d (survivors at day 15 were included in f for that day), and N is the number of mice in a group. The ED50 was determined as the dose of antibiotic (mg/kg/day) required for survival of 50% of infected mice at day 15, calculated as described above.
To study the clearance of PSSP bacteria from the lungs, infected mice received either tedizolid phosphate at 10 mg/kg QD or linezolid at 5 mg/kg BID, as described above. At 24 and 48 h after initiating treatments (28 and 52 h postinfection), five mice per treatment group were sacrificed by CO2 asphyxiation and exsanguinated by cardiac puncture, and lungs were removed from each mouse and homogenized in 10 ml of saline at 4°C. Untreated infected mice were sacrificed at time zero, 24 h, and 48 h to serve as controls (n = 5 at each time point). Total CFU counts in whole-lung homogenates were determined by plating 10-fold serial dilutions onto agar containing 5% horse serum and incubating the plates for 18 h at 37°C. The results are expressed as the mean log10 number of CFU (± standard deviation) per lung for each treatment group.
Two mice per treatment group (tedizolid phosphate at 10 mg/kg QD, linezolid at 5 mg/kg BID, infected untreated controls, and uninfected controls) were sacrificed at 48 h for histology. Lungs were perfused with saline, fixed in formalin, embedded in paraffin, and sectioned. Sections were stained with hematoxylin-eosin and scored for inflammation and edema, using a scale of from 0 to 3 for each (0, absent; 1, mild; 2, moderate; and 3, severe). One pathologist, blinded to the treatment group, scored all slides.
RESULTS
In vitro susceptibility of PRSP to tedizolid and linezolid.
Agar dilution experiments demonstrated that all 28 clinical isolates of PRSP were inhibited by tedizolid at 0.25 μg/ml (MIC range, 0.125 to 0.25 μg/ml) and all were inhibited by linezolid at 1 μg/ml (MIC range, 0.125 to 1 μg/ml) (Table 1). Tedizolid was 4-fold more potent than linezolid against PRSP; MIC90s were 0.25 μg/ml with tedizolid and 1 μg/ml with linezolid.
Table 1.
MICs for tedizolid and linezolid against PRSPa
| Antimicrobial agent | MIC (μg/ml) |
||
|---|---|---|---|
| Range | 50% | 90% | |
| Tedizolid | 0.125–0.25 | 0.25 | 0.25 |
| Linezolid | 0.125–1 | 0.5 | 1 |
Twenty-eight isolates were tested. Penicillin resistance was determined on the basis of the oral penicillin resistance MIC breakpoint for nonmeningitis pneumococcal isolates (≥2 μg/ml). For penicillin G tested against these isolates, the MIC range was 2 to 4 μg/ml, the MIC50 was 2 μg/ml, and the MIC90 was 4 μg/ml.
PRSP systemic lethal infection.
Four PRSP isolates, each with an MIC of 0.125 μg/ml for tedizolid and one of 0.5 μg/ml for linezolid, were used in experiments to compare the activities of the two agents when delivered orally or parenterally in the murine systemic infection model. Overall, oral tedizolid phosphate was approximately 2-fold more potent than oral linezolid (Table 2). Although ED50 values for oral tedizolid phosphate against strains DR9, DR10, and DR11 (5.70, 3.19, and 7.63 mg/kg/day, respectively) were lower than those of linezolid (11.06, 6.38, and 14.85 mg/kg/day), the 95% confidence intervals (CIs) for each strain overlapped. The potencies of oral tedizolid phosphate and oral linezolid against PRSP isolate DR14 were similar (ED50 values, 11.53 and 12.98 mg/kg/day, respectively). Tedizolid phosphate delivered i.v. was 6.5-, 5.0-, and 3.0-fold more potent than i.v. linezolid for strains DR9, DR10, and DR11, respectively, with no overlap of 95% CIs. Although i.v. tedizolid phosphate was nearly 4-fold more potent than linezolid against PRSP isolate DR14, there was considerable variability and the 95% CIs overlapped.
Table 2.
Activities of tedizolid phosphate and linezolid against PRSP isolates in a murine systemic lethal infection model
| PRSPb isolate (no. of CFU/mouse) | Antimicrobial agent | In vitro MICc (μg/ml) | Oral dosea |
Intravenous dosea |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. (%) of mice surviving after administration of dose (mg/kg/day) of: |
ED50 (mg/kg/day)d | No. (%) of mice surviving at day 7 after administration of dose (mg/kg/day) of: |
ED50 (mg/kg/day)d | |||||||||
| 1.48 | 4.44 | 13.33 | 40 | 1.48 | 4.44 | 13.33 | 40 | |||||
| PRSP DR9 (5 × 104) | Tedizolid phosphate | 0.125 | 0 (0.0) | 2 (25.0) | 8 (100) | 8 (100) | 5.70 (3.45–9.38) | 1 (12.5) | 3 (37.5) | 7 (87.5) | 8 (100) | 4.89 (2.95–8.04) |
| Linezolid | 0.5 | 0 (0.0) | 2 (25.0) | 3 (37.5) | 8 (100) | 11.06 (6.73–18.22) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 5 (62.5) | 31.84 (18.16–59.08) | |
| PRSP DR10 (7 × 102) | Tedizolid phosphate | 0.125 | 1 (12.5) | 6 (75.0) | 7 (87.5) | 8 (100) | 3.19 (1.63–5.94) | 2 (25.0) | 3 (37.5) | 8 (100) | 8 (100) | 3.52 (1.82–6.50) |
| Linezolid | 0.5 | 1 (12.5) | 2 (25.0) | 6 (75.0) | 8 (100) | 6.38 (3.48–11.55) | 0 (0.0) | 2 (25.0) | 2 (25.0) | 6 (75.0) | 17.62 (9.51–34.24) | |
| PRSP DR11 (2 × 104) | Tedizolid phosphate | 0.125 | 0 (0.0) | 1 (12.5) | 7 (87.5) | 8 (100) | 7.63 (4.81–12.09) | 0 (0.0) | 0 (0.0) | 6 (75.0) | 8 (100) | 10.19 (6.38–16.14) |
| Linezolid | 0.5 | 0 (0.0) | 1 (12.5) | 3 (37.5) | 7 (87.5) | 14.85 (6.38–16.14) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 5 (62.5) | 30.83 (18.93–52.29) | |
| PRSP DR14 (2.5 × 104) | Tedizolid phosphate | 0.125 | 0 (0.0) | 0 (0.0) | 5 (62.5) | 8 (100) | 11.53 (3.90–32.77) | 0 (0.0) | 1 (12.5) | 5 (62.5) | 8 (100) | 10.01 (3.56–28.53) |
| Linezolid | 0.5 | 0 (0.0) | 1 (12.5) | 3 (37.5) | 8 (100) | 12.98 (4.65–37.90) | 0 (0.0) | 1 (12.5) | 1 (12.5) | 2 (25) | 39.53 (9.98–233.33) | |
n = 8 mice per dose group. Survival data are for day 7 after infection.
Penicillin resistance was determined on the basis of the oral penicillin resistance MIC breakpoint for nonmeningitis pneumococcal isolates (>2 μg/ml).
The MICs reported are for tedizolid, which is formed in vivo after administration of its prodrug, tedizolid phosphate.
Data in parentheses are 95% CIs.
PSSP pneumonia model.
The majority (80%) of the untreated control mice infected with PSSP type III succumbed to the infection within 7 days. For infected mice receiving 48-h treatment with tedizolid phosphate at 2.5 mg/kg QD and 5 mg/kg QD (total daily doses, 2.5 mg/kg and 5 mg/kg, respectively), the 15-day cumulative survival rates were 50% and 80%, respectively (Table 3). Treatment with linezolid at 2.5 mg/kg BID and 5 mg/kg BID for 48 h (total daily doses, 5 mg/kg and 10 mg/kg, respectively) resulted in 15-day cumulative survival rates of 30% and 70%, respectively. A 100% survival rate was achieved with tedizolid phosphate at a minimum total daily dose of 10 mg/kg (regimen, 10 mg/kg QD), which was 4-fold lower than the 40-mg/kg total daily dose of linezolid (regimen, 20 mg/kg BID) needed to obtain 100% survival. On the basis of the ED50 values in the murine pneumococcal pneumonia model, tedizolid phosphate was nearly 3-fold more potent than linezolid, with ED50s of 2.80 mg/kg/day (95% CI, 1.41 to 4.44) versus 8.09 mg/kg/day (95% CI, 4.74 to 11.91) (Table 3).
Table 3.
In vivo efficacies of tedizolid phosphate and linezolid against S. pneumoniae (PSSP) in a murine pneumonia model according to 15-day cumulative survival rates
| Treatment | Regimen (total daily dose)a | Survival rate (%) | MSDb (day) | ED50 (mg/kg/day)c |
|---|---|---|---|---|
| Tedizolid phosphate | 2.5 mg/kg QD (2.5) | 50 | 9.7 | 2.80 (1.41–4.44) |
| 5 mg/kg QD (5) | 80 | 12.2 | ||
| 10 mg/kg QD (10) | 100 | 14.0 | ||
| 20 mg/kg QD (20) | 100 | 14.0 | ||
| Linezolid | 2.5 mg/kg BID (5) | 30 | 7.0 | 8.09 (4.74–11.91) |
| 5 mg/kg BID (10) | 70 | 11.1 | ||
| 10 mg/kg BID (20) | 90 | 13.0 | ||
| 20 mg/kg BID (40) | 100 | 14.0 | ||
| No treatment (infected control) | Not applicable | 10 | 5.7 | Not applicable |
Oral antimicrobial treatment was initiated at 4 h postinfection and was administered for 48 h at the indicated regimens. Total daily doses are given in milligrams per kilogram.
MDS, mean survival day, which is equal to [f(d − 1)]/N, where f is the number of mice recorded to have expired on day d (survivors on day 15 were included in f for that day) and N is the number of mice in a group.
Data in parentheses are 95% CIs.
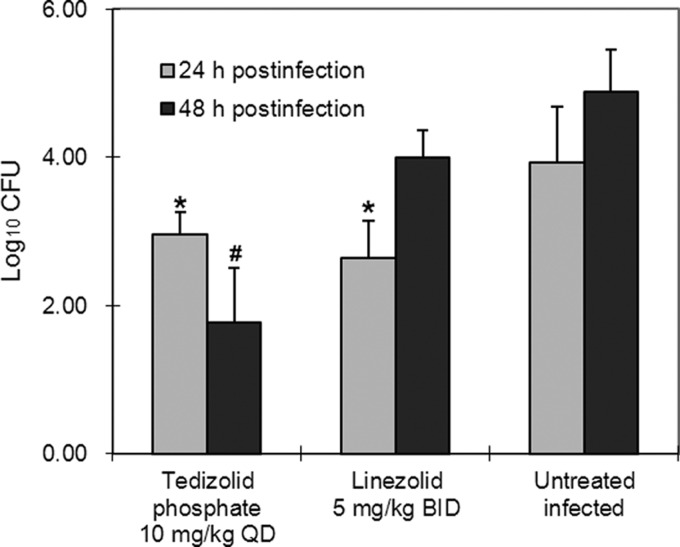
In untreated control mice infected with PSSP, pneumococcal counts in lung homogenates were 3.93 log10 CFU/ml at 24 h and 4.88 log10 CFU/ml at 48 h after initiating treatment (Fig. 1). Treatment with tedizolid phosphate or linezolid reduced the counts by 1 log unit at 24 h (P < 0.05 for each versus the control). At 48 h, however, the pneumococcal titers in the lungs of mice treated with tedizolid phosphate at 10 mg/kg QD were approximately 3 orders of magnitude lower than those in the lungs of control mice (P < 0.001), while lung homogenate titers from mice treated with linezolid were not significantly different from those for the controls (P = 0.64).
Fig 1.
Pneumococcal clearance from lungs of S. pneumoniae-infected mice by tedizolid phosphate. Oral antimicrobial treatment was started at 4 h postinfection. *, P < 0.05 versus untreated control at the same time point; #, P < 0.001 versus uninfected control at the same time point.
Histopathology of lungs harvested at 48 h revealed that the alveolar spaces in lungs from mice infected with PSSP that received no treatment or linezolid were infiltrated with large numbers of inflammatory cells, mainly neutrophils. Lungs of infected mice treated with tedizolid phosphate showed less severe inflammation and edema, as indicated by the mean scores for inflammation and edema (Table 4).
Table 4.
Mean histopathologic scores of tedizolid phosphate and linezolid in lung tissue of mice with S. pneumoniae pneumonia at 48 h postinfection
| Antimicrobial agent (regimen)a | Inflammation scoreb | Edema scoreb |
|---|---|---|
| Tedizolid phosphate (10 mg/kg QD) | 1.0 | 0.5 |
| Linezolid (5 mg/kg BID) | 2.1 | 1.9 |
| Untreated control | 2.6 | 1.1 |
| Uninfected control | 0 | 0 |
Oral antimicrobial treatment was initiated at 4 h postinfection.
Inflammation and edema were each graded on a scale of from 0 to 3 (0, absent; 1, mild; 2, moderate; and 3, severe).
DISCUSSION
Our results of agar dilution susceptibility testing of 28 penicillin-resistant S. pneumoniae clinical isolates from a hospital in South Korea were similar to those in recent reports of isolates collected in the United States, Europe, and Australia (2, 17). We derived MIC90 values of 0.25 μg/ml for tedizolid and 1 μg/ml for linezolid, as did Schaadt et al., who used the agar dilution method for 38 PSSP, 35 PRSP, and 37 intermediate-susceptibility isolates from the United States, Great Britain, France, Germany, and Australia (17). Another study of 133 strains isolated in the United States used broth dilution to determine MIC90 values of 0.25 μg/ml for tedizolid and 2.0 μg/ml for linezolid; these did not vary when PSSP, PRSP, and intermediate-susceptibility isolates were analyzed separately or pooled (2).
In this study, tedizolid was more effective than linezolid in protecting against PRSP systemic lethal infection in mice when either was administered orally or parenterally and survival was assessed at day 7. Tedizolid was 3- to 8-fold more potent than linezolid (among the 4 isolates) by intravenous administration and 2-fold more potent by oral administration for 3 of the 4 strains. Tedizolid was also 2.9-fold more potent than linezolid in the mouse PSSP pneumonia survival model.
The doses selected for the in vivo studies were in the range of equivalent doses efficacious for humans. Pharmacokinetic studies have found similar plasma exposures for mice administered oral tedizolid phosphate at 10 mg/kg/day and humans treated orally with 200 mg/day, while a 60-mg/kg/day dose of linezolid in the mouse is equivalent to a 600-mg/day dose in humans (1, 13). Although the maximum dose of linezolid used in this study was lower at 40 mg/kg/day, 90% and 100% 15-day survival rates were achieved with 20 mg/kg/day and 40 mg/kg/day, respectively, in the PSSP pneumonia model, where only 10% of untreated mice survived.
Recent studies have demonstrated excellent penetration of tedizolid into pulmonary epithelial lining fluid (ELF). Intravenous tedizolid at 20 mg/kg/day in mice achieved ELF concentrations similar to those achieved in humans receiving 200 mg/day i.v., while linezolid at 120 mg/kg BID in mice was comparable to linezolid at 600 mg BID in humans (18). The greater potency of tedizolid phosphate than linezolid in the mouse pneumonia model in this study may reflect better penetration into ELF and alveolar macrophages. In healthy adult volunteers who received 200 mg oral tedizolid phosphate per day for 3 days, exposure in ELF was 40 times and that in alveolar macrophages was 20 times the free-drug exposure in plasma (9). In another study, uninfected adult patients were treated with oral linezolid phosphate at 600 mg BID (5 doses total) before undergoing elective diagnostic bronchoscopy (6). Steady-state concentrations of linezolid were approximately 4-fold higher in ELF than plasma but were only 15% of plasma levels in alveolar cells (6). After comparing results from the two studies, Housman et al. (9) determined that 24-h exposure in ELF was nearly 9-fold greater for tedizolid than linezolid (960 μg · h/ml versus 109.3 μg · h/ml), at doses of 200 mg/day for tedizolid and 1,200 mg/day for linezolid.
Furthermore, studies by Drusano et al. (7) demonstrated a granulocyte-mediated enhanced killing effect of tedizolid against Staphylococcus aureus in a mouse thigh infection model; the potency of tedizolid in reducing intramuscular bacterial counts was greater in mice with an intact immune system than in mice rendered granulocytopenic by cyclophosphamide treatment (7, 13). Mechanistically, intracellular penetration of tedizolid and intracellular killing of bacteria, e.g., in macrophages and granulocytes, have been proposed to contribute to the efficacy of tedizolid (7, 11).
In conclusion, this study showed that tedizolid was 4-fold more potent than linezolid against PRSP isolates in vitro and that tedizolid phosphate administered to mice orally or intravenously was effective in vivo against systemic infection with PRSP isolates. Oral tedizolid phosphate was also effective in vivo against pneumonia induced with a PSSP strain. These results warrant further investigation into the pharmacodynamics of tedizolid phosphate in the respiratory tract, as well as clinical evaluation of tedizolid phosphate for the treatment of pneumococcal infections.
ACKNOWLEDGMENTS
This work was supported by Dong-A Pharmaceutical Co. and Trius Therapeutics, Inc., and conducted under the direction of Dong-A Pharmaceutical Co. Medical writing assistance with preparing the manuscript was provided by Synchrogenix Information Strategies, Inc., and by Sharon Dana, an employee of Trius Therapeutics.
Footnotes
Published ahead of print 19 June 2012
REFERENCES
- 1. Andes D, van Ogtrop ML, Peng J, Craig WA. 2002. In vivo pharmacodynamics of a new oxazolidinone (linezolid). Antimicrob. Agents Chemother. 46:3484–3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown SD, Traczewski MM. 2010. Comparative in vitro antimicrobial activities of torezolid (TR-700), the active moiety of a new oxazolidinone, torezolid phosphate (TR-701), determination of tentative disk diffusion interpretive criteria, and quality control ranges. Antimicrob. Agents Chemother. 54:2063–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Campbell GD, Jr, Silberman R. 1998. Drug-resistant Streptococcus pneumoniae. Clin. Infect. Dis. 26:1188–1195 [DOI] [PubMed] [Google Scholar]
- 4. Centers for Disease Control and Prevention 2008. Effects of new penicillin susceptibility breakpoints for Streptococcus pneumoniae-–United States, 2006-2007. MMWR Morb. Mortal. Wkly. Rep. 57:1353–1355 [PubMed] [Google Scholar]
- 5. Clinical and Laboratory Standards Institute 2008. Performance standards for antimicrobial susceptibility testing; 18th informational supplement. M100-S18. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 6. Conte JE, Jr, Golden JA, Kipps J, Zurlinden E. 2002. Intrapulmonary pharmacokinetics of linezolid. Antimicrob. Agents Chemother. 46:1475–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Drusano GL, Liu W, Kulawy R, Louie A. 2011. Impact of granulocytes on the antimicrobial effect of tedizolid in a mouse thigh infection model. Antimicrob. Agents Chemother. 55:5300–5305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Farrell DJ, Mendes RE, Ross JE, Sader HS, Jones RN. 2011. LEADER Program results for 2009: an activity and spectrum analysis of linezolid using 6,414 clinical isolates from 56 medical centers in the United States. Antimicrob. Agents Chemother. 55:3684–3690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Housman ST, et al. 2012. Pulmonary disposition of tedizolid following administration of once-daily oral 200-milligram tedizolid phosphate in healthy adult volunteers. Antimicrob. Agents Chemother. 56:2627–2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leach KL, et al. 2007. The site of action of oxazolidinone antibiotics in living bacteria and in human mitochondria. Mol. Cell 26:393–402 [DOI] [PubMed] [Google Scholar]
- 11. Lemaire S, Van Bambeke F, Appelbaum PC, Tulkens PM. 2009. Cellular pharmacokinetics and intracellular activity of torezolid (TR-700): studies with human macrophage (THP-1) and endothelial (HUVEC) cell lines. J. Antimicrob. Chemother. 64:1035–1043 [DOI] [PubMed] [Google Scholar]
- 12. Lin AH, Murray RW, Vidmar TJ, Marotti KR. 1997. The oxazolidinone eperezolid binds to the 50S ribosomal subunit and competes with binding of chloramphenicol and lincomycin. Antimicrob. Agents Chemother. 41:2127–2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Louie A, Liu W, Kulawy R, Drusano GL. 2011. In vivo pharmacodynamics of torezolid phosphate (TR-701), a new oxazolidinone antibiotic, against methicillin-susceptible and methicillin-resistant Staphylococcus aureus strains in a mouse thigh infection model. Antimicrob. Agents Chemother. 55:3453–3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lynch JP, III, Zhanel GG. 2009. Streptococcus pneumoniae: does antimicrobial resistance matter? Semin. Respir. Crit. Care Med. 30:210–238 [DOI] [PubMed] [Google Scholar]
- 15. National Committee for Clinical Laboratory Standards 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, NCCLS document M7-A5, 5th ed, vol 20, no. 2 National Committee for Clinical Laboratory Standards, Wayne, PA [Google Scholar]
- 16. Pfizer March 2012, posting date Zyvox® prescribing Information. Pfizer, New York, NY [Google Scholar]
- 17. Schaadt R, Sweeney D, Shinabarger D, Zurenko G. 2009. In vitro activity of TR-700, the active ingredient of the antibacterial prodrug TR-701, a novel oxazolidinone antibacterial agent. Antimicrob. Agents Chemother. 53:3236–3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tessier PR, Keel RA, Hagihara M, Crandon JL, Nicolau DP. 2012. Comparative in vivo efficacies of epithelial lining fluid exposures of tedizolid, linezolid, and vancomycin for methicillin-resistant Staphylococcus aureus in a mouse pneumonia model. Antimicrob. Agents Chemother. 56:2342–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yum JH, et al. 2010. Comparative in vitro activities of torezolid (DA-7157) against clinical isolates of aerobic and anaerobic bacteria in South Korea. Antimicrob. Agents Chemother. 54:5381–5386 [DOI] [PMC free article] [PubMed] [Google Scholar]