Abstract
Biofilms causing biomaterial-associated infection resist antibiotic treatment and usually necessitate the replacement of infected implants. Here we relate bacterial adhesion forces and the antibiotic susceptibility of biofilms on uncoated and polymer brush-coated silicone rubber. Nine strains of Staphylococcus aureus, Staphylococcus epidermidis, and Pseudomonas aeruginosa adhered more weakly to brush-coated silicone rubber (−0.05 ± 0.03 to −0.51 ± 0.62 nN) than to uncoated silicone rubber (−1.05 ± 0.46 to −5.1 ± 1.3 nN). Biofilms of weakly adhering organisms on polymer brush coatings remained in a planktonic state, susceptible to gentamicin, unlike biofilms formed on uncoated silicone rubber.
TEXT
Biomaterial-associated infections (BAI) remain the number one cause of failure of biomaterial implants or devices despite the development of various strategies to control BAI during implantation, like, e.g., modern, ventilated operating theaters and impermeable personnel clothing (14). Microbial adhesion is considered to be the onset of BAI and can lead to the formation of a biofilm, in which microorganisms embed themselves in a complex matrix of extracellular polymeric substances (EPS), which provides protection against antibiotic treatment and the host immune system (5, 7). Surface modifications can significantly reduce microbial adhesion and biofilm formation to biomaterial surfaces (4). Polymer brush coatings are currently the most promising nonadhesive coatings, as they reduce the adhesion of various bacterial strains by orders of magnitude (12). These coatings, however, do not completely suppress microbial adhesion and even the few bacteria adhering to a polymer brush have been demonstrated to be able to form a weakly adhering biofilm (12).
In this study, we hypothesized that bacteria on polymer brush coatings remain in a planktonic state because of weak forces of interaction with highly hydrated polymer brush coatings and hence remain susceptible to antibiotics. This hypothesis, if proven right, would open a new pathway to combat BAI.
Nine bacterial strains, representing Staphylococcus aureus (799, 835, ATCC 12600), Staphylococcus epidermidis (ATCC 35984, HBH 276, 138), and Pseudomonas aeruginosa (#3, 6487, ATCC 19582), were used in this study. Strains were either established type strains or clinical isolates taken from patients with implant- or device-related infections. Bacteria were grown and harvested as described before (12). Implant grade silicone rubber sheets (thickness, 0.5 mm; water contact angle, 110 ± 1°; Medin, Groningen, The Netherlands) were used as substrata. Pluronic F-127 (molecular weight, 12,600; Sigma-Aldrich) was used to prepare polymer brush-coated surfaces (12). Bacterial adhesion forces on uncoated and polymer brush-coated silicone rubber were recorded by using atomic force microscopy (AFM; BioScope Catalyst atomic force microscope with ScanAsyst [Veeco Instruments Inc., Camarillo, CA]). Before each measurement, cantilevers were calibrated by the thermal tuning method. Bacterial probes were prepared by immobilizing single bacteria on an NP-O10 tipless cantilever by using electrostatic attraction (2). All adhesion force measurements were performed in phosphate-buffered saline (PBS) at room temperature under a loading force of 5 nN at three randomly chosen spots and analyzed per strain using a mixed-effects model, taking the absence or presence of the polymer brush coating and probe employed as fixed effects and the spot chosen as a random one. The variance components were separately estimated for coated and uncoated surfaces. Maximum likelihood was used as the estimation method, and a type III test was used to evaluate a significant effect of the polymer brush coating on bacterial adhesion forces.
Bacterial growth and biofilm formation were monitored in a parallel-plate flow chamber (12) for one strain of each species. MICs of gentamicin against these strains were determined by using the Etest (AB bioMérieux, Solna, Sweden), and all strains were found susceptible to gentamicin with MICs of <4 μg/ml (1). After initial bacterial adhesion for 30 min at room temperature by a bacterial suspension (3 × 108 bacteria per ml) in PBS under flow (shear rate, 11 s−1), the flow was switched for 3.5 h to 10% tryptone soya broth at 37°C under reduced flow (shear rate, 5 s−1) to grow a biofilm, after which the chamber was perfused for 16 h with medium containing different concentrations (0.5, 5, and 50 μg/ml, i.e., below, at, and above the MIC) of gentamicin sulfate (Sigma-Aldrich).
From the images taken during the course of an experiment, the percentage of the surface covered by biofilm, indicative of the presence of both dead and live bacteria in the biofilm, was determined. The percentage of live bacteria in 20-h-old biofilms was determined by fluorescence microscopy (Leica, Wetzlar, Germany) after dispersal of the biofilms and live/dead staining of the organisms as an indicator of antibiotic susceptibility of biofilm organisms (12) and to calculate the surface coverage by live bacteria. All experiments for quantitative biofilm analysis were done in duplicate with separately grown bacterial cultures. In a separate set of experiments, intact biofilms were visualized using a Leica TCS SP2 confocal laser scanning microscope (CLSM; Leica Microsystems GmbH, Heidelberg, Germany). Twenty-hour-old biofilms were stained with live/dead stain mixed with calcofluor white, which was used to visualize EPS. For surface coverage, an analysis of variance was conducted for each bacterial strain. If an overall effect of the surface coating on the outcomes was significant, Fisher's least-significant-difference test was used to investigate the effect of the coating at each antibiotic concentration. All tests were conducted two sided, and a significance level of P < 0.05 was used.
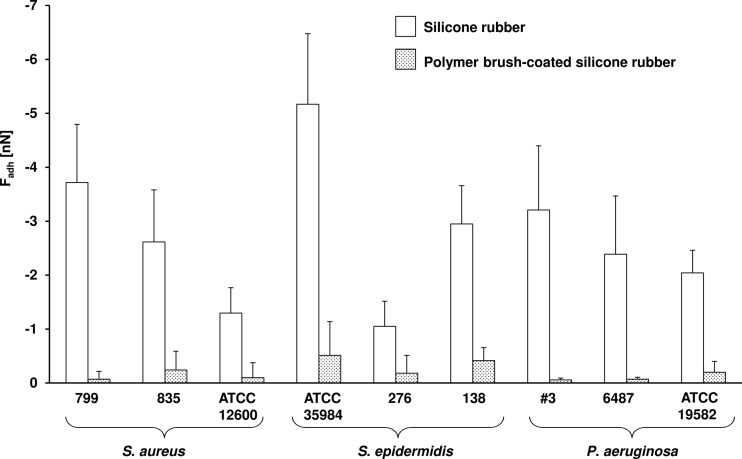
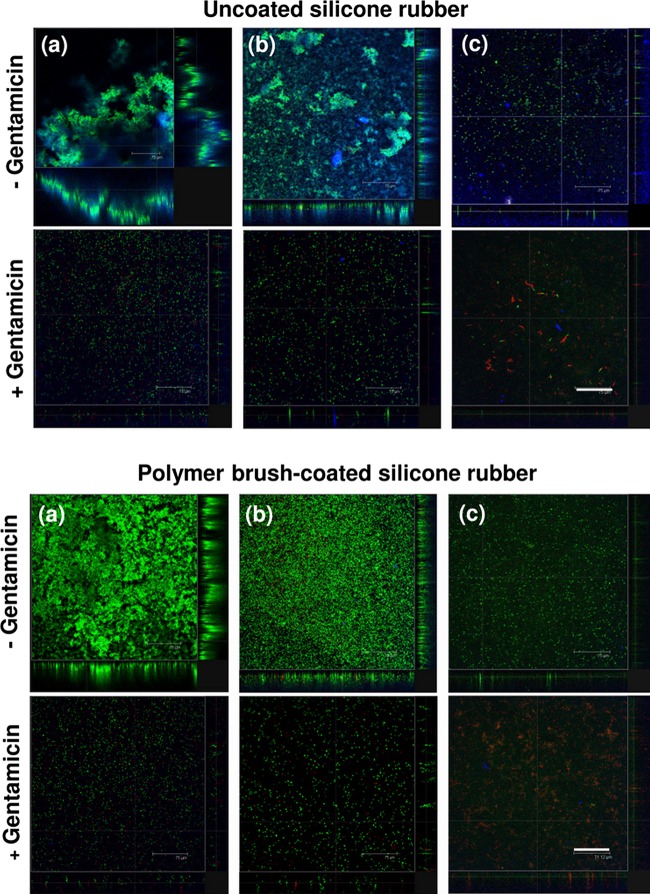
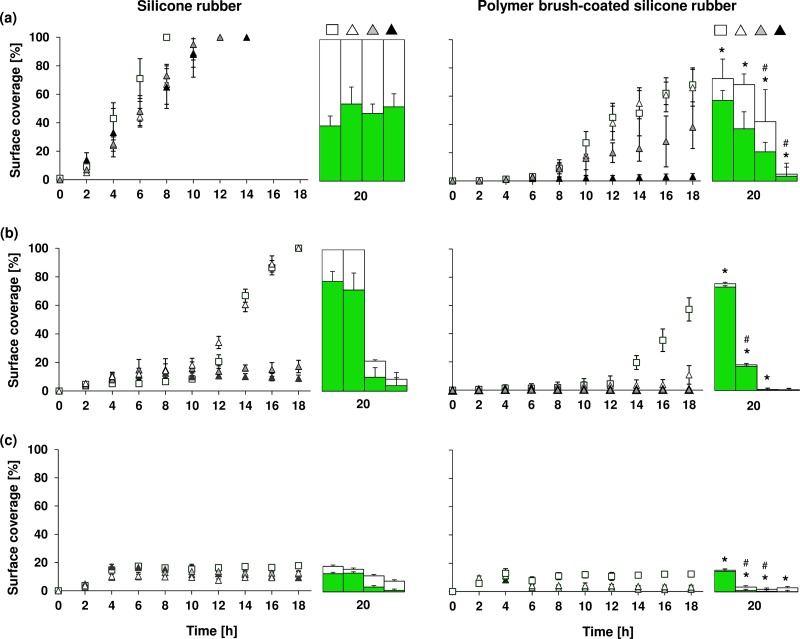
The adhesion forces of all strains were lower on polymer brush-coated silicone rubber (−0.05 ± 0.03 to −0.51 ± 0.62 nN) than on uncoated silicone rubber (−1.05 ± 0.46 to −5.1 ± 1.3 nN), representing a significant (P < 0.05) reduction (Fig. 1). Biofilm formation of selected strains representing each of the three different species on uncoated silicone rubber was accompanied by the production of EPS in large amounts, especially for the staphylococcal biofilms, while EPS production was virtually absent on polymer brush-coated silicone rubber (Fig. 2). Biofilms of both staphylococcal strains in the absence of antibiotics achieved full surface coverage of uncoated silicone rubber within 14 to 16 h, while full coverage of polymer brush-coated silicone rubber was not reached within 20 h (Fig. 3). Such a difference in growth kinetics was absent in the case of P. aeruginosa, yielding less than 20% surface coverage even on uncoated silicone rubber, possibly as a result of its rod-shaped morphology and motility. Importantly, biofilm growth in the presence of various concentrations of gentamicin was reduced significantly more strongly on polymer brush-coated silicone rubber than on uncoated silicone rubber. Surface coverage by P. aeruginosa remained similarly low in the presence of gentamicin than in its absence. Moreover, after 20 h of growth, the coverage by live bacteria in the absence of antibiotics was higher on polymer brush-coated silicone rubber than on uncoated silicone rubber, while in the presence of gentamicin, we saw less coverage by live bacteria on the polymer brush coating, with little or no efficacy of the antibiotic on biofilms formed on silicone rubber, depending on the strain considered (Fig. 3).
Fig 1.
Forces of bacterial adhesion (Fadh) to uncoated and polymer brush-coated silicone rubber, showing significant reductions in adhesion forces (P < 0.05) for all nine strains after the silicone rubber surface was coated with a polymer brush.
Fig 2.
CLSM overlay images and optical sections of 20-h-old intact biofilms grown in the absence (−) or presence (+) of 50 μg/ml gentamicin on uncoated silicone rubber or polymer brush-coated silicone rubber. Live and dead bacteria show green and red fluorescence, respectively, while EPS yields blue fluorescent patches. Bars, 75 μm. Panels: a, S. aureus ATCC 12600; b, S. epidermidis 138; c, P. aeruginosa #3.
Fig 3.
Surface coverage as a function of time on uncoated silicone rubber and polymer brush-coated silicone rubber by biofilms grown in the absence or presence of various concentrations of gentamicin (open squares, no antibiotic; open triangles, 0.5 μg/ml gentamicin; gray triangles, 5 μg/ml gentamicin; black triangles, 50 μg/ml gentamicin) and coverage by live organisms after 20 h of growth (green bars). Gentamicin was introduced after 4 h of growth. Error bars represent standard deviations of two separate experiments. Panels: a, S. aureus ATCC 12600; b, S. epidermidis 138; c, P. aeruginosa #3. An asterisk indicates a significant difference (P < 0.05) between uncoated silicone rubber and polymer brush-coated silicone rubber in surface coverage by biofilm after 20 h of growth. The symbol # indicates a significant difference (P < 0.05) between the numbers of live bacteria in 20-h-old biofilms on silicone rubber and polymer brush-coated silicone rubber.
Furthermore, the amount of EPS produced on polymer brush coatings was smaller, explaining the higher susceptibility to gentamicin. Thus, in addition to the known lower biofilm formation on polymer brush coatings than on common biomaterials, this study is the first to demonstrate that bacterial biofilms on a polymer brush coating remain susceptible to antibiotics, regardless of the molecular basis of the resistance mechanism. This phenomenon has enormous clinical implications, as it shows an original pathway toward a biomaterial implant coating that allows antibiotic treatment to prevent biofilm formation and thereby reduce the risk of BAI.
Upon bacterial adhesion, a cascade of genotypic and phenotypic changes are induced that result in a biofilm-specific phenotype (8, 10, 13). Changes in gene regulation occur within minutes after bacterial attachment to a solid surface (6), suggesting that adhering bacteria may sense a solid surface, leading to a signaling cascade that causes genes to be up- or downregulated and the production of EPS (9), rendering the organisms more resistant to antimicrobial agents (8, 11, 15). Recently it has been argued that in the absence of visual, auditory, and olfactory perception, adhering bacteria react to membrane stresses arising from minor deformations due to the adhesion forces felt to make them aware of their adhering state on a surface and change from a planktonic to a biofilm phenotype (3). The adhesion forces of nine different bacterial strains are clearly much higher on silicone rubber than on polymer brush coatings, and in fact, on the polymer brush, these forces are so low that it can be argued that bacteria, though weakly adhering, are unable to sense the surface as they do on silicone rubber. As a result, they remain in their antibiotic-susceptible state, whereas on silicone rubber, they adopt a biofilm mode of growth with full protection against a gentamicin concentration of 50 μg/ml, far above the MIC.
This is the first study providing a link between bacterial adhesion forces and the susceptibility of bacterial biofilms, providing a clear clue as to why the susceptibility of biofilms of bacteria of the same strain may differ on different biomaterials. In fact, on the basis of the present study, it can be concluded that the transition of microorganisms from a planktonic state to their protected biofilm mode of growth is not merely dictated by the absence or presence of a substratum material but depends on the forces exerted on the organisms by the substratum surface. This is clinically relevant, as it suggests that antimicrobial treatment of BAI could be more effective in cases where infection occurs after the implantation of a polymer brush-coated implant or device.
ACKNOWLEDGMENT
This work was supported by the University Medical Center, Groningen, The Netherlands.
Footnotes
Published ahead of print 25 June 2012
REFERENCES
- 1. Andrews JM. 2009. BSAC standardized disc susceptibility testing method (version 8). J. Antimicrob. Chemother. 64:454–489 [DOI] [PubMed] [Google Scholar]
- 2. Bolshakova AV, et al. 2001. Comparative studies of bacteria with an atomic force microscopy operating in different modes. Ultramicroscopy 86:121–128 [DOI] [PubMed] [Google Scholar]
- 3. Busscher HJ, Van der Mei HC. 2012. How do bacteria know they are on a surface and regulate their response to an adhering state? PLoS Pathog. 8:e1002440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cheng G, Zhang Z, Chen SF, Bryers JD, Jiang S. 2007. Inhibition of bacterial adhesion and biofilm formation on zwitterionic surfaces. Biomaterials 28:4192–4199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Costerton JW, Stewart PS, Greenberg EP. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322 [DOI] [PubMed] [Google Scholar]
- 6. Davies DG, Geesey GG. 1995. Regulation of the alginate biosynthesis gene algC in Pseudomonas aeruginosa during biofilm development in continuous culture. Appl. Environ. Microbiol. 61:860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. del Pozo JL, Patel R. 2007. The challenge of treating biofilm-associated bacterial infection. Clin. Pharmacol. Ther. 82:204–209 [DOI] [PubMed] [Google Scholar]
- 8. Drenkard E, Ausubel FM. 2002. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotypic variation. Nature 416:740–743 [DOI] [PubMed] [Google Scholar]
- 9. Jefferson KK. 2004. What drives bacteria to produce a biofilm? FEMS Microbiol. Lett. 236:163–173 [DOI] [PubMed] [Google Scholar]
- 10. Mah TFC, O'Toole GA. 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9:34–39 [DOI] [PubMed] [Google Scholar]
- 11. Mah TF, et al. 2003. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 426:306–310 [DOI] [PubMed] [Google Scholar]
- 12. Nejadnik MR, Van der Mei HC, Norde Busscher WHJ. 2008. Bacterial adhesion and growth on a polymer brush-coating. Biomaterials 29:4117–4121 [DOI] [PubMed] [Google Scholar]
- 13. Schachter B. 2003. Slimy business—the biotechnology of biofilms. Nat. Biotechnol. 21:361–365 [DOI] [PubMed] [Google Scholar]
- 14. Verkkala K, et al. 1998. The conventionally ventilated operating theatre and air contamination control during cardiac surgery—bacteriological and particulate matter control garment options for low level contamination. Eur. J. Cardiothorac. Surg. 14:206–210 [DOI] [PubMed] [Google Scholar]
- 15. Whiteley M, et al. 2001. Gene expression in Pseudomonas aeruginosa biofilms. Nature 413:860–864 [DOI] [PubMed] [Google Scholar]