Abstract
Background:
Microalbuminuria in hypertension has been described as an early sign of kidney damage and a predictor for end stage renal disease and cardiovascular disease. Thus, it is of great importance to study urinary albumin creatinine ratio and progression of kidney disease in hypertensive patients.
Aims:
The present study was undertaken to find out the prevalence and association of microalbuminuria in newly diagnosed essential hypertension.
Materials and Methods:
Newly diagnosed essential hypertensive cases (n = 106) and normotensive controls (n = 106) were enrolled. Hypertension was defined according to Joint national committee-VII definitions. Microalbuminuria was measured using an U-Albumin (NycoCard, Norway) and adjusted for urine creatinine. Descriptive statistics and testing of hypothesis were used for the analysis using SPSS 16 software.
Results:
51.88% of hypertension cases and 13.2% of normotensive controls had microalbuminuria in total population (odds ratio 7.086, P-value <0.001). 46.67% of cases and 12.08% of controls had microalbuminuria in male population (odds ratio 6.375, P-value <0.001). Similarly, 58.7% of cases and 14.58% of controls had microalbuminuria in female population (odds ratio 8.32, P-value <0.001).
Conclusions:
By showing strong association between microalbuminuria and hypertension, our findings suggest that microalbuminuria could be a useful marker to assess risk management of cardiovascular disease and renal disease.
Keywords: Albumin creatinine ratio, Hypertension, Normotension, Microalbuminuria
Introduction
Hypertension is the growing issues of public health problem of adult population in both developed as well as developing world, affecting single person in every four people.[1] The exact cause for hypertension is difficult to predict because hypertension results from a complex interaction of genes and environmental factors.[1] Microalbuminuria (MAU) in essential hypertension is associated with the increased mortality. Microalbuminuria is the independent risk factor to develop cardiovascular and cerebrovascular diseases. Furthermore, MAU has been described as an early sign of kidney damage and a redactor for end stage renal disease (ESRD) and cardiovascular disease.[2] MAU is defined as the increased urinary excretion of albuminuria (30-300 mg/24 h) which cannot be detected by routine protein dipstick method.[3] Measurement of MAU can be done by using random spot urine sample. Due to the variation in urinary flow rate and concentration, the excreted urinary albumin can be adjusted to creatininuria. Thus, obtaining urinary albumin creatinine ratio (ACR) of 30-300 mg albumin/g creatinine, corresponding to 3.4-33.9 mg albumin/ mmol creatine can be considered positive ACR or microalbuminuria.[4]
Thus, it is of great importance to study urinary ACR and progression of kidney disease in hypertensive patients. This type of study has not been reported in Nepalese population so far. Thus, the objective of our study was to find out the prevalence and association of urinary microalbuminuria in newly diagnosed essential hypertensive patients in Nepalese population.
Materials and Methods
This hospital based cross-sectional study was conducted in the Department of Biochemistry in collaboration with Department of Internal Medicine (nephrology unit), Tribhuvan University Teaching Hospital (TUTH), Nepal from 2008 February to 2010 August. Ethical approval was taken from the ethical board of TUTH, Kathmandu, Nepal. Written consent was taken from the participants. We enrolled 106 essential hypertensive cases of age between 25 years and 65 years and similar numbers as well as age of normotensive healthy controls were also enrolled.
Data collection
A medical history was taken and a physical examination was performed by a physician. Age, sex, and weight were collected from the participants. BMI was calculated by weight in kilogram divided by height in meter squared. We measured biochemical profile including total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), triglyceride (TG), creatinine, and uric acid from all participants. The urine sample was also processed on the same day and estimated for urinary microalbuminuria and creatinine. Laboratory standard operation procedures were maintained for all laboratory analysis. Internal quality control sera, both normal, and pathological, were also run for each lot of the test, for the validation of the results.
Inclusion criteria
Subjects having age of more than 25 years and less than 65 years with essential hypertension were enrolled as cases. Similarly, the age of more than 25 years and less than 65 years without hypertension were enrolled as a healthy control group.
Exclusion criteria
Subjects having age less than 25 years and more than 65 years, diabetes mellitus, chronic kidney disease, end stage renal failure, nephrotic syndrome, pregnancy, and under steroid therapy were excluded from study.
Definition
Hypertension was categorized according to blood pressure readings by JNC-VII definitions: normal (systolic <120 mm Hg and diastolic <80 mm Hg), prehypertension (systolic 120 to 139 mm Hg or diastolic 80 to 89 mm Hg), hypertension stage I (systolic 140 to 159 mm Hg or diastolic 90 to 99 mm Hg), and hypertension stage II (systolic ≥160 or diastolic ≥100 mm Hg).[5]
In spot urine sample albumin was measured quantitatively and adjusted to creatininuria then interpreted as albumin creatinine ratio (ACR) <3.4 mg albumin/mmol creatinine as normal albuminuria, ≥3.4-33.9 mg albumin/ mmol creatinine as microalbuminuria, and >33.9 mg albumin/ mmol creatinine as macroalbuminuria.[6]
The formula of Cockcroft and Gault equation was used to calculate eGFR.[7]
Calculation of eGFR in males:
eGFR = 140-age (in years) × weight (in kg) × 88.4/[72 × serum creatinine (μmol/L)] .
A companion equation for women, based on their 15% lower muscle mass (on average):
eGFR = 140-age (in years) × weight (in kg) × 88.4 ×0.85/[72 × serum creatinine (μmol/L)].
Statistical analysis
The data were analyzed using Excel 2003, R 2.8.0 Statistical Package for the Social Sciences (SPSS) for Windows Version 16.0 (SPSS Inc; Chicago, IL, USA). Association between hypertension and microalbuminuria was tested by Chi-square test. Odds ratio was also calculated. Comparison of mean of continuous data between stages different group of urinary ACR level was tested by ANOVA test. A P-value of <0.05 (two-tailed) was used to establish statistical significance.
Results
Table 1 shows the categorical association between hypertension and microalbuminuria. To define microalbuminuria, we had taken cut off point for urinary ACR ≥3.4 mg/mmol. Microalbuminuria in male hypertensive cases, female hypertensive cases, and total hypertensive cases were found to be 46.67, 58.7, and 51.88%, respectively. Similarly, microalbuminuria in male normotensive healthy controls, female normotensive healthy controls, and total normotensive healthy controls were found to be 12.08, 14.58, and 13.2%, respectively. The association between hypertension and microalbuminuria was very strong (P-value <0.001).
Table 1.
Distribution of study population according to microalbuminuria in male and Female (categorized by taking cut off point ≥3.4 mg albumin/mmol creatinine for microalbuminuria)
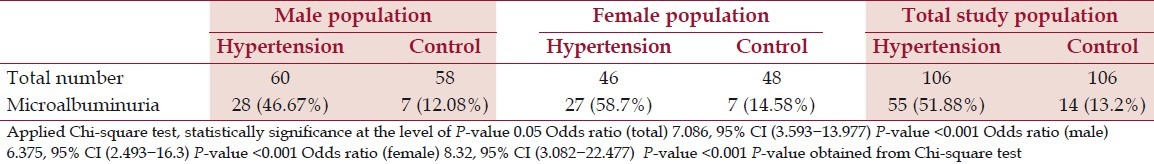
Table 2 shows the prevalence of microalbuminuria according to sex and age in different stages of blood pressure. Prevalence of microalbuminuria increases with the level of blood pressure. Microalbuminuria was found in higher number with the advances of ages and it was again worse in female.
Table 2.
Prevalence (in percentage) of microalbuminuria in persons with hypertension
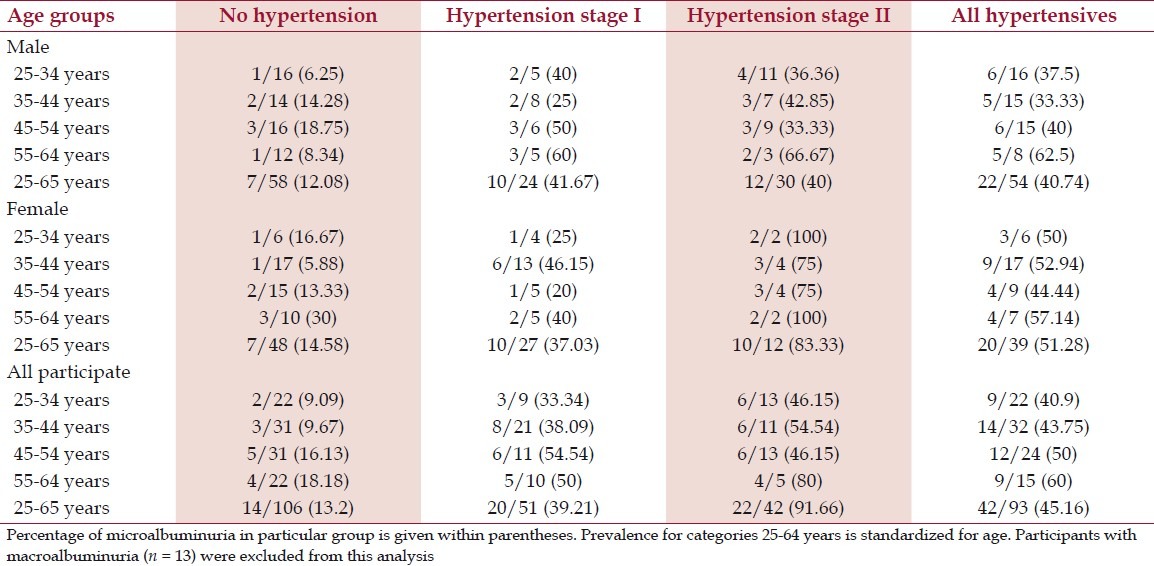
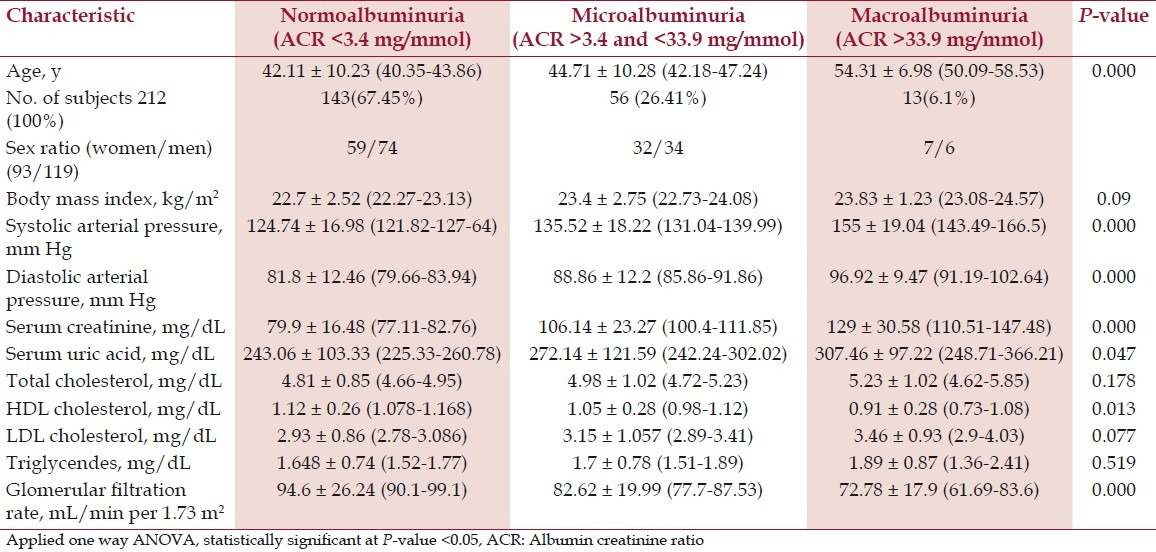
Table 3 shows the comparison of mean value of demographic, clinical, and biochemical characteristics to different level of ACR group. Serum level of total cholesterol (P-value 0.178), TG (P-value 0.519), and LDL-C (P-value 0.077) were found to be insignificantly increased with the increased level of urinary ACR. However, serum level of HDL-C (P-value 0.013) was found to be significantly decreased with the increased level of urinary ACR. Similarly, serum level of uric acid (P-value 0.07) and creatinine (P-value <0.001) were found to be significantly increased with the increased level of urinary ACR. With the increases of age (P-value <0.001), the level of urinary ACR was also increased. Systolic blood pressure (P-value <0.001) and diastolic blood pressure (P-value <0.001) were significantly increased with the level of urinary ACR whereas eGFR (P-value <0.001) level was significantly decreased with increased level of urinary ACR.
Table 3.
Demographic, clinical, and renal characteristics of participants with different level of ACR
Discussion
Among the 106 hypertensive patients, 51 patients with non CKD had normoalbuminuria, 42 patients had microalbuminuria, and 13 patients had macroalbuminuria. We found 51.88% of overall hypertensive patients had microalbuminuira positive and 13.2% of general population had microalbuminuria positive by taking the cut off point of ACR 3.4 mg albumin/mmol creatinine to define microalbuminuria. Some of the study showed approximately 30% of hypertensive patients had microalbuminuria positive[8,9] and some other studies reported even higher prevalence of microalbuminuria in hypertensive patients.[10,11] Strong association between microalbuminuria and hypertension was found. The association between MAU and hypertension is independent of renal function markers. Some factors bring about the high prevalence of MAU in our study. The newly diagnosed essential hypertension (with BP>140/90 mmHg) of age 25-65 years visiting TUTH were enrolled in this study and the presence of obesity was not considered in both normal healthy controls who were enrolled after normal blood pressure (<120/80 mmHg) as well as hypertensive cases. Excess body weight has been reported to be associated with MAU and albuminuria independently of other risk factors such as hypertension and diabetes. We reported high prevalence of microalbuminuira (13.2%) in normotensive healthy controls and our report suggests that the presence of microalbuminuria may be due to different causes other than hypertension and diabetes.[12]
The prevalence of microalbuminuria in female hypertensive population and male hypertensive population were 58.7 and 46.67%, respectively. However, the prevalence of microalbuminuria between genders in normotensive population was hardly different which accounts for 14.58 and 12.08% of microalbuminuria in normotensive female population and normotensive male population, respectively. The prevalence of MAU in female hypertensive population was higher than in male hypertensive population of our study whereas the reverse was found in some other populations.[13] Increased prevalence of MAU in female than in male might be related to lower muscle mass (approximately 15% less) hence low creatinuria in women. Therefore, some authors have suggested the use of sex-related cut-off points to define MAU (2.5-30 mg albumin/ mmol creatinine for male and 3.5-30 mg albumin/mmol creatinine for female).[14,15] However, we have not used the sex-specific cut-off point of ACR. Moderately higher prevalence of microalbuminuria in male and lower microalbuminuria in female would results in case of using a sex-specific cut off point for urinary ACR. However, the overall prevalence of microalbuminuria might not be altered to a large extent. Such sex-specific criteria might have a particular impact on the prevalence of microalbuminuria in the youngest age group in which muscle mass is high which may trigger somewhat increased urinary creatinine excretion. Besides chosen cut off points for urinary ACR, this difference may also be consistent with a increased prevalence of obesity in female hypertensive population than in male hypertensive population, but it was not considered in our study population . Obesity has been found to be an independent risk factor of microalbuminuria. Zheng et al. reported a possible association between a BMI and albuminuria.[16] The mean value of body mass index (BMI) was found to be increased insignificantly (P-value 0.09) with the increased level of ACR. Our result gives some support to the findings of Zheng et al. but insignificant.
We found that serum level of uric acid (P-value 0.047) and creatinine (P-value <0.001) were increased with the increased level of urinary ACR, although, both the parameters fell well within the normal reference range. An increasing urinary albumin excretion was noted with the severity of hypertension. The higher level of uric acid and creatinine with the increased level of urinary ACR may be due to subclinical ultra structural changes in the glomeruli of hypertensive patients.[17] Higher level of urinary ACR was noted with the advances of age (P-value <0.001). Proteinuria represents a predictor of progressive renal impairment in all form of glomerulonephritis.[18] Some studies reported that the presence of microalbuminuria in early stage of hypertension can be taken as an important independent predictor for progression of renal disease.[6,9,11]
Conclusion
We found strong association between microalbuminuria and hypertension. Hypertension is the established risk factor for cardiovascular disease and renal disease. By showing strong association between MAU and hypertension, our findings suggest that MAU could be a useful marker to assess risk management of cardiovascular disease and renal disease. Further studies in large scale population are required to further verification of MAU as a screening tool to predict cardiovascular and renal disease.
Footnotes
Source of Support: Department of Biochemistry, Institute of Medicine, Kathmandu, Nepal.
Conflict of Interest: None declared.
References
- 1.Balam-Ortiz E, Esquivel-Villarreal A, Huerta-Hernandez D, Fernandez-Lopez JC, Alfaro-Ruiz L, Muñoz-Monroy O, et al. Hypercontrols in genotype-phenotype analysis reveal ancestral haplotypes associated with essential hypertension. Hypertension. 2012;59:847–53. doi: 10.1161/HYPERTENSIONAHA.111.176453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duran M, Kalay N, Ardic I, Yarlioglues M, Kayaalti F, Yilmaz Y, et al. Microalbuminuria is not associated with endothelial dysfunction and coronary atherosclerosis in patients with acute coronary syndromes. Ren Fail. 2010;32:659–65. doi: 10.3109/0886022X.2010.485288. [DOI] [PubMed] [Google Scholar]
- 3.Incerti J, Zelmanovitz T, Camargo JL, Gross JL, de Azevedo MJ. Evaluation of tests for microalbuminuria screening in patients with diabetes. Nephrol Dial Transplant. 2005;20:2402–7. doi: 10.1093/ndt/gfi074. [DOI] [PubMed] [Google Scholar]
- 4.Croal BL, Mutch WJ, Clark BM, Dickie A, Church J, Noble D, et al. The clinical application of a urine albumin:creatinine ratio point-of-care device. Clin Chim Acta. 2001;307:15–21. doi: 10.1016/s0009-8981(01)00450-8. [DOI] [PubMed] [Google Scholar]
- 5.Lenfant C, Chobanian AV, Jones DW, Roccella EJ. Seventh report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7): Resetting the hypertension sails. Hypertension. 2003;41:1178–9. doi: 10.1161/01.HYP.0000075790.33892.AE. [DOI] [PubMed] [Google Scholar]
- 6.Pruijm MT, Madeleine G, Riesen WF, Burnier M, Bovet P. Prevalence of microalbuminuria in the general population of Seychelles and strong association with diabetes and hypertension independent of renal markers. J Hypertens. 2008;26:871–7. doi: 10.1097/HJH.0b013e3282f624d9. [DOI] [PubMed] [Google Scholar]
- 7.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 8.Schrader J, Lüders S, Kulschewski A, Hammersen F, Züchner C, Venneklaas U, et al. Microalbuminuria and tubular proteinuria as risk predictors of cardiovascular morbidity and mortality in essential hypertension: Final results of a prospective long-term study (MARPLE Study) J Hypertens. 2006;24:541–8. doi: 10.1097/01.hjh.0000209991.48928.c4. [DOI] [PubMed] [Google Scholar]
- 9.McKenna MJ, Arias C, Feldkamp CS, Whitehouse FW. Microalbuminuria in clinical practice. Arch Intern Med. 1991;151:1745–7. [PubMed] [Google Scholar]
- 10.Losito A, Fortunati F, Zampi I, Del Favero A. Impaired renal functional reserve and albuminuria in essential hypertension. Br Med J (Clin Res Ed) 1988;36:1562–4. doi: 10.1136/bmj.296.6636.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giaconi S, Levanti C, Fommei E, Innocenti F, Seghieri G, Palla L, et al. Microalbuminuria and casual and ambulatory blood pressure monitoring in normotensives and in patients with borderline and mild essential hypertension. Am J Hypertens. 1989;2:259–61. doi: 10.1093/ajh/2.4.259. [DOI] [PubMed] [Google Scholar]
- 12.Bovet P, Shamlaye C, Gabriel A, Riesen W, Paccaud F. Prevalence of cardiovascular risk factors in a middle income country and estimated cost of a treatment strategy. BMC Public Health. 2006;6:9. doi: 10.1186/1471-2458-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joshi VD, Nandkumar M, Lim J. Prevalence and risk factors of undetected proteinuria in an eldery South-East Asian population. Nephrology (Carlton) 2006;11:347–54. doi: 10.1111/j.1440-1797.2006.00593.x. [DOI] [PubMed] [Google Scholar]
- 14.Mattix HJ, Hsu CY, Shaykevich S, Curhan G. Use of albumin/creatinine ratio to detect microalbuminuria: Implications of sex and race. J Am Soc Nephrol. 2002;13:1034–9. doi: 10.1681/ASN.V1341034. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs DR, Murtaugh MA, Steffes M, Yu X, Roseman J, Goetz FC. Gender and race-specific determination of albumin-to-creatinine ratio in single, untimed urine specimens: The Coronary Artery Risk Development in Young Adults Study. Am J Epidemiol. 2002;155:1114–9. doi: 10.1093/aje/155.12.1114. [DOI] [PubMed] [Google Scholar]
- 16.Zheng J, Ye P, Wang X, Xiao WK, Wu HM. The relationship between obesity and microalbuminuria among general population in Beijing area. Zhonghua Nei Ke Za Zhi. 2011;50:388–92. [PubMed] [Google Scholar]
- 17.Rodilla E, Pérez-Lahiguera F, Costa JA, González C, Miralles A, Moral D, et al. Association between serum uric acid, metabolic syndrome and microalbuminuria in previously untreated essential hypertensive patients. Med Clin (Barc) 2009;132:1–6. doi: 10.1016/j.medcli.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Maschio G, Oldrizzi L, Rugin C, Valvo E, Lupo A, Loschiavo C, et al. Factors affecting progression of ranl failure in patients on long terms dietary protein restriction. Kidney Int Suppl. 1987;22:S49–52. [PubMed] [Google Scholar]


